Significance
Treatment of the lysosomal storage disease mucopolysaccharidosis type I (MPS I) is currently based on hematopoietic stem cell transplantation (HSCT) or weekly infusions of the deficient enzyme. To circumvent the morbidity and mortality associated with HSCT and the economic and quality of life costs of lifelong enzyme replacement therapy, we tested liver-directed gene therapy as a means of achieving endogenous enzyme expression in a feline model of MPS I. We found that hepatic gene transfer not only generated therapeutic levels of circulating enzyme, but in most cases also resulted in complete resolution of storage lesions in the cardiac valves, a tissue that is refractory to currently available therapies and responsible for much of the residual morbidity and mortality in treated patients.
Keywords: AAV, lysosomal storage disease
Abstract
Patients with mucopolysaccharidosis type I (MPS I), a genetic deficiency of the lysosomal enzyme α-l-iduronidase (IDUA), exhibit accumulation of glycosaminoglycans in tissues, with resulting diverse clinical manifestations including neurological, ocular, skeletal, and cardiac disease. MPS I is currently treated with hematopoietic stem cell transplantation or weekly enzyme infusions, but these therapies have significant drawbacks for patient safety and quality of life and do not effectively address some of the most critical clinical sequelae, such as life-threatening cardiac valve involvement. Using the naturally occurring feline model of MPS I, we tested liver-directed gene therapy as a means of achieving long-term systemic IDUA reconstitution. We treated four MPS I cats at 3–5 mo of age with an adeno-associated virus serotype 8 vector expressing feline IDUA from a liver-specific promoter. We observed sustained serum enzyme activity for 6 mo at ∼30% of normal levels in one animal, and in excess of normal levels in three animals. Remarkably, treated animals not only demonstrated reductions in glycosaminoglycan storage in most tissues, but most also exhibited complete resolution of aortic valve lesions, an effect that has not been previously observed in this animal model or in MPS I patients treated with current therapies. These data point to clinically meaningful benefits of the robust enzyme expression achieved with hepatic gene transfer that extend beyond the economic and quality of life advantages over lifelong enzyme infusions.
Mucopolysaccharidosis type I (MPS I) is a recessive genetic disorder caused by deficiency of the lysosomal enzyme α-l-iduronidase (IDUA). In the absence of IDUA, cells are unable to catabolize the ubiquitous glycosaminoglycans (GAGs) heparan and dermatan sulfates. The resulting lysosomal GAG storage causes multisystem organ pathology and diverse clinical manifestations, including bone and joint deformity, upper airway obstruction, hepatosplenomegaly, corneal clouding, and cognitive impairment (1). Most patients also develop cardiac disease, which arises from the combined effects of GAG deposition in the myocardium, coronary arteries, and left-sided heart valves (2). Without treatment, median survival in patients with the severe form of the disease is less than 7 y (3).
Care of MPS I patients has been vastly improved by the introduction of two disease-modifying therapies—hematopoietic stem cell transplantation (HSCT) and enzyme replacement therapy (ERT). Both treatments are based on the principle of cross-correction: that cells can efficiently endocytose extracellular lysosomal enzymes bearing a mannose-6-phosphate residue, allowing IDUA secreted from donor-derived cells after HSCT or recombinant enzyme delivered i.v. to correct the metabolic defect in many tissues (4, 5). The introduction of HSCT has increased the survival of MPS I patients and has demonstrated improvements in growth, mobility, hepatosplenomegaly, and some aspects of cardiac disease such as left ventricular hypertrophy (2, 6–9). ERT has shown a similar capacity to improve many of the clinical features of MPS I (7, 10, 11). ERT is favored in patients with an attenuated disease phenotype because of the high mortality associated with HSCT, although HSCT remains the first-line intervention for patients less than 2 y of age owing to the beneficial effect of early transplantation on cognitive outcomes (7).
Despite the enormous advances that have been made in the treatment of MPS I, significant shortcomings remain. Neurological symptoms do not improve with ERT and are highly variable after HSCT (7, 9). Skeletal disease is incompletely treated by both therapies. ERT and HSCT may improve heart disease but do not reverse valvular GAG deposition, often leaving treated patients with persistent aortic and mitral valve insufficiency or stenosis (2, 12). Additionally, the current treatment options are fundamentally limited by the morbidity and mortality associated with HSCT and the need for lifelong, expensive, weekly enzyme infusions in ERT.
For diseases such as MPS I that require lifelong systemic enzyme replacement, liver-directed gene therapy has emerged as a potential therapeutic option. The high synthetic capacity of the liver, coupled with the discovery of adeno-associated virus (AAV) vectors capable of safe and efficient hepatic targeting, make this a feasible alternative to exogenous enzyme infusion (13, 14). The first clinical success of AAV-mediated liver gene therapy was recently demonstrated in a trial for hemophilia B, in which some patients were able to discontinue prophylactic factor IX injections (15). Apart from the potential safety and quality of life benefits over HSCT and ERT, respectively, we hypothesized that liver-directed gene therapy could have three potential benefits specific to MPS I. First, the liver is a therapeutic target in MPS I, making the high local concentrations of enzyme potentially useful for efficiently treating hepatomegaly due to GAG storage. Second, liver-mediated expression could theoretically result in circulating concentrations of IDUA higher than those achieved with HSCT, and more stable than those achieved with i.v. infusion of the enzyme, which has a serum half-life of less than 4 h (11). Maintaining high levels of serum IDUA could drive greater enzyme uptake and improve efficacy in difficult-to-treat tissues. Finally, antibody responses to IDUA, which develop in the vast majority of patients receiving ERT, seem to limit treatment efficacy (16, 17). Evidence from mouse models suggests that AAV-mediated hepatic expression of an enzyme is less immunogenic than i.v. delivery of the recombinant protein, indicating that this approach could exhibit improved efficacy by eliciting less-robust immune responses to IDUA (18).
In the present study we tested liver-directed gene therapy in the naturally occurring feline model of MPS I, which recapitulates many of the clinical and pathological features of the disease, including progressive cardiac valve involvement (19–23). Four animals were treated at 3–5 mo of age with an i.v. injection of an AAV serotype 8 vector expressing feline IDUA from a liver-specific promoter. Three of the animals exhibited sustained supraphysiologic IDUA expression, with subsequent GAG clearance from all tissues examined. Remarkably, aortic valve lesions were reversed in these three animals, indicating the potential utility of this approach for targeting treatment refractory tissues in MPS I.
Results
Efficient AAV8-Mediated Liver Transduction in MPS I Cats.
MPS I cats carry a 3-bp in-frame deletion producing the omission of a single aspartate residue from the IDUA protein, resulting in a complete loss of catalytic activity (24). These animals exhibit GAG storage in most tissues and develop substantial orthopedic, corneal, and cardiac disease within the first 6 mo of life (19–21). We treated four MPS I cats between 3 and 5 mo of age (Table 1) with an i.v. injection of 5 × 1012 genome copies (GC)/kg of an AAV8 vector expressing feline IDUA from the liver-specific thyroid-binding globulin (TBG) promoter (Fig. S1). Serum samples were collected for measurement of IDUA activity throughout the 6-mo study period, after which the animals were killed for histological and biochemical analyses. Three untreated MPS I cats and five normal cats (IDUA +/− or +/+) served as controls. One treated cat (9021) exhibited an elevation in serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) (215 and 92 U/L, respectively) 7 d after vector injection, which resolved within 48 h (ALT 147 and AST 43 U/L). The transaminase elevation was not associated with clinically apparent signs or loss of transgene expression. There were no other adverse clinical or biochemical events during the course of the study.
Table 1.
Summary of study subjects
| Animal no. | Genotype | Treatment | Dose, GC/kg | Weight at injection, kg | Sex | Age at study end, mo |
| 9110 | WT | Untreated | — | — | M | 7 |
| 9115 | WT | Untreated | — | — | F | 7 |
| 7704 | MPS I Het | Untreated | — | — | F | 58 |
| 8991 | MPS I Het | Untreated | — | — | F | 6 |
| 8992 | MPS I Het | Untreated | — | — | F | 6 |
| 8922 | MPS I | Untreated | — | — | F | 13 |
| 9052 | MPS I | Untreated | — | — | F | 10 |
| 9055 | MPS I | Untreated | — | — | M | 10 |
| 8958 | MPS I | AAV8-TBG-fIDUA | 5 × 1012 | 2.03 | F | 11 |
| 8994 | MPS I | AAV8-TBG-fIDUA | 5 × 1012 | 1.66 | M | 9 |
| 9021 | MPS I | AAV8-TBG-fIDUA | 5 × 1012 | 2.23 | F | 11 |
| 9056 | MPS I | AAV8-TBG-fIDUA | 5 × 1012 | 1.69 | M | 9 |
The normal control animals (9110, 9115, 7704, 8991, 8992) and the untreated MPS I control animals (8922, 9052, 9055) were previously described (30). M, male; F, female.
The AAV8 vector demonstrated a strong hepatic tropism in the MPS I cats, with up to 100 vector GC per host diploid genome (Table 2). More than one vector genome per 10 diploid genomes was also noted in the heart and spleen; minimal vector deposition was detected in lung, kidney, and cerebral cortex.
Table 2.
Vector biodistribution
| Animal no. | Heart | Lung | Liver | Spleen | Renal medulla | Renal cortex | Brain |
| 8958 | 0.24 | 0.05 | 89.32 | 1.48 | 0.05 | 0.09 | 0.03 |
| 8994 | 0.11 | 0.01 | 33.93 | 0.13 | 0.003 | 0.02 | 0.03 |
| 9021 | 0.13 | 0.20 | 116.62 | 0.32 | 0.04 | 0.06 | 0.003 |
| 9056 | 0.17 | 0.04 | 183.44 | 0.20 | 0.02 | 0.13 | 0.07 |
| 9115* | <0.0005† | <0.0005 | <0.0005 | <0.0005 | <0.0005 | <0.0005 | <0.0005 |
Six months after i.v. injection of 5 × 1012 GC/kg AAV8 vector, MPS I cats were killed, and tissue DNA was extracted for quantification of vector genomes by Taqman PCR. The brain sample was taken from frontal cortex. Values are vector genome copies per host diploid genome.
Untreated control.
Limit of detection.
Elevated Serum IDUA Activity in Treated MPS I Cats.
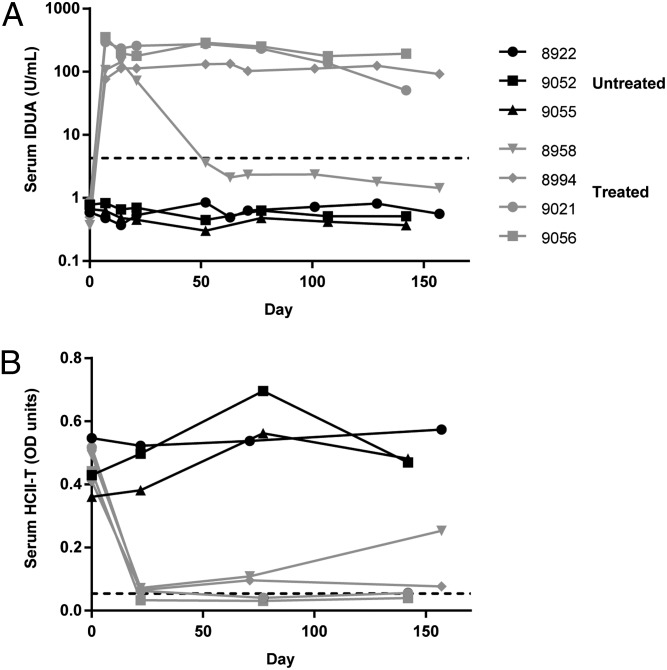
All treated cats exhibited a rapid elevation in serum IDUA activity to more than 30-fold normal levels by day 21 (Fig. 1A). High level expression was maintained for the duration of the study in three of the cats. The fourth animal (8958) exhibited a decline in serum IDUA activity by day 52 but remained at 30% of normal levels throughout the study.
Fig. 1.
Increased serum IDUA activity and reduction of serum HCII-T complex concentration. (A) IDUA activity was measured in serum collected from treated and untreated MPS I cats. (B) The covalent complex formed from thrombin and heparin cofactor II (HCII-T), a proposed biomarker for MPS I (26), was measured in serum samples by sandwich ELISA. The dashed lines indicate mean values for normal animals.
Normalization of Serum Heparin Cofactor II–Thrombin Complex.
Formation of the covalent complex between heparin cofactor II and thrombin is catalyzed by GAGs such as dermatan sulfate, and serum concentrations of this complex have been suggested as a biomarker for MPS I (25, 26). Serum concentrations of the heparin cofactor II–thrombin complex (HCII-T) were measured by ELISA (Fig. 1B). Normalization of serum HCII-T was observed by day 21 in all treated animals. The serum HCII-T concentration later increased in the animal with low serum IDUA activity but remained below pretreatment levels.
Systemic IDUA Uptake and Reversal of Storage Lesions.
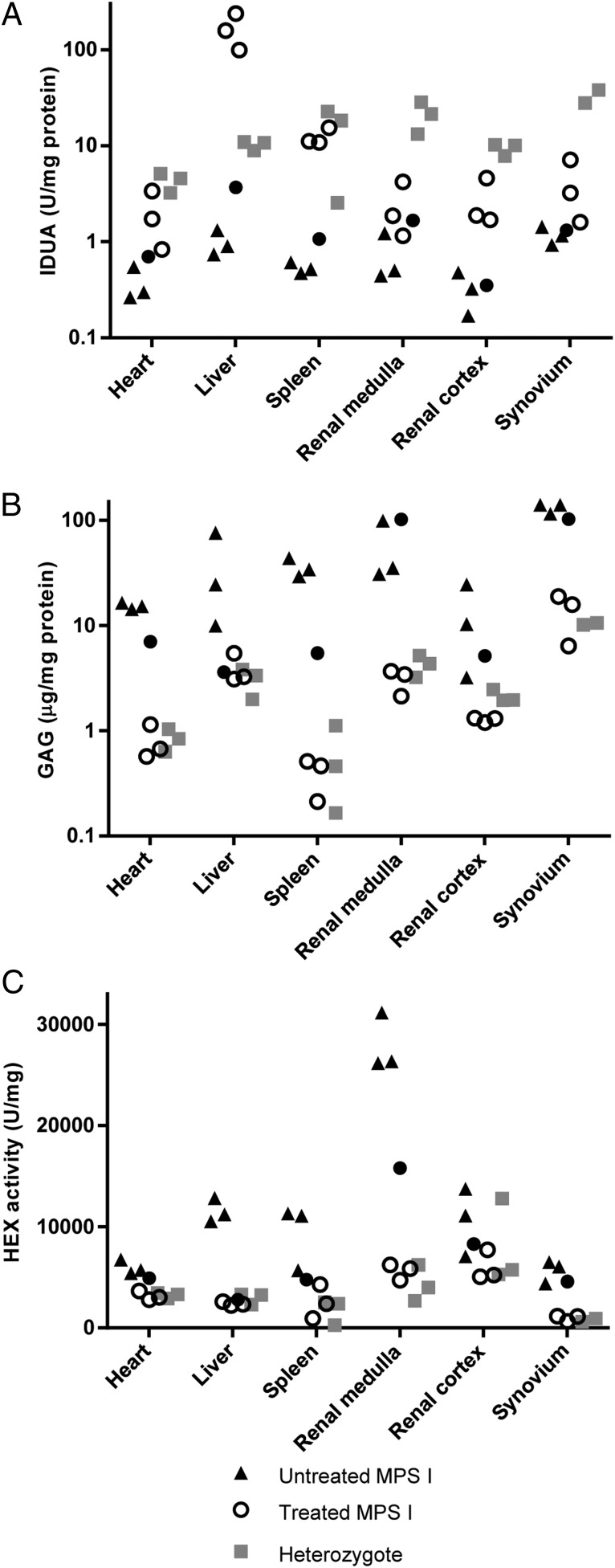
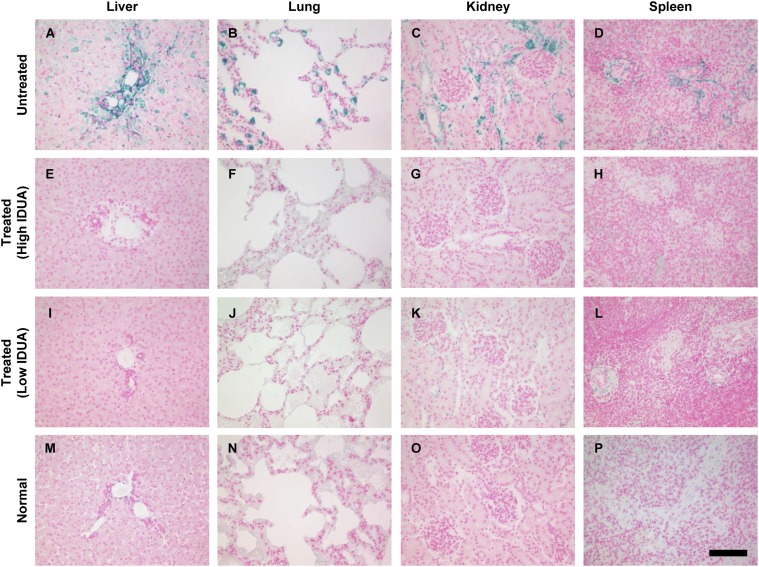
Tissue IDUA activity was extremely elevated in the livers of treated cats, reflective of local production of the enzyme (Fig. 2A). Other tissues exhibited modest elevations of IDUA activity compared with untreated cats. The animal with declining serum IDUA activity had residual enzyme in liver, but activity in extrahepatic tissues was not detectable. GAG storage and activity of the lysosomal enzyme hexosaminidase, which is typically overexpressed by IDUA-deficient cells, were normalized in the treated animals, although the animal with low serum activity exhibited incomplete correction of GAG storage in all tissues except for liver (Fig. 2 B and C). Systemic GAG clearance was supported by histology, which revealed reduced Alcian blue staining in the liver, lung, kidney, and spleen of treated animals (Fig. 3). The animal with low serum IDUA activity likewise demonstrated decreased GAG storage by histology, although some residual GAG storage was evident in the kidney and spleen (Fig. 3 K and L).
Fig. 2.
IDUA uptake in tissues and normalization of GAG storage and hexosaminidase activity. (A) IDUA activity was measured in tissue lysates from treated (circles) and untreated (triangles) MPS I cats and normal (heterozygous) controls (squares). For synovium data, normal controls are two wild-type cats (9110 and 9115). Filled circles designate the animal that exhibited declining serum IDUA activity (8958). (B) GAG storage was assessed in tissue lysates by dimethylmethylene blue binding assay. (C) Hexosaminidase activity was measured in tissue lysates using the fluorescent substrate 4-methylumbelliferyl-2-acetamido-2-deoxy-β-D-glucopyranoside.
Fig. 3.
Histological assessment of tissue GAG storage. Tissues were stained with Alcian blue for detection of sulfated GAGs. (A–D) Representative sections from untreated MPS I cats. (M–P) Sections from normal cat tissues. (E–H) Representative of the three animals with sustained serum IDUA activity. (I–L) Representative sections from the treated animal with low serum IDUA activity (8958). (Scale bar, 100 µm.)
Correction of Cardiovascular Lesions.
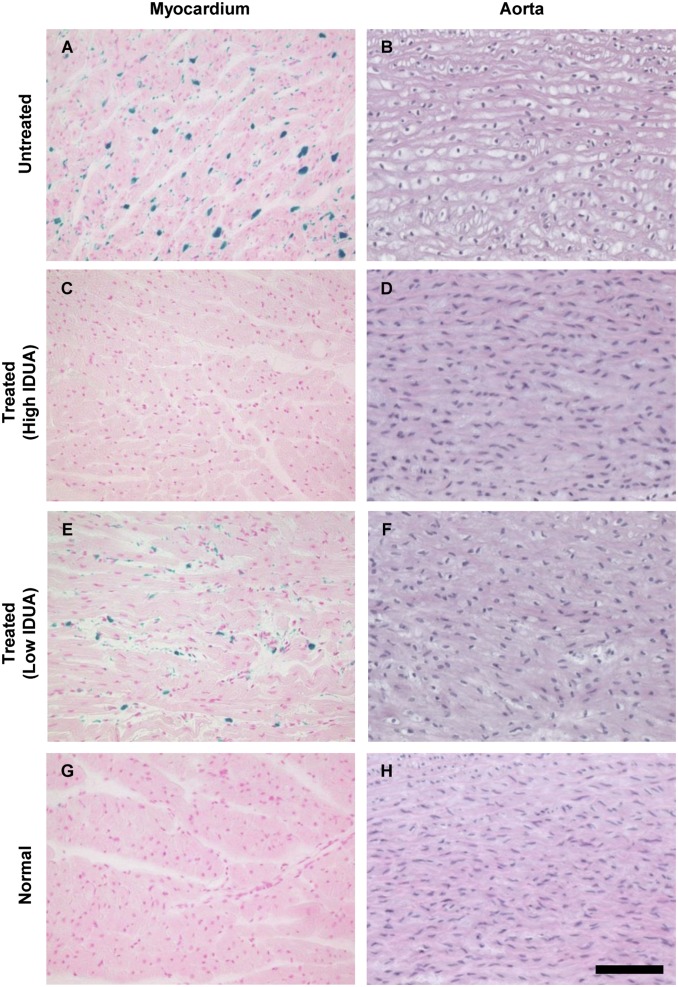
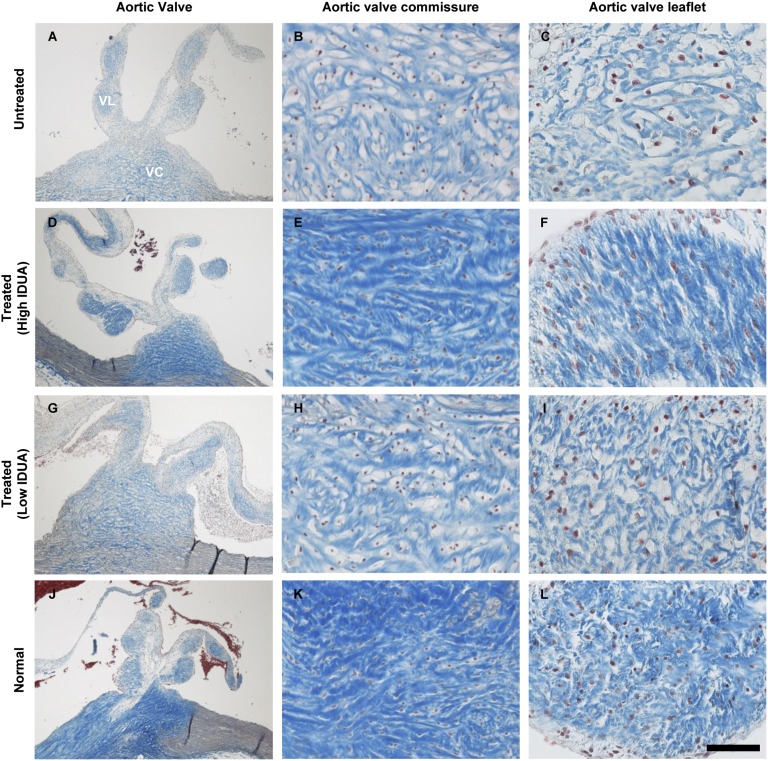
Myocardial GAG storage and valve abnormalities similar to those of MPS I patients have previously been described in MPS I cats (19, 21, 27). We noted complete correction of storage lesions in myocardium and aorta of the three cats with stable serum IDUA activity, and moderate improvement in the animal with reduced serum IDUA activity (Fig. 4). Remarkably, the aortic valves also exhibited near complete resolution of storage pathology (Fig. 5). The cytoplasmic clearing visible by trichrome stain in the aortic valve commissures and leaflets was eliminated in treated animals, and the collagen structure of the fibrosa layer (dark blue) of the valve exhibited the densely packed collagen bands typical of valves from normal controls. Modest improvement was evident in the aortic valve of the animal with lower serum IDUA activity (Fig. 5 G–I).
Fig. 4.
Reduced storage lesions in myocardium and aorta. (A, C, E, and G) Sections of myocardium were stained for GAGs using Alcian blue. (B, D, F, and H) Ascending aorta sections were stained with H&E. (Scale bar, 100 µm.)
Fig. 5.
Correction of aortic valve lesions. Cross-sections of aortic valve were prepared with trichrome stain. (A, D, G, and J) Low-magnification images show one valve commissure (VC) with the two associated leaflets (VL). High-magnification images show the valve commissure (B, E, H, and K) or the fibrosa layer of the valve leaflet (C, F, I, and L). (Scale bar, 500 µm for A, D, G, and J; 100 µm for B, E, H, and K; and 50 µm for C, F, I, and L.)
Incomplete Storage Correction in Brain and Cornea.
The brain and cornea are clinically important target tissues in MPS I that are incompletely treated by current therapies (27, 28). Although we observed substantial improvement in meningeal GAG storage by Alcian blue stain, there was no evidence of reduced ganglioside storage pathology in the brain parenchyma (Fig. S2). The corneas of treated animals likewise exhibited persistent storage lesions (Fig. S3).
Discussion
In the present study we evaluated the effect of AAV8-mediated hepatic gene transfer on systemic storage pathology in MPS I cats. In three of the four treated animals, we observed sustained supraphysiologic serum IDUA activity and extensive correction of GAG storage throughout the body. In the fourth treated animal, serum IDUA activity peaked at 3 wk after injection, then fell to below normal levels. The reason for this decline was not clear. The high vector copy numbers in the liver samples collected at necropsy rule out death of transduced hepatocytes as a cause of this decline as was seen previously when the normal canine IDUA cDNA was used in a retroviral vector (29). The kinetics of the loss of IDUA activity were identical to those observed in a previous study of CNS-directed gene therapy in feline MPS I, which were linked to the induction of antibodies against IDUA (30). Antibodies to IDUA were not detected by ELISA in the animal that exhibited a decline in expression in the present study (Fig. S4), although this may be attributable to interference in the assay from high serum levels of IDUA antigen. Additional studies will be required to determine whether antibody induction was responsible for the decline in IDUA activity and whether the remarkable efficacy noted in the other three animals was due to the lack of such an immune response to the enzyme. Intriguingly, if this is the case it would indicate a rate of antibody induction of only 25% in this cohort. In human trials of ERT, the incidence of antibodies against IDUA is greater than 90%, even in attenuated MPS I patients who, like the MPS I cat, express residual mutant protein (31). This may support the relatively nonimmunogenic nature of AAV-mediated hepatic expression of therapeutic proteins.
In our examination of the cardiovascular effects of liver-directed gene therapy, it was notable that not only the myocardium and aorta exhibited correction of storage pathology, but even the typically treatment-refractory aortic valves demonstrated nearly complete histological normalization. A previous study examining the effect of 6 mo of weekly high-dose enzyme replacement therapy in MPS I cats of similar ages found no histological evidence of improvement in heart valves, in agreement with clinical outcomes after ERT (20). This is an important proof of concept for the potential of gene transfer to address the cardiac valve disease associated with MPS I, which carries significant morbidity and mortality. This also raises the possibility that the high sustained levels of IDUA expression achieved with liver-directed gene therapy could have benefits for other tissues that do not respond readily to treatment, such as the bones and joints. Intriguingly, the three animals that maintained high circulating levels of IDUA had elevated enzyme activity in synovium, which coincided with normalization of both synovial GAG and hexosaminidase activity. This suggests effective enzyme uptake within the joint space, which could allow for effective treatment of the joint disease that is incompletely addressed by current therapies. Future studies should address the histological, radiographic, and clinical effects of liver-directed gene therapy on orthopedic disease in MPS I cats.
The present study demonstrates several features of liver-directed gene transfer for MPS I that could have considerable therapeutic value. A single i.v. injection of an AAV8 vector resulted in sustained serum IDUA expression at supraphysiologic levels in three MPS I cats, and at ∼30% of normal in a fourth animal. The ability to achieve stable circulating IDUA activity could make this a viable alternative to HSCT or weekly enzyme infusions, potentially reducing treatment-related morbidity and improving patient quality of life. The level of IDUA expression achieved with this approach resulted in widespread reductions in GAG storage as well as correction of aortic valve lesions, indicating the potential for clinical benefits that are not achieved with HSCT or ERT. The high sustained serum activity in three of the treated animals may be due to a relatively low incidence of antibodies against IDUA associated with liver-mediated expression, which could offer a further advantage over ERT. The safety and clinical feasibility of hepatic gene replacement for MPS I are supported by the recent successful trial of AAV8-mediated gene therapy for hemophilia B, which demonstrated long-term expression without antibody induction. Together, the present findings indicate that this approach could serve as a safe and effective treatment for MPS I, as well as other lysosomal storage diseases requiring lifelong systemic enzyme replacement.
Materials and Methods
Animals.
The MPS I cat colony was maintained at the University of Pennsylvania School of Veterinary Medicine under National Institutes of Health and US Department of Agriculture guidelines for the care and use of animals in research. All study protocols were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania. For i.v. vector injections, 5 × 1012 GC/kg AAV8-TBG-fIDUA was diluted in 1 mL saline and slowly injected via the cephalic vein. Killing was performed using an i.v. overdose of sodium barbiturate, 80 mg/kg.
Vector.
The feline IDUA sequence available through GenBank (accession no. XP_003985663) appeared to contain errors in the 5′ region according to alignment to protein sequences from other species. We therefore cloned the 5′ region of the IDUA gene from cat liver genomic DNA using a genome walking strategy with noise suppressive primer design, as previously described (32). The full-length feline IDUA sequence (accession no. KM404168) was then cloned by RT-PCR from feline liver RNA. A codon-optimized feline IDUA sequence was cloned into an expression construct containing the TBG promoter, an artificial intron, and a rabbit globin polyadenylation sequence (Fig. S1). The expression construct, flanked by AAV2 inverted terminal repeats, was packaged in an AAV serotype 8 capsid and purified on an iodixanol gradient as previously described (33).
Histology.
Tissues were processed and stained as previously described (30). Trichrome staining was performed with a kit (Sigma Aldrich) according to the manufacturer’s instructions.
HCII-T ELISA.
The HCII-T ELISA was performed as previously described (26) with the following modifications: the ELISA was developed with 150 µL 3,3′,5,5′-tetramethylbenzidine substrate for 20 min at room temperature, the reaction was stopped with 50 µL 2N sulfuric acid, and absorbance was recorded at 450 nm.
Enzyme Assays.
IDUA and hexosaminidase assays were performed as previously described (30).
ELISA for Anti-IDUA Antibodies.
Purification of feline IDUA and indirect ELISA were performed as previously described (30).
Vector Biodistribution.
DNA was extracted from tissues and vector genomes quantified by Taqman PCR as previously described (33).
Supplementary Material
Acknowledgments
We thank Hongwei Yu for expert histology services; and the vector, immunology, and animal models cores of the Gene Therapy Program (Department of Pathology and Laboratory Medicine, Perelman School of Medicine, University of Pennsylvania) for their support. This work was funded by National Institutes of Health Grants P40-OD010939 and DK25759 (to M.E.H.) and by ReGenX Holdings (J.M.W.).
Footnotes
Conflict of interest statement: J.M.W. is an advisor to ReGenX Biosciences and Dimension Therapeutics, and is a founder of, holds equity in, and receives grants from ReGenX Biosciences and Dimension Therapeutics. In addition, he is a consultant to several biopharmaceutical companies and is an inventor on patents licensed to various biopharmaceutical companies. M.E.H. is a stockholder of BioMarin Pharmaceuticals.
This article is a PNAS Direct Submission.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. KM404168).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1413645111/-/DCSupplemental.
References
- 1.Parker EI, Xing M, Moreno-De-Luca A, Harmouche E, Terk MR. Radiological and clinical characterization of the lysosomal storage disorders: Non-lipid disorders. Br J Radiol. 2014;87(1033):20130467. doi: 10.1259/bjr.20130467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braunlin EA, et al. Usefulness of bone marrow transplantation in the Hurler syndrome. Am J Cardiol. 2003;92(7):882–886. doi: 10.1016/s0002-9149(03)00909-3. [DOI] [PubMed] [Google Scholar]
- 3.Moore D, Connock MJ, Wraith E, Lavery C. The prevalence of and survival in Mucopolysaccharidosis I: Hurler, Hurler-Scheie and Scheie syndromes in the UK. Orphanet J Rare Dis. 2008;3:24. doi: 10.1186/1750-1172-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dahms NM, Lobel P, Kornfeld S. Mannose 6-phosphate receptors and lysosomal enzyme targeting. J Biol Chem. 1989;264(21):12115–12118. [PubMed] [Google Scholar]
- 5.Kakkis ED, Matynia A, Jonas AJ, Neufeld EF. Overexpression of the human lysosomal enzyme alpha-L-iduronidase in Chinese hamster ovary cells. Protein Expr Purif. 1994;5(3):225–232. doi: 10.1006/prep.1994.1035. [DOI] [PubMed] [Google Scholar]
- 6.Boelens JJ, et al. Outcomes of hematopoietic stem cell transplantation for Hurler’s syndrome in Europe: A risk factor analysis for graft failure. Bone Marrow Transplant. 2007;40(3):225–233. doi: 10.1038/sj.bmt.1705718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Ru MH, et al. Enzyme replacement therapy and/or hematopoietic stem cell transplantation at diagnosis in patients with mucopolysaccharidosis type I: Results of a European consensus procedure. Orphanet J Rare Dis. 2011;6:55. doi: 10.1186/1750-1172-6-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Souillet G, et al. Outcome of 27 patients with Hurler’s syndrome transplanted from either related or unrelated haematopoietic stem cell sources. Bone Marrow Transplant. 2003;31(12):1105–1117. doi: 10.1038/sj.bmt.1704105. [DOI] [PubMed] [Google Scholar]
- 9.Whitley CB, et al. Long-term outcome of Hurler syndrome following bone marrow transplantation. Am J Med Genet. 1993;46(2):209–218. doi: 10.1002/ajmg.1320460222. [DOI] [PubMed] [Google Scholar]
- 10.Sifuentes M, et al. A follow-up study of MPS I patients treated with laronidase enzyme replacement therapy for 6 years. Mol Genet Metab. 2007;90(2):171–180. doi: 10.1016/j.ymgme.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 11.Wraith JE, et al. Enzyme replacement therapy in patients who have mucopolysaccharidosis I and are younger than 5 years: Results of a multinational study of recombinant human alpha-L-iduronidase (laronidase) Pediatrics. 2007;120(1):e37–e46. doi: 10.1542/peds.2006-2156. [DOI] [PubMed] [Google Scholar]
- 12.Clarke LA, et al. Long-term efficacy and safety of laronidase in the treatment of mucopolysaccharidosis I. Pediatrics. 2009;123(1):229–240. doi: 10.1542/peds.2007-3847. [DOI] [PubMed] [Google Scholar]
- 13.Gao GP, et al. Novel adeno-associated viruses from rhesus monkeys as vectors for human gene therapy. Proc Natl Acad Sci USA. 2002;99(18):11854–11859. doi: 10.1073/pnas.182412299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao G, et al. Clades of Adeno-associated viruses are widely disseminated in human tissues. J Virol. 2004;78(12):6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nathwani AC, et al. Adenovirus-associated virus vector-mediated gene transfer in hemophilia B. N Engl J Med. 2011;365(25):2357–2365. doi: 10.1056/NEJMoa1108046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dickson P, et al. Immune tolerance improves the efficacy of enzyme replacement therapy in canine mucopolysaccharidosis I. J Clin Invest. 2008;118(8):2868–2876. doi: 10.1172/JCI34676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kakavanos R, Turner CT, Hopwood JJ, Kakkis ED, Brooks DA. Immune tolerance after long-term enzyme-replacement therapy among patients who have mucopolysaccharidosis I. Lancet. 2003;361(9369):1608–1613. doi: 10.1016/S0140-6736(03)13311-9. [DOI] [PubMed] [Google Scholar]
- 18.LoDuca PA, Hoffman BE, Herzog RW. Hepatic gene transfer as a means of tolerance induction to transgene products. Curr Gene Ther. 2009;9(2):104–114. doi: 10.2174/156652309787909490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ellinwood NM, et al. Bone marrow transplantation for feline mucopolysaccharidosis I. Mol Genet Metab. 2007;91(3):239–250. doi: 10.1016/j.ymgme.2007.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kakkis ED, et al. Enzyme replacement therapy in feline mucopolysaccharidosis I. Mol Genet Metab. 2001;72(3):199–208. doi: 10.1006/mgme.2000.3140. [DOI] [PubMed] [Google Scholar]
- 21.Sleeper MM, et al. Clinical characterization of cardiovascular abnormalities associated with feline mucopolysaccharidosis I and VI. J Inherit Metab Dis. 2008;31(3):424–431. doi: 10.1007/s10545-008-0821-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haskins ME, Jezyk PF, Desnick RJ, McDonough SK, Patterson DF. Mucopolysaccharidosis in a domestic short-haired cat—a disease distinct from that seen in the Siamese cat. J Am Vet Med Assoc. 1979;175(4):384–387. [PubMed] [Google Scholar]
- 23.Haskins ME, Jezyk PF, Desnick RJ, McDonough SK, Patterson DF. Alpha-L-iduronidase deficiency in a cat: A model of mucopolysaccharidosis I. Pediatr Res. 1979;13(11):1294–1297. doi: 10.1203/00006450-197911000-00018. [DOI] [PubMed] [Google Scholar]
- 24.He X, et al. Identification and characterization of the molecular lesion causing mucopolysaccharidosis type I in cats. Mol Genet Metab. 1999;67(2):106–112. doi: 10.1006/mgme.1999.2860. [DOI] [PubMed] [Google Scholar]
- 25.Langford-Smith KJ, et al. Heparin cofactor II-thrombin complex and dermatan sulphate:chondroitin sulphate ratio are biomarkers of short- and long-term treatment effects in mucopolysaccharide diseases. J Inherit Metab Dis. 2011;34(2):499–508. doi: 10.1007/s10545-010-9254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Randall DR, et al. Heparin cofactor II-thrombin complex: A biomarker of MPS disease. Mol Genet Metab. 2008;94(4):456–461. doi: 10.1016/j.ymgme.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 27.Haskins ME, Aguirre GD, Jezyk PF, Desnick RJ, Patterson DF. The pathology of the feline model of mucopolysaccharidosis I. Am J Pathol. 1983;112(1):27–36. [PMC free article] [PubMed] [Google Scholar]
- 28.Alroy J, Haskins M, Birk DE. Altered corneal stromal matrix organization is associated with mucopolysaccharidosis I, III and VI. Exp Eye Res. 1999;68(5):523–530. doi: 10.1006/exer.1998.0622. [DOI] [PubMed] [Google Scholar]
- 29.Ponder KP, et al. Mucopolysaccharidosis I cats mount a cytotoxic T lymphocyte response after neonatal gene therapy that can be blocked with CTLA4-Ig. Mol Ther. 2006;14(1):5–13. doi: 10.1016/j.ymthe.2006.03.015. [DOI] [PubMed] [Google Scholar]
- 30.Hinderer C, et al. Intrathecal gene therapy corrects CNS pathology in a feline model of mucopolysaccharidosis I. Mol Ther. 2014 doi: 10.1038/mt.2014.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wraith JE, et al. Enzyme replacement therapy for mucopolysaccharidosis I: A randomized, double-blinded, placebo-controlled, multinational study of recombinant human α-L-iduronidase (laronidase) J Pediatr. 2004;144(5):581–588. doi: 10.1016/j.jpeds.2004.01.046. [DOI] [PubMed] [Google Scholar]
- 32.Matz M, et al. Amplification of cDNA ends based on template-switching effect and step-out PCR. Nucleic Acids Res. 1999;27(6):1558–1560. doi: 10.1093/nar/27.6.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L, et al. Impact of pre-existing immunity on gene transfer to nonhuman primate liver with adeno-associated virus 8 vectors. Hum Gene Ther. 2011;22(11):1389–1401. doi: 10.1089/hum.2011.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.