Significance
The unfolded protein response (UPR) maintains protein-folding homeostasis in the endoplasmic reticulum (ER). The UPR is involved in diseases such as triple-negative breast cancer, developmental processes such as B-cell activation, and the decision to commit to apoptosis. In principle, the UPR could restore homeostasis to the ER by increasing folding capacity or reducing the load of newly synthesized proteins. Here we report that budding yeast augments the UPR with PKA signaling and reduces the load of newly synthesized proteins, a role functionally analogous to the PKR-like ER kinase (PERK) and regulated inositol-requiring enzyme 1 (IRE1)-dependent mRNA decay (RIDD) pathways in other organisms. Accounting for all the pathways that contribute to stress resistance is necessary to understand how perturbations, including targeted therapies, propagate through the cell.
Abstract
During environmental, developmental, or genetic stress, the cell’s folding capacity can become overwhelmed, and misfolded proteins can accumulate in all cell compartments. Eukaryotes evolved the unfolded protein response (UPR) to counteract proteotoxic stress in the endoplasmic reticulum (ER). Although the UPR is vital to restoring homeostasis to protein folding in the ER, it has become evident that the response to ER stress is not limited to the UPR. Here, we used engineered orthogonal UPR induction, deep mRNA sequencing, and dynamic flow cytometry to dissect the cell’s response to ER stress comprehensively. We show that budding yeast augments the UPR with time-delayed Ras/PKA signaling. This second wave of transcriptional dynamics is independent of the UPR and is necessary for fitness in the presence of ER stress, partially due to a reduction in general protein synthesis. This Ras/PKA-mediated effect functionally mimics other mechanisms, such as translational control by PKR-like ER kinase (PERK) and regulated inositol-requiring enzyme 1 (IRE1)-dependent mRNA decay (RIDD), which reduce the load of proteins entering the ER in response to ER stress in metazoan cells.
Endoplasmic reticulum (ER) protein-folding homeostasis requires sufficient protein-folding capacity to meet the secretory demands of the cell. The ER needs to contain enough volume, chaperone proteins, glycosylation enzymes, oxidation enzymes, and degradation machinery to keep up with the influx of newly synthesized proteins (1). Misfolded proteins accumulate in the ER when the protein-folding capacity is overwhelmed, a condition known as ER stress. Eukaryotic cells evolved a set of signaling pathways collectively known as the unfolded protein response (UPR) to counteract ER stress (2).
In budding yeast, the UPR consists of a single pathway, initiated by activation of the ER-resident transmembrane protein inositol-requiring enzyme 1 (Ire1) (3, 4). Under ER stress conditions, misfolded proteins directly bind to the ER-luminal stress-sensing domain of Ire1, triggering its oligomerization (5, 6). Oligomerization of the luminal domain activates Ire1’s cytoplasmic effector domains, including its RNase function (7), that upon recruitment of HAC1 mRNA excises the nonconventional intron, alleviating translational repression (8, 9). Translation of the ligated exons produces Hac1, a transcription factor that induces the UPR target genes that serve to increase the protein folding and degradation capacity of the ER (10). Once the response is sufficient to counteract the stress and ER homeostasis is restored, the UPR turns off as Ire1 deoligomerizes (6, 11–13).
Although the UPR in fission yeast also uses Ire1 to initiate the response to ER stress, Ire1 activation does not induce a transcriptional response (14). Rather, fission yeast relies on a process known as regulated Ire1-dependent mRNA decay (RIDD), in which Ire1 degrades ER-associated mRNAs and thereby decreases translation and protein influx (14). Thus, the UPR can restore homeostasis either by increasing the protein-folding capacity of the ER, as in budding yeast, or by decreasing the protein-folding demand by reducing the influx of newly synthesized proteins, as in fission yeast, or by using a combination of both mechanisms, as in metazoan cells.
Metazoan cells have elaborated the UPR into three branches: the IRE1, ATF6, and PKR-like ER kinase (PERK) branches (2). The IRE1 branch both increases protein-folding capacity by activating the transcription factor XBP1 and decreases protein influx via RIDD (15, 16). The ATF6 branch induces target genes that increase ER folding capacity (17). The PERK branch reduces protein influx by reducing global translation initiation through phosphorylation of eIF2α (18), but also induces a transcriptional response through the selective translation of transcription factors like ATF4 (19). The extent, duration, and mode of ER stress can result in complex dynamics and interplay between these three branches that ultimately determine whether homeostasis is restored or whether cells commit to apoptosis (2).
The dynamic response to ER stress in mammalian cells is multifaceted, yet it has become increasingly clear that, even in budding yeast, coping with ER stress involves more than just Ire1 regulation. In addition to the UPR, three mitogen-activated protein kinase (MAPK) pathways—the Slt2-mediated cell wall integrity pathway, the Hog1-mediated hyperosmotic stress response, and the Kss1-mediated invasive growth pathway—have been implicated in the response to ER stress (20–22). Moreover, microarray studies revealed that the transcriptional response to ER stress includes target genes induced in many other stress conditions (23). A subset of this plethora of targets constitute the general stress response (GSR) controlled by the transcription factors Msn2 and Msn4 (Msn2/4), which are in turn regulated by protein kinase A (PKA) (24). In addition to controlling the GSR, PKA regulates translation and ribosome biogenesis (25). The small GTPase Ras2 regulates PKA via cAMP production. Ras2 is also upstream of Kss1 in the invasive growth pathway (26).
To integrate the various signaling pathways implicated in the response to ER stress into a unified model, we explored the transcriptional changes in yeast cells experiencing ER stress. Through engineered activation of the UPR transcription factor Hac1 in the absence of ER stress, we account for Hac1-dependent and -independent transcriptional changes without the pleiotropic effects of deletion strains. We show that budding yeast complements the Hac1-dependent response with a second, Hac1-independent transcriptional program mediated by PKA signaling. Hac1 activation and PKA deactivation together account for the majority of the response to ER stress.
Results
ER Stress Activates a Hac1-Independent Transcriptional Response.
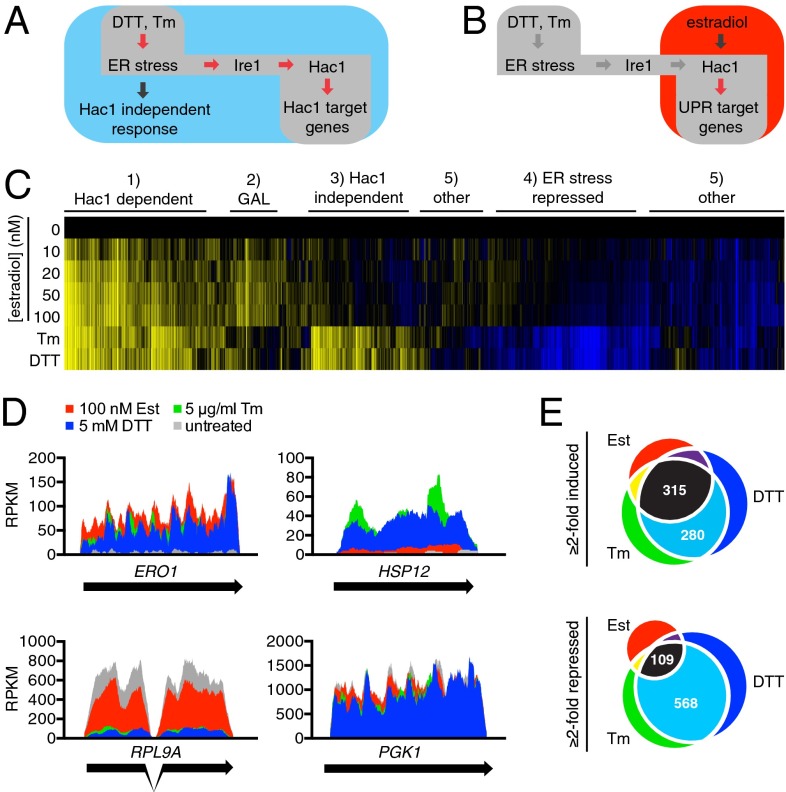
To obtain a broad overview of the various programs elicited by ER stress and determine their Hac1-dependence, we used mRNA deep sequencing (RNA seq). In this experiment, we compared the transcriptional profile of cells experiencing ER stress to cells in which we induced the ectopic expression of the UPR transcription activator HAC1 (Fig. 1 A and B). To control production of Hac1 in the absence of ER stress, we fused an ORF encoding HAC1i to the GAL1 promoter. HAC1i encodes an intron-less mRNA that is translated into active Hac1 protein, bypassing the requirement for Ire1 activity (27). We integrated PGAL1–HAC1i into the genome of a yeast strain expressing a chimeric transcription factor comprising the DNA-binding domain of Gal4, the ligand-binding domain of the estrogen receptor, and a transcription-activation domain. This chimeric transcription factor induces expression of genes containing UASGAL motifs in their promoters in proportion to the concentration of estradiol in the medium (Fig. S1 and Tables S1 and S2). In two distinct ER stress conditions—addition of the reducing agent DTT (5 mM final concentration) or the N-linked glycosylation inhibitor tunicamycin (5 µg/mL final concentration)—we observed largely overlapping changes in the transcriptome (Fig. 1C and Dataset S1). By contrast, expression of Hac1 in the absence of ER stress led to a qualitatively different transcriptional response (Fig. 1C and Dataset S1).
Fig. 1.
ER stress activates a Hac1-independent transcriptional response. (A) Schematic of the RNA-seq samples from ER-stressed cells. (B) Schematic of RNA-seq samples from specific activation of the UPR in the absence of ER stress via ectopic expression of Hac1 by the addition of estradiol (Est). (C) Clustered heat map of the fold change of gene expression ectopic Hac1 expression (0–100 nM estradiol for 2 h), treatment with 5 mM DTT and 5 µg/mL tunicamycin (Tm) for 4 h. Yellow indicates up-regulated genes; blue indicates down-regulated genes. Cells were collected after 3 h of treatment, and the mRNA was purified and sequenced. (D) Coverage plots of mapped reads for ERO1, HSP12, RPL9A, and PGK1. (E) Venn diagram of the number of genes induced (Upper) or repressed (Lower) by estradiol, tunicamycin, and DTT.
Clustering analysis revealed five major categories of genes (Fig. 1C): (i) genes up-regulated in an estradiol (i.e., Hac1) dose-dependent manner that were also induced by DTT and tunicamycin (“Hac1-dependent”; for example, ERO1), a set that largely matched the UPR targets identified by previous studies; (ii) genes activated during ER stress that were not induced by estradiol (“Hac1-independent”; for example, HSP12); (iii) genes that were repressed by DTT and tunicamycin but were unchanged by estradiol (“ER stress repressed”; for example, RPL9A); (iv) genes induced by estradiol but not by ER stress (“GAL”—a consequence of the Gal4-based ectopic expression system); and (v) two groups of other genes that remained largely unchanged (for example, PGK1) (Fig. 1 C and D). Specifically, we identified 315 genes as transcriptional targets of Hac1 (induced greater than or equal to twofold in estradiol, DTT, and tunicamycin), 280 genes as up-regulated under ER stress independently of Hac1, and 568 genes as repressed by ER stress independently of Hac1 (greater than or equal to twofold repressed in both DTT and tunicamycin conditions, but not in estradiol-treated cells) (Fig. 1E).
The Hac1-Independent ER Stress Response Has the Hallmarks of PKA Deactivation.
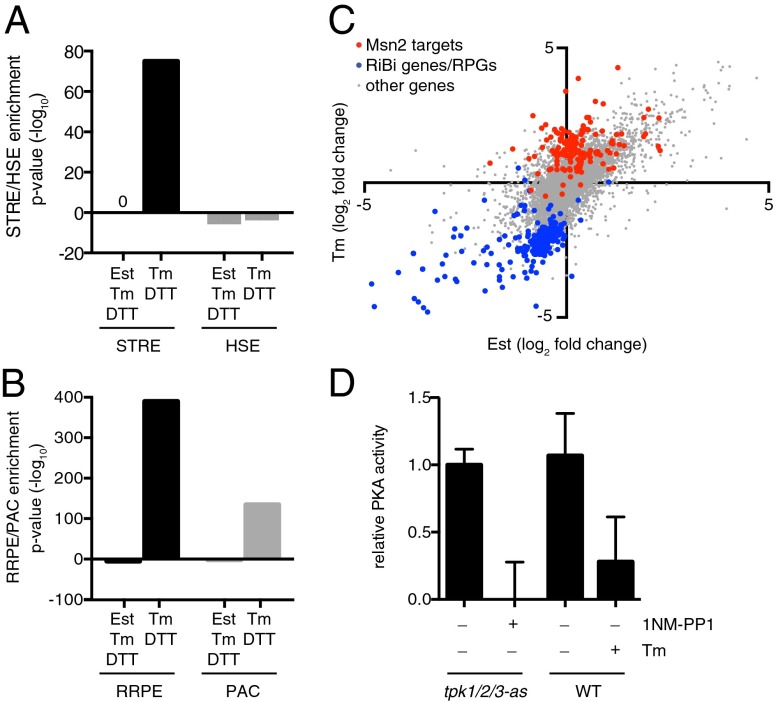
To identify putative regulators of the genes whose transcript levels changed upon ER stress in a Hac1-independent fashion, we performed an unbiased search for enriched sequence motifs in the promoters of the Hac1-independent and ER stress-repressed genes using the SCOPE web interface (28). This analysis revealed that the promoter sequences of the Hac1-independent genes are enriched with the stress response element (STRE), which is known to be the binding site for the GSR transcription factors Msn2/4 (Fig. 2A) (24). By contrast, when directed to search for the heat shock element (HSE), the binding site for the heat shock factor Hsf1, no enrichment was observed (Fig. 2A). SCOPE analysis also revealed that the promoters of the ER stress-repressed genes are enriched for the ribosomal RNA processing element (RRPE; the binding site for the transcriptional repressor Stb3), and the polymerase A and C motif (PAC; the binding site for the repressors Dot6 and Tod6) (Fig. 2B and ref. 29). Quantitatively, many canonical targets of Msn2 were induced significantly more by DTT and tunicamycin than by estradiol, whereas ribosomal protein genes and assembly factors were significantly more repressed by DTT and tunicamycin than by estradiol (Fig. 2C). Msn2/4, Stb3, Dot6, and Tod6 are phosphorylated and inhibited by PKA, suggesting that PKA is deactivated during ER stress (24, 30).
Fig. 2.
The Hac1-independent ER stress response has the hallmarks of PKA deactivation. (A) Enrichment of the STRE (Msn2/4 binding site) motif in the group of 280 Hac1-independent genes targets. The HSE (Hsf1 binding site) was not enriched. (B) Enrichment of the RRPE (Stb3 binding site) and PAC (Dot6/Tod6 binding site) motifs. (C) Fold change of gene expression with 100 nM estradiol (Est) vs. 5 µg/mL tunicamycin (Tm). Msn2/4 targets are highlighted in red; genes annotated as ribosomal protein genes or genes involved in ribosome biogenesis are highlighted in blue. (D) Relative PKA activity in cell extracts from a tpk1/2/3-as strain treated with 1NM-PP1 or a wild-type strain treated with 5 µg/mL tunicamycin for 4h.
To test this notion directly, we measured PKA kinase activity in cell lysates using a biochemical assay monitoring loss of protease-dependent fluorescence upon phosphorylation of a peptide containing the PKA consensus sequence. We observed a decrease of PKA activity for a strain in which the wild-type alleles of the PKA catalytic unit (TPK1/2/3) have been replaced with analog-sensitized alleles whose protein products can be inhibited with the ATP competitive analog 1-naphthylmethyl-4-amino-1-tert-butyl-3-(p-methylphenyl) pyrazolo[3,4-d]pyrimidine (1NM-PP1; tpk1/2/3-as) (Fig. 2D and ref. 31), validating the approach. Using this assay, we determined that cells treated with tunicamycin indeed had diminished PKA activity (Fig. 2D).
The GSR Is Activated in a Second Wave of Transcription During ER Stress.
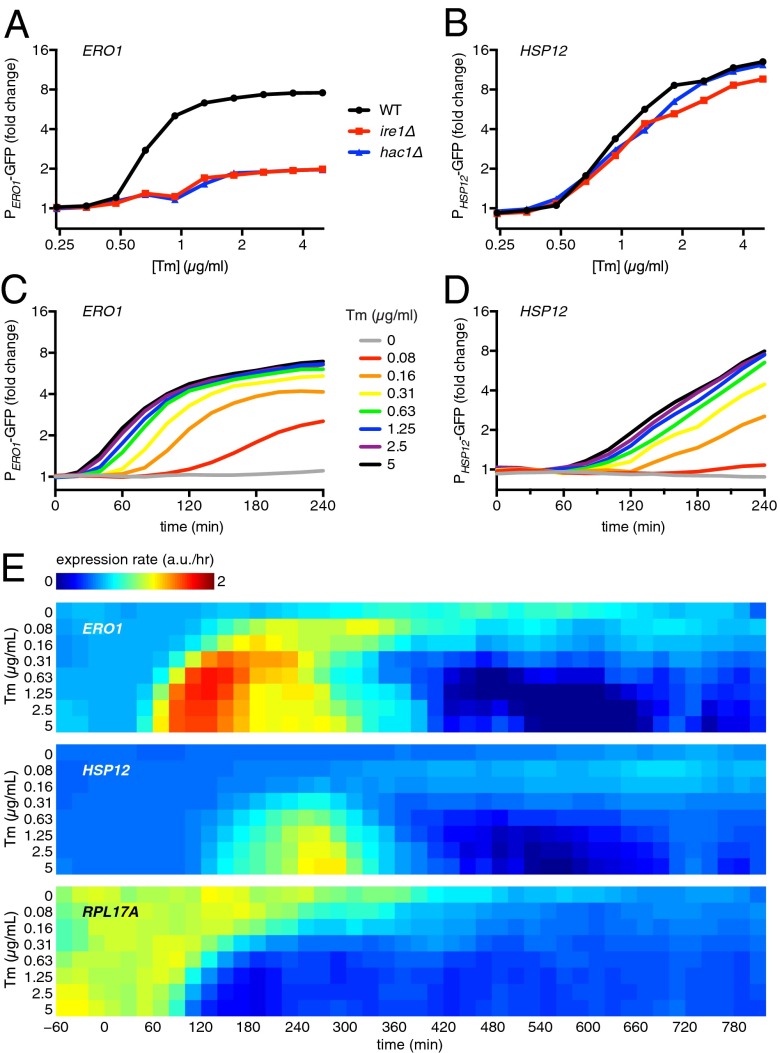
To dissect the relationship between the Hac1 and PKA components in the response to ER stress, we monitored the activation state of PKA using the transcriptional activity of the PKA-responsive regulators Msn2/4. Upon PKA deactivation, Msn2 and Msn4 are rapidly dephosphorylated and translocate to the nucleus to induce transcription of the GSR target genes (31). We measured the transcriptional activity of the Msn2/4 target HSP12 by flow cytometry in cells bearing a fluorescent reporter consisting of the HSP12 promoter fused to GFP and compared its dose–response after tunicamycin treatment to that of the UPR target gene ERO1 in wild-type, ire1∆, and hac1∆ cells. Both reporters were induced significantly by tunicamycin in wild-type cells (Fig. 3 A and B). Moreover, the activation of both reporters increased as a function of tunicamycin dose, indicating that PKA deactivation is a bona fide aspect of the homeostatic response to ER stress and not merely a consequence of lethal doses of ER stress (Fig. 3 A and B). As expected, ERO1 induction was nearly abolished in ire1∆ and hac1∆ cells (Fig. 3A). However, HSP12 was induced almost identically to wild type in ire1∆ and hac1∆ cells (Fig. 3B). Thus, Hac1 is dispensable for HSP12 induction in response to ER stress.
Fig. 3.
The GSR is activated in a second UPR-independent wave of transcription during ER stress. (A and B) Mean volume-corrected fluorescence from a transcriptional reporter of the UPR target ERO1 (A) or a reporter of the Msn2/4 target HSP12 (B) in wild-type cells (solid line) or ire1∆ cells (dashed line). Samples were measured by flow cytometry 4 h after treatment with tunicamycin (Tm). (C and D) Mean volume-corrected fluorescence per cell of a strain containing the reporter for ERO1 (C) or HSP12 (D) measured over time by automated sampling. (E) Calculated expression rates from the data shown in C and D (SI Materials and Methods).
We next asked whether the two programs occurred concurrently by monitoring PERO1–GFP and PHSP12–GFP as a function of time following ER stress. We used a robotic flow-cytometry setup that samples cultures every 20 min to measure time-dependent dose–responses for different tunicamycin concentrations in an automated fashion (32). The activation of HSP12 was delayed compared with ERO1 at all doses of tunicamycin (Fig. 3 C and D). To clearly represent the response dynamics, we calculated an expression rate for both reporters. The expression rate captures the underlying transcription dynamics in a reporter time course as measured by the rate of change of fluorescence over time, corrected for growth rate and differential fluorophore dilution. The expression rates confirm that ERO1 induction occurred before HSP12 (Fig. 3E). Furthermore, the rates revealed that both responses were transient. Once initiated, HSP12 induction ramped up much more slowly than ERO1 to reach its maximum rate (Fig. 3E). For both reporters, higher doses of tunicamycin led to earlier responses, but ERO1 always induced ahead of HSP12. In addition to HSP12, we measured a reporter of RPL17A to monitor a gene in the ER stress repressed cluster. Consistent with the HSP12 data, RPL17A levels decreased in response to tunicamycin subsequent to ERO1 induction (Fig. 3E). These observations suggest a model in which the homeostatic response to ER stress begins with Hac1 activation and is followed by PKA deactivation, leading to a second wave of transcriptional changes.
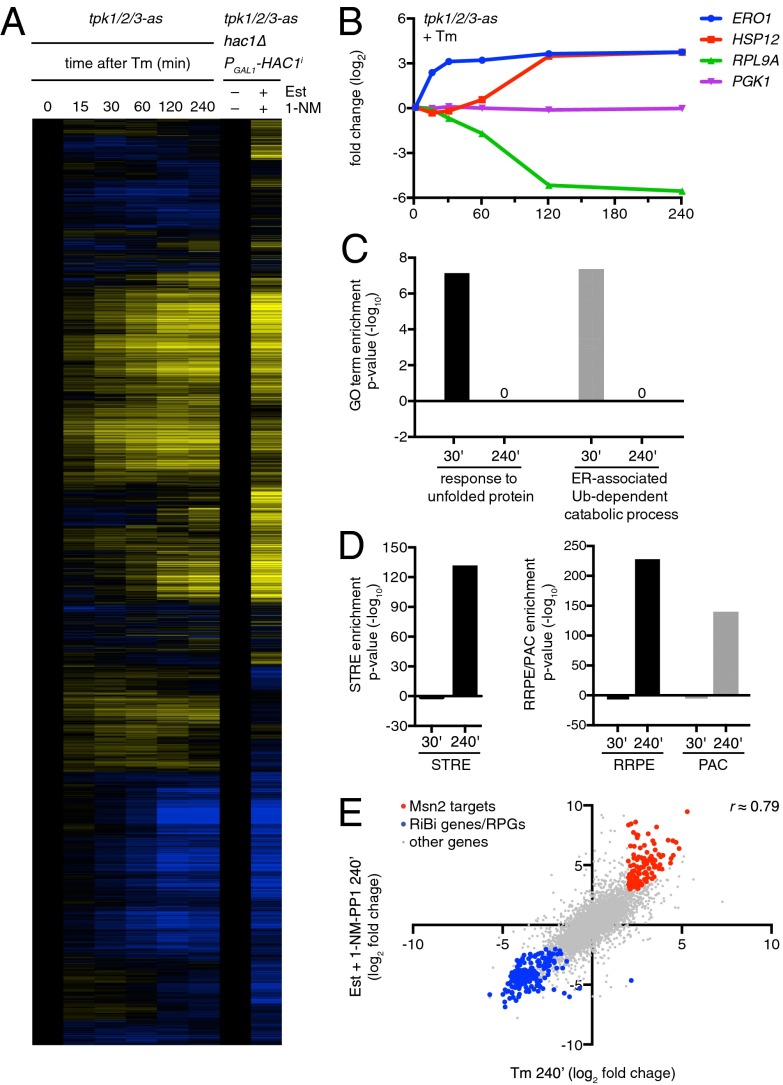
To establish that these trends are global, we quantified the transcriptome over time in response to tunicamycin using RNA seq and examined enrichment of genes induced greater than or equal to fourfold at the 30- and 240-min time points (Fig. 4 A and B and Dataset S2). We found that the UPR-associated Gene Ontology (GO) terms “response to unfolded protein” and “ER-associated ubiquitin-dependent catabolic process” were enriched at the 30-min time point, but not at the 240-min time point, consistent with the notion that the UPR is induced early in response to tunicamycin and then masked by other transcriptional dynamics later in the response (Fig. 4C). For example, in accordance with the reporter assays, we observed early induction of the endogenous ERO1 transcript, which reached its maximum within 30 min of treatment with tunicamycin (Fig. 4B). We also looked for enrichment of the STRE motif in the promoters of genes induced greater than or equal to fourfold at the 30- and 240-min time points and enrichment of the RRPE and PAC motifs in the genes repressed greater than or equal to fourfold at the 30- and 240-min time points. As predicted, we observed no enrichment of the STRE, PAC, or RRPE motifs at the 30-min time point and large enrichments of all three motifs at the 240-min time point (Fig. 4D). HSP12 and RPL9A, for example, reached their maximum and minimum levels, respectively, 120 min after treatment with tunicamycin. A control gene, PGK1, remained constant throughout the experiment (Fig. 4B).
Fig. 4.
Ectopic activation of UPR and deactivation of PKA are sufficient to explain the transcriptional program elicited by tunicamycin. (A) Clustered heat map of the fold change of gene expression upon ER stress (tunicamycin; Tm) or synthetic Hac1 activation/PKA deactivation. Samples were taken at different times after treatment with tunicamycin and at 240 min after estradiol (Est) + 1NM-PP1 addition. (B) Fold change in ERO1, HSP12, RPL9A, and PGK1 expression from cells treated with 5 µg/mL tunicamycin. (C) Enrichment of UPR-related GO terms in the group of genes induced at early times (greater than fourfold at 30 min after tunicamycin treatment) or genes induced only at later times (greater than fourfold at 240 min and less than fourfold at 30 min after tunicamycin treatment). (D) Enrichment of the STRE (Left) and RRPE or PAC (Right) motifs in the group of genes induced at early times or only at later times (as in C). (E) Fold change in gene expression for Hac1 activation/PKA deactivation vs. tunicamycin treatment 240 min after treatment. Msn2/4 targets are highlighted in red. Ribosomal protein and biogenesis genes are highlighted in blue.
Hac1 Expression Combined with PKA Inhibition Recapitulates the Majority of the Transcriptional Program Elicited by ER Stress.
To quantify the Hac1-mediated vs. PKA-mediated contributions in the response to ER stress, we compared the transcriptional response to tunicamycin with that of the combined ectopic Hac1 expression and PKA inhibition in the absence of ER stress. To do so, we introduced the system in which we could control expression of HAC1i into the tpk1/2/3-as strain. In this fashion, we could simultaneously induce expression of HAC1i with estradiol and inhibit PKA by adding 1NM-PP1. RNA-seq analysis following this experiment showed qualitatively similar expression patterns to those from cells treated with tunicamycin for 240 min (Fig. 4A). More quantitatively, genome-wide comparison of these samples indicated high similarity in fold change, yielding a correlation coefficient of 0.79 (Fig. 4E). These data indicate that the combined activation of the Hac1 and deactivation of PKA explain the majority of the transcriptional response to ER stress.
PKA Deactivation Contributes to Fitness During ER Stress.
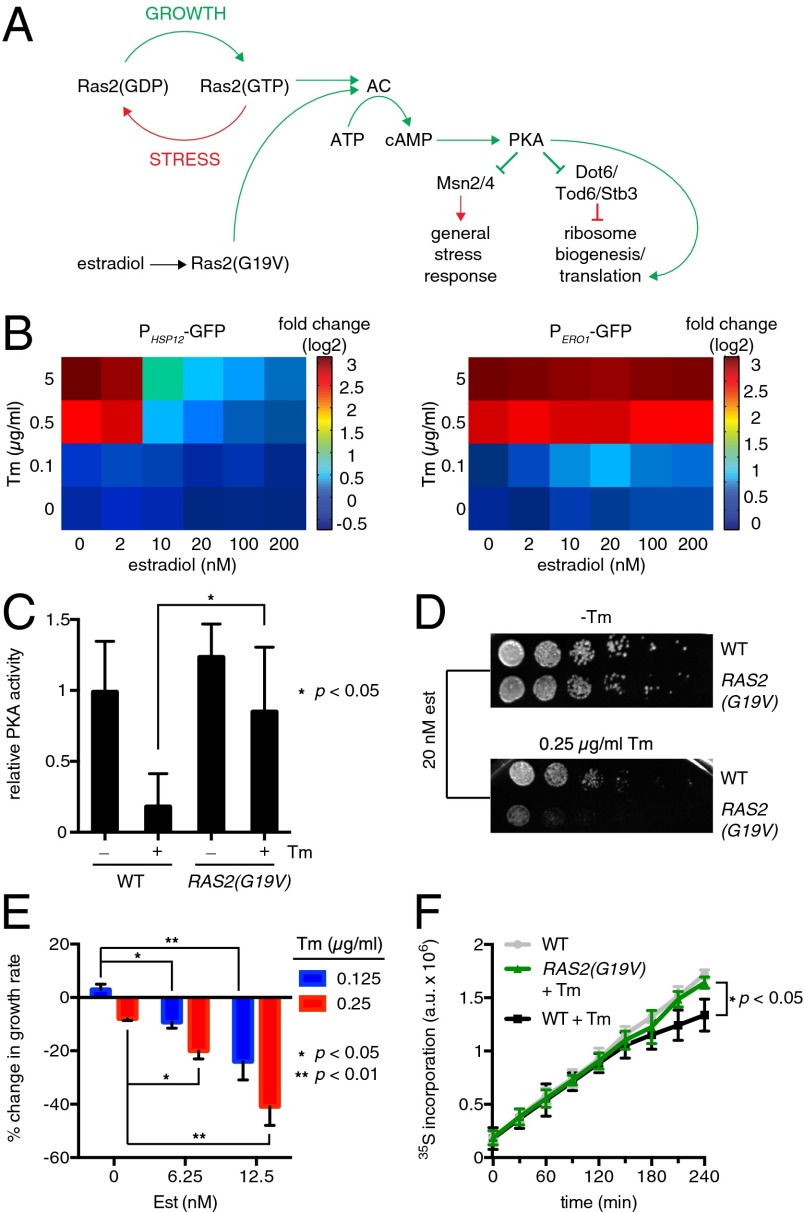
To determine the role of PKA deactivation on cellular fitness in ER stress conditions, we introduced a genetic system to conditionally manipulate PKA activity. To prevent PKA from deactivating, we ectopically expressed a constitutively active allele of the upstream PKA regulator RAS2 via estradiol control. The allele encodes Ras2(G19V), a mutant locked in a GTP-bound state that decouples cAMP production from cell state and thereby keeps PKA active (Fig. 5A). Even at intermediate expression levels (e.g., 20 nM estradiol), Ras2(G19V) abrogated the expression of the HSP12 reporter at both intermediate and high levels of tunicamycin, indicating that Msn2/4 remained inactive in the presence of active PKA (Fig. 5 B, Left). By contrast, even at the highest expression levels, Ras2(G19V) did not impair the activation of the ERO1 reporter (Fig. 5B). To confirm that Ras2(G19V) acted upstream of PKA, we measured PKA kinase activity. Whereas tunicamycin decreased PKA activity in wild-type cells, expression of Ras2(G19V) prevented tunicamycin-mediated PKA deactivation (Fig. 5C). Thus, Ras2(G19V) provides a tool to decouple PKA regulation from ER stress, while leaving UPR regulation intact.
Fig. 5.
PKA deactivation contributes to fitness during ER stress. (A) Experimental strategy to keep PKA active during ER stress. (B) Mean volume-corrected fluorescence of a transcriptional reporter of HSP12 (Left) or ERO1 (Right) measured by flow cytometry as function of Ras2(G19V) expression level (estradiol) and tunicamycin (Tm). Fluorescence was measured 4 h after treatment. (C) PKA activity in cell extracts from wild-type cells and cells expressing Ras2(G19V) treated with tunicamycin for 4 h. (D) Dilution series spot assay for wild type and an isogenic strain containing the Ras2(G19V) construct. (E) Relative growth rates for the strain expressing Ras2(G19V) at different levels in the presence of different concentrations of tunicamycin. (F) Culture density-normalized 35S incorporation in cells treated with 5 µg/mL tunicamycin and 20 nM estradiol driving the expression of Ras2(G19V).
We used Ras2(G19V) to assay cellular fitness in two ways. First, we performed a dilution series spot assay of cells grown in the presence and absence of tunicamycin with and without expression of Ras2(G19V). Expression of Ras2(G19V) had a detrimental effect on cell growth, specifically in ER stress conditions (Fig. 5D). Second, we performed a quantitative competitive growth assay at two different expression levels of Ras2(G19V) and two different concentrations of tunicamycin. Although higher levels of expression of Ras2(G19V) slowed growth in the absence of stress, the growth rate of cells expressing Ras2(G19V) was up to 40% lower than that of its matched control in the presence of tunicamycin (Fig. 5E and Fig. S2A).
Because PKA controls both induction of Hac1-independent target genes through Msn2/4 and the down-regulation of the ER stress-repressed genes through Dot6, Tod6, and Stb3, we sought to isolate the contribution of the two classes of regulation on cellular fitness in the presence of ER stress. To this end, we assayed msn2∆ msn4∆ cells and dot6∆ tod6∆ cells in the presence of tunicamycin. Deletion of STB3 significantly reduced cellular fitness, even in the absence of stress, and therefore could not be directly compared. Msn2∆ msn4∆ cells showed modest growth impairment in the presence of tunicamycin compared with wild type, as did dot6∆ tod6∆ cells (Fig. S2B). Neither double deletion was as impaired as cells expressing Ras2(G19V). Thus, it is likely that both aspects of PKA deactivation—induction of the GSR and reduction of ribosome biogenesis—contribute to fitness during ER stress.
PKA Deactivation After ER Stress Leads to Decreased Protein Synthesis.
In addition to activating the Msn2/4-mediated GSR, PKA deactivation is responsible for repressing transcription of the ribosomal protein genes and biosynthesis machinery (Fig. 5A). Thus, we reasoned that tunicamycin, by deactivating PKA and thereby decreasing ribosome biogenesis, would decrease global protein translation. To test this notion, we measured incorporation of 35S as a function of time in untreated cells and cells treated with tunicamycin. Cells treated with tunicamycin incorporated less 35S over time compared with untreated cells, but they also divided fewer times compared with the untreated cells. To account for the decrease in cell division in the stressed cells, we normalized the 35S counts by the density of the culture. After controlling for cell growth, tunicamycin-treated cells incorporated moderately less 35S per unit density than wild-type cells after several hours of treatment with tunicamycin (Fig. 5F), consistent with the notion that PKA deactivation resulted in decreased translation. To test the role of PKA, we measured 35S incorporation in tunicamycin-treated cells expressing Ras2(G19V). Although these cells also divided less than untreated cells, 35S incorporation per unit density more closely matched the untreated cells than the cells treated only with tunicamycin (Fig. 5F). Together, these data indicate that tunicamycin decreases protein synthesis through PKA deactivation, albeit moderately.
Discussion
ER stress directly triggers activation of Hac1 when misfolded proteins bind to Ire1. We have shown here that ER stress subsequently leads to the deactivation of PKA, which initiates a second wave of transcriptional dynamics (Figs. 2 and 3). By combining synthetic induction of Hac1 and inhibition of PKA in the absence of ER stress, we recapitulated the majority of the transcriptional response to ER stress (Fig. 4). These two distinct stress response programs, originating in separate cellular compartments, both contribute to cellular fitness in the presence of ER-specific stress (Fig. 5).
PKA deactivation in budding yeast induces expression of the GSR and reduces expression of ribosome biogenesis genes, thereby decreasing the cell’s protein translation capacity (25). Induction of the GSR buffers cytosolic protein-folding homeostasis, alters metabolism, and is known to be important in myriad stress conditions (23). The other aspect of PKA deactivation—decreasing protein synthesis by repressing ribosome biogenesis—provides an interesting parallel to the UPR in mammalian cells and fission yeast. In addition to increasing protein-folding capacity in the ER, the mammalian UPR, via RIDD and PERK, inhibits protein synthesis to reduce the load of unfolded proteins entering the ER (18, 33). Fission yeast exclusively uses RIDD to reduce the load of newly synthesized proteins to alleviate stress, foregoing a transcriptional response to increase ER capacity altogether (14). PKA-mediated repression of ribosome biogenesis in budding yeast could serve a functionally analogous role to metazoan PERK and RIDD in metazoans and fission yeast.
However, as opposed to PERK activation and RIDD, which occur as early steps in the response to ER stress, PKA deactivation in budding yeast occurs as a second wave of the response to ER stress after Hac1 is activated (Fig. 3). The kinetic delay in PKA deactivation during ER stress may be a valuable feature of the budding yeast ER stress response. By first activating Ire1 to induce the UPR before deactivating PKA to decrease global protein synthesis, the target genes that increase the folding capacity of the ER are expressed, and the proteins they encode are produced before translation is inhibited. Once the folding capacity is increased, a subsequently decreased influx of newly synthesized proteins would serve to further ameliorate the folding conditions in the ER to promptly restore homeostasis. Additionally, gene-specific translational regulation could ensure that UPR targets are translated and used to cope with ER stress, as has been shown in other conditions (34–36).
The mechanistic details of how ER stress could affect PKA activity remain to be elucidated. It is possible that PKA is sensing the accumulation of misfolded proteins in the cytoplasm, oxidative stress, or plasma membrane stress. Because of the high demand for ER-associated degradation during ER stress, the pool of proteasomes in the cell may be monopolized by ER clients, leading to a backlog in the normal turnover of cytoplasmic proteins and the accumulation of misfolded proteins in the cytoplasm. Likewise, ER stress impairs redox homeostasis in the ER, which could propagate beyond the ER lumen, leading to oxidative cytosolic stress (37). Furthermore, the cell wall composition and properties can be impaired by ER stress, activating the Pkc1–Slt2 pathway (38, 39), which in turn could deactivate PKA (40–45). Another possibility is that ER stress interferes with the maturation of integral membrane proteins, thereby imposing plasma membrane stress, which could disrupt Ras2 activation. The full scope and distribution of the effects of ER stress on the cell, as well as the signals that regulate PKA, remain to be defined.
Although the combination of Hac1 activation and PKA inhibition is sufficient to explain most of the transcriptional response to ER stress, some aspects of the response remain unexplained. Although we were unable to define the remaining subset of induced target genes with GO terms or promoter motif enrichment, it is possible that the Slt2 and Hog1 MAPK pathways contribute to regulating some of these genes, as might the oxidative stress transcription factors Yap1–7 (20, 21, 23). In addition, 109 genes were repressed when Hac1i was expressed. Although it is possible that Hac1 directly represses these genes, it is likely that they are repressed by an alternative mechanism such as the action of Hac1 targets.
More broadly, the dynamic interplay between ER stress and PKA signaling contributes to an emerging paradigm in which communication networks connect all of the compartments within the cell. Fitness in the midst of fluctuating extracellular and intracellular demands requires integration of information about the status of all cellular subsystems. A quantitative and mechanistic description of how the major growth control regulators—such as PKA, PKC, AMP-activated protein kinase (AMPK), and mechanistic target of rapamycin (mTOR)—interface with the major stress response pathways, such as the UPR and the heat shock response, is necessary to understand how perturbations, including targeted therapies, propagate through the cell.
Materials and Methods
Plasmid and yeast strain construction was performed by using standard techniques as described in SI Materials and Methods. Quantitative transcription and growth assays are described in detail in SI Materials and Methods. Details of PKA activity assay, 35S incorporation, RNA sequencing, and bioinformatics analysis are also in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Joanna Krakowiak for technical assistance; Marcy Diaz for advice and protocols for making the initial RNA sequencing libraries; Sebastian “Schucky” Schuck for lengthy discussion at critical early phases of the project; Carmela Sidrauski for commenting on the manuscript; and members of the H.E.-S. and P.W. laboratories for feedback throughout. D.P. was supported by a National Science Foundation (NSF) graduate fellowship. H.E.-S. was supported by the NSF, the National Institutes of Health, the National Cancer Institute, the Packard foundation, the Sandler family foundation, and the Allen Foundation. P.W. is an Investigator for the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1409588111/-/DCSupplemental.
References
- 1.Braakman I, Hebert DN. Protein folding in the endoplasmic reticulum. Cold Spring Harb Perspect Biol. 2013;5(5):a013201. doi: 10.1101/cshperspect.a013201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gardner BM, Pincus D, Gotthardt K, Gallagher CM, Walter P. Endoplasmic reticulum stress sensing in the unfolded protein response. Cold Spring Harb Perspect Biol. 2013;5(3):a013169. doi: 10.1101/cshperspect.a013169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cox JS, Shamu CE, Walter P. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell. 1993;73(6):1197–1206. doi: 10.1016/0092-8674(93)90648-a. [DOI] [PubMed] [Google Scholar]
- 4.Mori K, Ma W, Gething MJ, Sambrook J. A transmembrane protein with a cdc2+/CDC28-related kinase activity is required for signaling from the ER to the nucleus. Cell. 1993;74(4):743–756. doi: 10.1016/0092-8674(93)90521-q. [DOI] [PubMed] [Google Scholar]
- 5.Gardner BM, Walter P. Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science. 2011;333(6051):1891–1894. doi: 10.1126/science.1209126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pincus D, et al. BiP binding to the ER-stress sensor Ire1 tunes the homeostatic behavior of the unfolded protein response. PLoS Biol. 2010;8(7):e1000415. doi: 10.1371/journal.pbio.1000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Korennykh AV, et al. The unfolded protein response signals through high-order assembly of Ire1. Nature. 2009;457(7230):687–693. doi: 10.1038/nature07661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aragón T, et al. Messenger RNA targeting to endoplasmic reticulum stress signalling sites. Nature. 2009;457(7230):736–740. doi: 10.1038/nature07641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rüegsegger U, Leber JH, Walter P. Block of HAC1 mRNA translation by long-range base pairing is released by cytoplasmic splicing upon induction of the unfolded protein response. Cell. 2001;107(1):103–114. doi: 10.1016/s0092-8674(01)00505-0. [DOI] [PubMed] [Google Scholar]
- 10.Travers KJ, et al. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell. 2000;101(3):249–258. doi: 10.1016/s0092-8674(00)80835-1. [DOI] [PubMed] [Google Scholar]
- 11.Chawla A, Chakrabarti S, Ghosh G, Niwa M. Attenuation of yeast UPR is essential for survival and is mediated by IRE1 kinase. J Cell Biol. 2011;193(1):41–50. doi: 10.1083/jcb.201008071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kimata Y, et al. Two regulatory steps of ER-stress sensor Ire1 involving its cluster formation and interaction with unfolded proteins. J Cell Biol. 2007;179(1):75–86. doi: 10.1083/jcb.200704166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rubio C, et al. Homeostatic adaptation to endoplasmic reticulum stress depends on Ire1 kinase activity. J Cell Biol. 2011;193(1):171–184. doi: 10.1083/jcb.201007077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimmig P, et al. The unfolded protein response in fission yeast modulates stability of select mRNAs to maintain protein homeostasis. eLife. 2012;1:e00048. doi: 10.7554/eLife.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hollien J, et al. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol. 2009;186(3):323–331. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107(7):881–891. doi: 10.1016/s0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 17.Adachi Y, et al. ATF6 is a transcription factor specializing in the regulation of quality control proteins in the endoplasmic reticulum. Cell Struct Funct. 2008;33(1):75–89. doi: 10.1247/csf.07044. [DOI] [PubMed] [Google Scholar]
- 18.Harding HP, Zhang Y, Ron D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature. 1999;397(6716):271–274. doi: 10.1038/16729. [DOI] [PubMed] [Google Scholar]
- 19.Harding HP, et al. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell. 2000;6(5):1099–1108. doi: 10.1016/s1097-2765(00)00108-8. [DOI] [PubMed] [Google Scholar]
- 20.Babour A, Bicknell AA, Tourtellotte J, Niwa M. A surveillance pathway monitors the fitness of the endoplasmic reticulum to control its inheritance. Cell. 2010;142(2):256–269. doi: 10.1016/j.cell.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bicknell AA, Tourtellotte J, Niwa M. Late phase of the endoplasmic reticulum stress response pathway is regulated by Hog1 MAP kinase. J Biol Chem. 2010;285(23):17545–17555. doi: 10.1074/jbc.M109.084681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang HY, Tatebayashi K, Yamamoto K, Saito H. Glycosylation defects activate filamentous growth Kss1 MAPK and inhibit osmoregulatory Hog1 MAPK. EMBO J. 2009;28(10):1380–1391. doi: 10.1038/emboj.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gasch AP, et al. Genomic expression programs in the response of yeast cells to environmental changes. Mol Biol Cell. 2000;11(12):4241–4257. doi: 10.1091/mbc.11.12.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Görner W, et al. Nuclear localization of the C2H2 zinc finger protein Msn2p is regulated by stress and protein kinase A activity. Genes Dev. 1998;12(4):586–597. doi: 10.1101/gad.12.4.586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klein C, Struhl K. Protein kinase A mediates growth-regulated expression of yeast ribosomal protein genes by modulating RAP1 transcriptional activity. Mol Cell Biol. 1994;14(3):1920–1928. doi: 10.1128/mcb.14.3.1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mösch HU, Roberts RL, Fink GR. Ras2 signals via the Cdc42/Ste20/mitogen-activated protein kinase module to induce filamentous growth in Saccharomyces cerevisiae. Proc Natl Acad Sci USA. 1996;93(11):5352–5356. doi: 10.1073/pnas.93.11.5352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kimata Y, Ishiwata-Kimata Y, Yamada S, Kohno K. Yeast unfolded protein response pathway regulates expression of genes for anti-oxidative stress and for cell surface proteins. Genes Cells. 2006;11(1):59–69. doi: 10.1111/j.1365-2443.2005.00921.x. [DOI] [PubMed] [Google Scholar]
- 28.Carlson JM, Chakravarty A, DeZiel CE, Gross RH. SCOPE: A web server for practical de novo motif discovery. Nucleic Acids Res. 2007;35(Web Server issue):W259–264. doi: 10.1093/nar/gkm310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liko D, Slattery MG, Heideman W. Stb3 binds to ribosomal RNA processing element motifs that control transcriptional responses to growth in Saccharomyces cerevisiae. J Biol Chem. 2007;282(36):26623–26628. doi: 10.1074/jbc.M704762200. [DOI] [PubMed] [Google Scholar]
- 30.Lippman SI, Broach JR. Protein kinase A and TORC1 activate genes for ribosomal biogenesis by inactivating repressors encoded by Dot6 and its homolog Tod6. Proc Natl Acad Sci USA. 2009;106(47):19928–19933. doi: 10.1073/pnas.0907027106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hao N, O’Shea EK. Signal-dependent dynamics of transcription factor translocation controls gene expression. Nat Struct Mol Biol. 2012;19(1):31–39. doi: 10.1038/nsmb.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zuleta IA, Aranda-Díaz A, Li H, El-Samad H. Dynamic characterization of growth and gene expression using high-throughput automated flow cytometry. Nat Methods. 2014;11(4):443–448. doi: 10.1038/nmeth.2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Walter P, Ron D. The unfolded protein response: From stress pathway to homeostatic regulation. Science. 2011;334(6059):1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 34.Kuhn KM, DeRisi JL, Brown PO, Sarnow P. Global and specific translational regulation in the genomic response of Saccharomyces cerevisiae to a rapid transfer from a fermentable to a nonfermentable carbon source. Mol Cell Biol. 2001;21(3):916–927. doi: 10.1128/MCB.21.3.916-927.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smirnova JB, et al. Global gene expression profiling reveals widespread yet distinctive translational responses to different eukaryotic translation initiation factor 2B-targeting stress pathways. Mol Cell Biol. 2005;25(21):9340–9349. doi: 10.1128/MCB.25.21.9340-9349.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Warringer J, Hult M, Regot S, Posas F, Sunnerhagen P. The HOG pathway dictates the short-term translational response after hyperosmotic shock. Mol Biol Cell. 2010;21(17):3080–3092. doi: 10.1091/mbc.E10-01-0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Merksamer PI, Trusina A, Papa FR. Real-time redox measurements during endoplasmic reticulum stress reveal interlinked protein folding functions. Cell. 2008;135(5):933–947. doi: 10.1016/j.cell.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bonilla M, Cunningham KW. Mitogen-activated protein kinase stimulation of Ca(2+) signaling is required for survival of endoplasmic reticulum stress in yeast. Mol Biol Cell. 2003;14(10):4296–4305. doi: 10.1091/mbc.E03-02-0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Scrimale T, Didone L, de Mesy Bentley KL, Krysan DJ. The unfolded protein response is induced by the cell wall integrity mitogen-activated protein kinase signaling cascade and is required for cell wall integrity in Saccharomyces cerevisiae. Mol Biol Cell. 2009;20(1):164–175. doi: 10.1091/mbc.E08-08-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Boorsma A, et al. Characterization of the transcriptional response to cell wall stress in Saccharomyces cerevisiae. Yeast. 2004;21(5):413–427. doi: 10.1002/yea.1109. [DOI] [PubMed] [Google Scholar]
- 41.García R, et al. The global transcriptional response to transient cell wall damage in Saccharomyces cerevisiae and its regulation by the cell integrity signaling pathway. J Biol Chem. 2004;279(15):15183–15195. doi: 10.1074/jbc.M312954200. [DOI] [PubMed] [Google Scholar]
- 42.Lagorce A, et al. Genome-wide analysis of the response to cell wall mutations in the yeast Saccharomyces cerevisiae. J Biol Chem. 2003;278(22):20345–20357. doi: 10.1074/jbc.M211604200. [DOI] [PubMed] [Google Scholar]
- 43.Li Y, Moir RD, Sethy-Coraci IK, Warner JR, Willis IM. Repression of ribosome and tRNA synthesis in secretion-defective cells is signaled by a novel branch of the cell integrity pathway. Mol Cell Biol. 2000;20(11):3843–3851. doi: 10.1128/mcb.20.11.3843-3851.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nierras CR, Warner JR. Protein kinase C enables the regulatory circuit that connects membrane synthesis to ribosome synthesis in Saccharomyces cerevisiae. J Biol Chem. 1999;274(19):13235–13241. doi: 10.1074/jbc.274.19.13235. [DOI] [PubMed] [Google Scholar]
- 45.Park JI, Collinson EJ, Grant CM, Dawes IW. Rom2p, the Rho1 GTP/GDP exchange factor of Saccharomyces cerevisiae, can mediate stress responses via the Ras-cAMP pathway. J Biol Chem. 2005;280(4):2529–2535. doi: 10.1074/jbc.M407900200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.