Significance
The standard view of neural signaling is that a neuron can influence its target cell by exciting or inhibiting it. An important aspect of the standard view is that learning consists of changing the efficacy of synapses, either strengthening (long-term potentiation) or weakening (long-term depression) them. In studying how cerebellar Purkinje cells change their responsiveness to a stimulus during learning of conditioned responses, we have found that these cells can learn the temporal relationship between two paired stimuli. The cells learn to respond at a particular time that reflects the time between the stimuli. This finding radically changes current views of both neural signaling and learning.
Keywords: cerebellum, eyeblink conditioning, temporal control, glutamate transmission
Abstract
The standard view of the mechanisms underlying learning is that they involve strengthening or weakening synaptic connections. Learned response timing is thought to combine such plasticity with temporally patterned inputs to the neuron. We show here that a cerebellar Purkinje cell in a ferret can learn to respond to a specific input with a temporal pattern of activity consisting of temporally specific increases and decreases in firing over hundreds of milliseconds without a temporally patterned input. Training Purkinje cells with direct stimulation of immediate afferents, the parallel fibers, and pharmacological blocking of interneurons shows that the timing mechanism is intrinsic to the cell itself. Purkinje cells can learn to respond not only with increased or decreased firing but also with an adaptively timed activity pattern.
Timing is an integral aspect of all movements, from tilting a coffee cup to pressing a piano key. Fine motor timing involves the cerebellum (1), as illustrated by eyeblink conditioning. If a neutral conditional stimulus is followed repeatedly at a fixed temporal interval (an interstimulus interval) by an unconditional blink-eliciting stimulus, the conditional stimulus acquires the ability to elicit a blink that will be timed to occur just before the unconditional stimulus. If the interstimulus interval is increased or decreased, the timing of the conditioned response will change accordingly after additional training (2). The cerebellar cortex is necessary for the generation of such timed conditioned responses (3, 4). The conditional stimulus is transmitted to the cerebellar cortex by the mossy and parallel fiber system, and the unconditional blink-eliciting stimulus is transmitted by the climbing fibers (5). During conditioning, tonically active Purkinje cells in a blink-controlling area of the cerebellar cortex acquire learned pauses in firing. These pauses, conditioned Purkinje cell responses, cause disinhibition of the cerebellar nuclei and thereby generate the overt conditioned response (6, 7). The conditioned Purkinje cell responses share a number of features with the overt conditioned blink responses. For instance, they are extinguished by unpaired presentations of conditional and unconditional stimuli and show savings on retraining after extinction (7), they are adaptively timed (8), their latencies respond to changes in stimulus parameters in the same way (9, 10), and they are not acquired with interstimulus intervals below about 100 ms (11).
In accordance with current views on learning, long-term depression of synapses between parallel fibers and Purkinje cells usually is considered to be the mechanism underlying conditioning. Strengthening or weakening of synapses alone cannot explain the timing of neural responses, however (12). Therefore the timing of conditioned Purkinje cell responses generally is believed to depend on a temporal code carried by the parallel fibers. If different parallel fiber afferents are active at different times during the interstimulus interval, and Purkinje cells could learn to respond differentially to particular parallel fibers, timing would follow automatically (1, 13).
The purpose of the present work was to determine if the timing of the conditioned Purkinje cell response depends on such a temporally patterned input. We show that it does not do so. Parallel fibers make synaptic contacts with Purkinje cells and cerebellar cortical interneurons without any intermediate synapses. By using direct stimulation of parallel fibers as the conditional stimulus, we can bypass any delays in the conditional stimulus signal to the Purkinje cells and ensure that no time code in the parallel fiber signal is possible. Nonetheless, we observed the acquisition of conditioned Purkinje cell responses, adaptively timed to a range of different interstimulus intervals from 150–300 ms.
Results
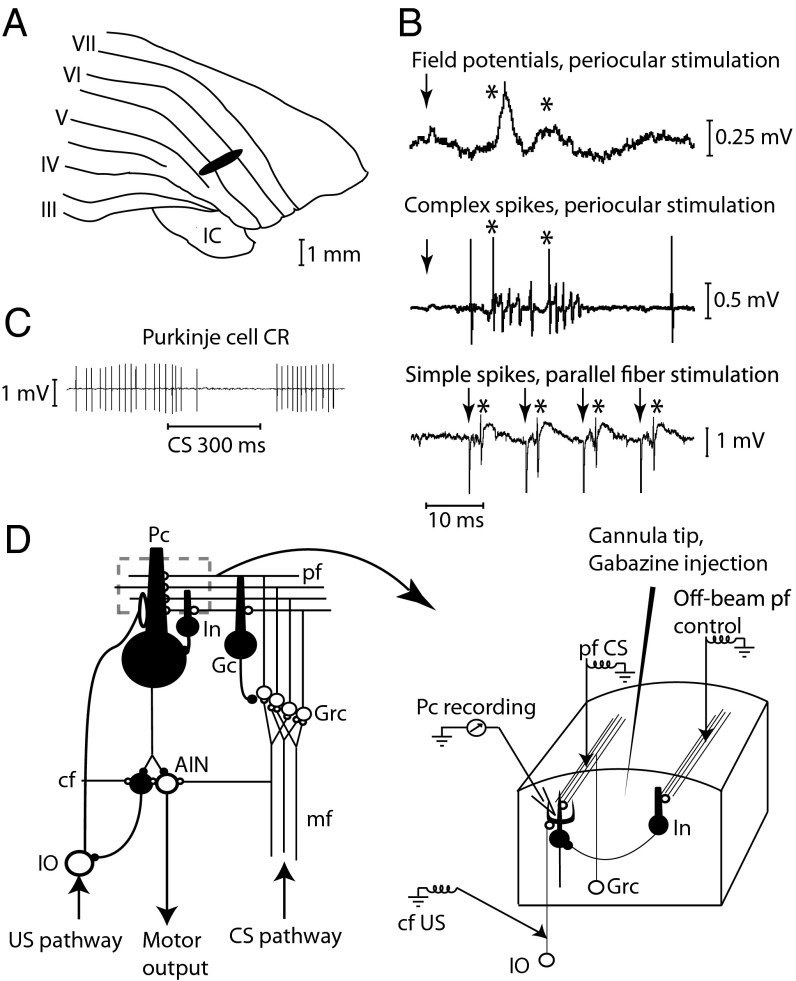
We first made extracellular recordings from 23 Purkinje cells in 19 decerebrate male ferrets, while using direct electrical stimulation (50 or 100 Hz) of parallel fibers as the conditional stimulus and stimulus of climbing fibers (500 Hz) as a proxy for the unconditional blink-eliciting stimulus (Fig. 1).
Fig. 1.
Experimental setup. (A) Blink-controlling area in cerebellar cortex. IC, inferior colliculus; Roman numerals indicate cerebellar lobules. (B) Periocular stimulation (1 pulse, 300 μA) elicits short-latency field potential responses on the cerebellar surface. Below are single-cell recordings of two complex spikes elicited by the periocular stimulation (1 mA) and simple spikes elicited by parallel fiber stimulation (4 μA). Arrows indicate stimulation; asterisks indicate responses. (C) Typical conditioned Purkinje cell response (CR). (D) Neuronal wiring diagram with stimulation, recording, and injection sites. AIN, anterior interpositus nucleus; CS, conditional stimulus; cf, climbing fiber; Gc; Golgi cell; Grc, granule cells; In, interneuron; IO, inferior olive; mf, mossy fibers; Pc, Purkinje cell; pf, parallel fibers; US, unconditional stimulus.
We monitored activity of Purkinje cells in an area in the C3 zone that controls the conditioned blink response (14, 15) for several hours during training to three different interstimulus intervals (150, 200, and 300 ms). Longer intervals were not studied because learning would be very much slower and difficult to obtain in the time span available in the decerebrate preparation.
In eight cells, the conditional stimulus coterminated with the unconditional stimulus, and in 15 cells the duration of the conditional stimulus outlasted the interstimulus interval by 150–600 ms. In the standard conditioning protocols, the conditional stimulus is terminated at the time of the unconditional stimulus. The fact that the conditioned Purkinje cell response ends at that time simply might reflect the termination of the conditional stimulus. By using long conditional stimuli, we can distinguish response features that are intrinsic to the conditioned response, in particular response offset, from direct effects of the conditional stimulus duration (8).
Naive cells (n = 19) responded to the conditional stimulus with increases or no change in simple spike firing (Figs. 2 A and F and 3D). All cells acquired conditioned responses during training, i.e., a significant reduction in simple spikes in response to the conditional stimulus during the interstimulus interval (P < 0.00001), illustrated in Fig. 3. Considering the robust difference between naive and trained responses both here and in hundreds of Purkinje cells in our previous publications (6–8, 10, 11, 16, 17), we felt justified in including four additional neighboring cells encountered after training, for which there were no naive data.
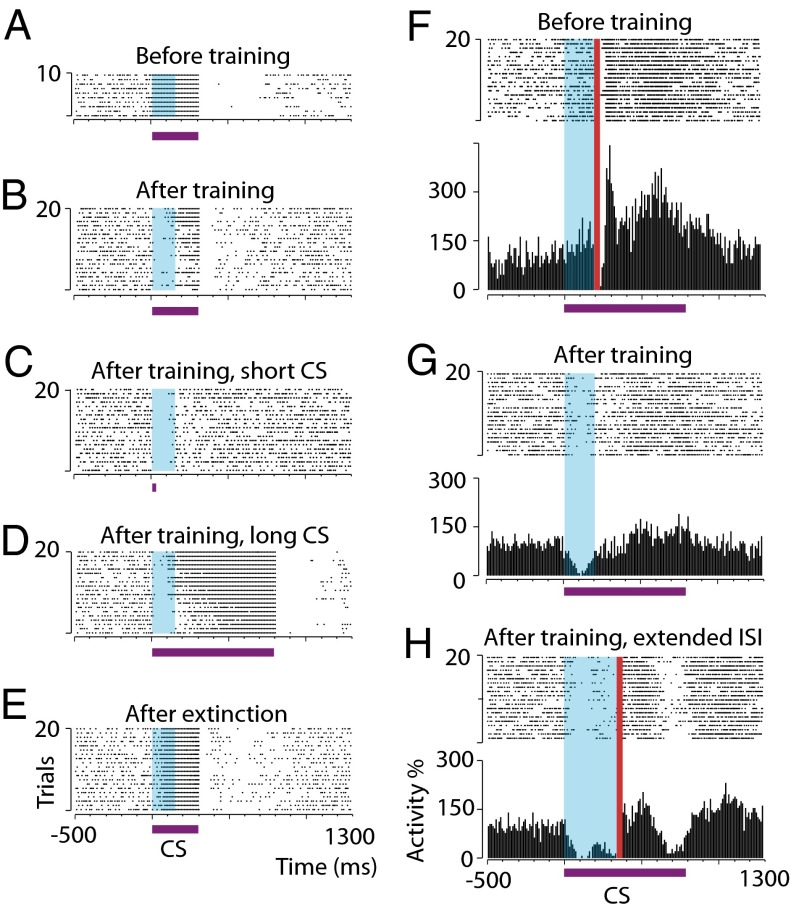
Fig. 2.
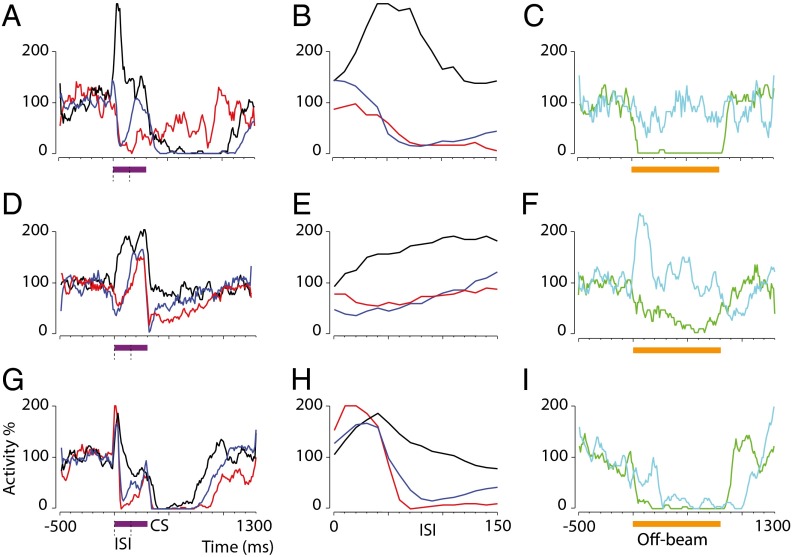
Conditioned Purkinje cell responses timed to interstimulus intervals. (A and B) Raster plots showing a typical Purkinje cell response to a 300-ms conditional stimulus before (A) and after (B) training with a 150-ms interstimulus interval (blue shading). (C and D) Responses of the cell in A and B to 17.5-ms (C) and 800-ms (D) conditional stimuli (CS) after training. (E) Response to a 300-ms conditional stimulus after extinction. (F–H) Raster plots and histograms illustrating responses of a Purkinje cell that was trained first with a 200-ms interstimulus interval and subsequently with a 350-ms interstimulus interval. Red vertical bars in F and H denote unconditional stimulus artifacts (data from paired conditional stimulus–unconditional stimulus trials). Purple horizontal bars indicate the conditional stimuli. ISI, interstimulus interval.
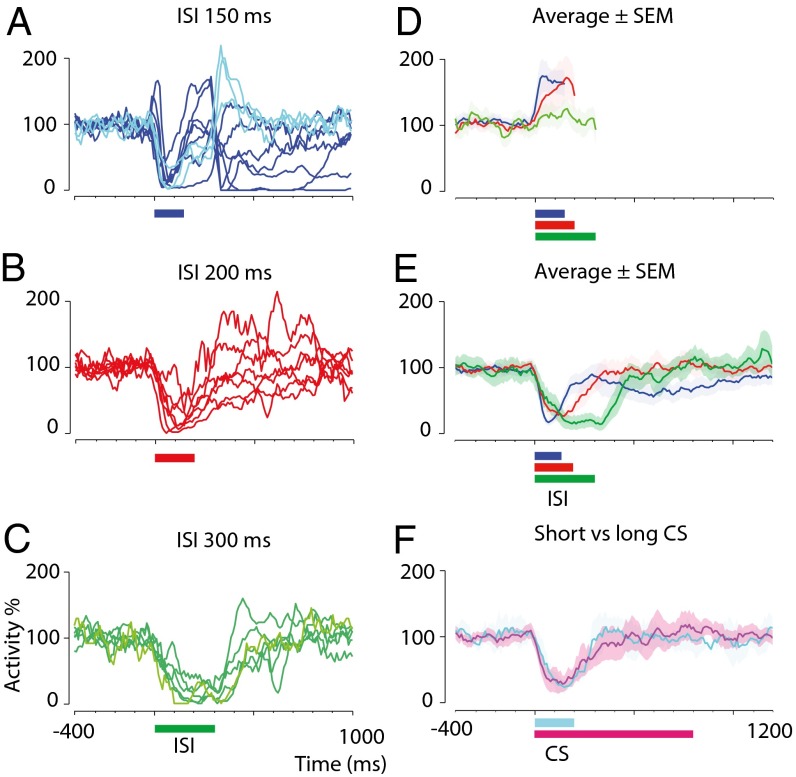
Fig. 3.
Time courses of conditioned responses after training with different interstimulus intervals and using conditional stimuli of different durations. (A–C) Smoothed and averaged simple spike activity after training with 150-ms (blue, n = 10) (A), 200-ms (red, n = 7) (B), and 300-ms (green, n = 6) (C) interstimulus intervals. Traces with lighter shading represent cells for which naive data are lacking. Colored horizontal bars indicate the interstimulus intervals. (D) Activity ± SEM for each interstimulus interval before training. Traces are truncated at the onset of the unconditional stimulus artifact, which prohibits identification of spikes. Abrupt downward inflections at the end of some traces reflect an effect of smoothing (0 identified spikes during the unconditional stimulus artifact). (E) Activity ± SEM for each interstimulus interval after training. (F) Activity ± SEM for cells trained with a 200-ms interstimulus interval and a coterminating conditional stimulus (cyan, n = 2) or an 800-ms conditional stimulus (magenta, n = 5).
To determine whether Purkinje cells trained with a parallel fiber conditional stimulus behaved as those trained with a forelimb or mossy fiber conditional stimulus (7, 8, 16), we performed a series of postacquisition manipulations. For three cells, recording conditions permitted additional hours of training with conditional stimulus only. As expected, the conditioned Purkinje cell responses gradually disappeared (see the example in Fig. 2E). For one cell, recorded for almost 10 h, we also were able to shift the interstimulus interval. After emitting conditioned responses to an interstimulus interval of 200 ms, subsequent training with a new 350-ms interstimulus interval caused the cell to acquire a bimodal conditioned response, with a second pause response close to the end of the new interstimulus interval (Fig. 2H). A similar observation has been made previously using a mossy fiber conditional stimulus (8).
Training to increasingly longer interstimulus intervals resulted in responses with increasingly delayed onsets, maxima, and offsets (Fig. 3). Conditioned response maxima ±1 SD for the different interstimulus intervals (79 ± 34, 146 ± 32, and 260 ± 36 ms, respectively) appear <75 ms before the anticipated onset of the unconditional stimulus. The estimated latencies to the conditioned response onset were 13 ± 11, 48 ± 34, and 73 ± 18 ms, respectively. The latencies to offset were 193 ± 62, 298 ± 82, and 477 ± 64 ms, respectively. There was a significant effect of interstimulus interval on latencies to response onset, maximum, and offset (P = 0.0006, 0.0002, and 0.0005, respectively; Kruskal–Wallis one-way ANOVA).
If, after training, the conditional stimulus was lengthened (n = 4) or shortened (n = 4) on a series of probe trials, it still elicited a response timed to the previously trained interstimulus interval (see the examples in Fig. 2 C and D). Also, the duration of the conditional stimulus used during training does not appear to have any effect on the temporal profile of the conditioned response. Cells conditioned to an interstimulus interval of 200 ms using a coterminating conditional stimulus or a conditional stimulus that outlasts the interstimulus interval by 600 ms show similar temporal response profiles (Fig. 3F). Thus, the timing of the conditioned Purkinje cell response does not depend on a time-coded input to the cell signaled by a temporal pattern in the conditional stimulus.
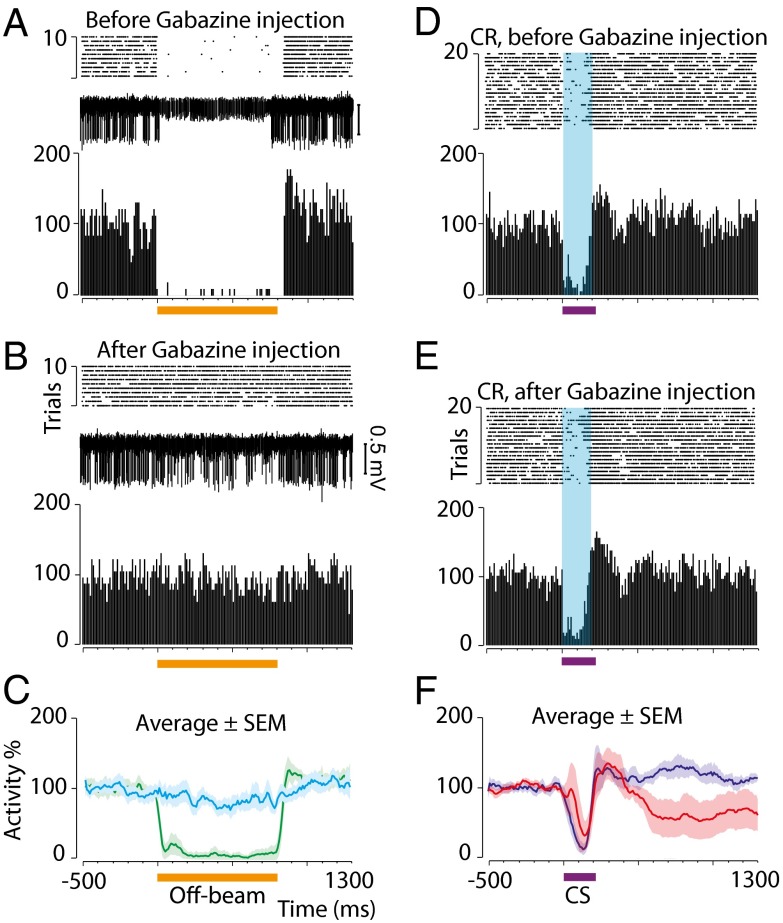
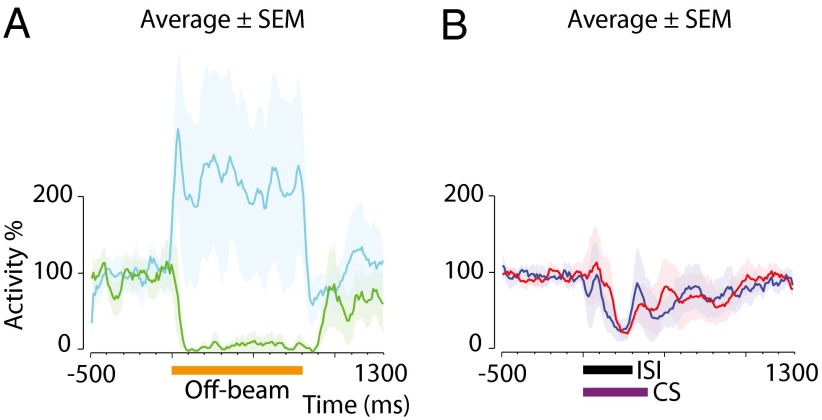
Therefore, the memory trace must reside either in the Purkinje cells or in molecular layer inhibitory interneurons. To examine the role of the latter, we tested the effect of a GABA-antagonist on conditioned Purkinje cell responses using two different interstimulus intervals (Figs. 4 and 5). Seven cells were trained until they reliably emitted conditioned responses (in this case with a forelimb conditional stimulus). Before local injection of the GABAA receptor antagonist gabazine, off-beam parallel fiber stimulation [i.e, stimulation of parallel fibers that do not terminate on the recorded Purkinje cell but which do excite interneurons that innervate that Purkinje cell (Fig. 1D)] effectively silenced the simple spike activity (Fig. 4A). Injection of the antagonist blocked interneuron inhibition from off-beam stimulation (Fig. 4 B and C), but the most important features of the conditioned responses remained essentially the same (Fig. 4 D and F). In two cases, the stimulation activated both excitatory and inhibitory input to the Purkinje cell, and the effect of gabazine was to remove inhibition and unmask an excitatory response (visible in Figs. 5A and 6F). In this case, too, there was no effect on the conditioned pause response. Similar experiments were performed using a direct parallel fiber conditional stimulus instead of the forelimb stimulation (Fig. 6).
Fig. 4.
Gabazine blocks interneuron inhibition of Purkinje cells but leaves conditioned responses to a 200-ms interstimulus interval intact. Orange horizontal bars indicate off-beam stimulation (800 ms, 81 pulses, 100 Hz). Purple horizontal bars indicate the conditional stimulus, and blue shading indicates the interstimulus interval (200 ms). (A and B) Purkinje cell responses to interneuron activation by off-beam parallel fiber stimulation (compare with Fig. 1D) before (A) and after (B) gabazine injection. Stimulation artifacts are masked. (C) Average (n = 4) responses ± SEM before (green) and after (cyan) gabazine injection. (D and E) Conditioned responses before (D) and after (E) gabazine injection in the same Purkinje cell shown in A and B. (F) The average response profile before (blue) and after (red) injection in the cells shown in C.
Fig. 5.
Gabazine blocks interneuron inhibition of Purkinje cells but leaves conditioned responses to a 300-ms interstimulus interval intact. (A) Average Purkinje cell responses (n = 3) ± SEM to interneuron activation by parallel fiber stimulation before (green) and after (cyan) injection of gabazine. The horizontal orange bar indicates off-beam stimulation (800 ms, 81 pulses, 100 Hz). (B) The average response profile to a 400-ms conditional stimulus before (blue) and after (red) in the cells shown in A. Black and purple horizontal bars indicate the interstimulus interval (300 ms) and conditional stimulus (CS, 400 ms), respectively.
Fig. 6.
Effects of different concentrations of gabazine on conditioned Purkinje cell responses to a parallel fiber conditional stimulus. Each row indicates one cell. (A, D, and G) Naive (black) and conditioned responses before (blue) and after (red) gabazine injection. Purple horizontal bars indicate a 300-ms conditional stimulus. Dashed lines indicate a 150-ms interstimulus interval. (B, E, and H) Magnification of the response during the interstimulus interval. (C, F, and I) Purkinje cell responses to interneuron activation by parallel fiber stimulation before (green) and after (cyan) gabazine injection. At 100 μM, gabazine distinctly blocks inhibition (C and F), whereas 10 μM gabazine blocks inhibition for the first 200 ms (I). Orange horizontal bars indicate off-beam stimulation (800 ms, 81 pulses, 100 Hz).
The gabazine experiments demonstrate that the main part of the conditioned Purkinje cell response is not mediated by interneuron inhibition but must be an effect of parallel fiber input to the Purkinje cells. Parallel fibers are glutamatergic, and a pause in firing might seem an unexpected response to this normally excitatory transmitter, but glutamate-evoked hyperpolarization through group II and III metabotropic glutamate receptors has been described previously (18).
Discussion
Although many different mechanisms, such as long-term depression or potentiation or changes in intrinsic excitability in Purkinje cells, perhaps working in synergy, probably participate in many forms of cerebellar motor learning in the behaving animal (19), the synaptic mechanism usually invoked to account for the learning of a Purkinje cell conditioned response is long-term depression of the parallel fiber to Purkinje cell synapses (13). Long-term potentiation of parallel fibers to interneurons that inhibit the Purkinje cell also has been suggested (20). Modulation of these synapses has been demonstrated with parallel and climbing fiber inputs that occur in close temporal proximity to each other (21–23). However, a challenge for both theories has been to explain how learning of conditioned responses could be adaptively timed and dependent on the conditional stimulus–unconditional stimulus interval. Mere strengthening or weakening of these synapses cannot account for the time course of the conditioned pause response (onset, maximum, offset) (12).
Most models (1, 13), with some notable exceptions (24), assume that delays in the granule cells, perhaps through interactions with Golgi cells, generate a temporal spike pattern in the granule cell responses to the mossy fiber input carrying the conditional stimulus signal. If granule cells have long-lasting variable activity states during the interstimulus interval, some cells in the population will have an activity peak with a temporal relation to the climbing fiber input that is maximally conducive to depression or potentiation of molecular layer synapses. When the same temporal pattern appears after learning, these synapses will automatically generate an appropriately timed conditioned response.
In the present investigation the conditional stimulus was delivered directly to the parallel fibers, thus bypassing any possible delays or temporal patterns in the granule cells. Therefore there can be no time code in the temporal pattern of the input to the Purkinje cells except the regular repetition provided by the train of parallel fiber stimuli. It could be argued that stimulation of parallel fibers caused antidromic activation of parallel fibers and granule cells and that a temporal input pattern might be generated via this route. This notion is extremely implausible, however. Identical electrical stimuli were delivered to the parallel fibers up to 81 times every 10 ms (800 ms, 100 Hz). The immediate effect of such stimulation is almost certain to drown or corrupt any specific temporal activity pattern in granule cell responses elicited by antidromic activation. Furthermore, in vivo recordings of granule cells and Golgi cells show that these cells do not exhibit the delayed signals that are necessary for the models (25, 26).
Furthermore, in agreement with previous findings on both overt and Purkinje cell conditioned responses (27, 16) using mossy fiber conditional stimuli, we observed the same response on posttraining probe trials whether we delivered eight pulses over 17.5 ms, 31 pulses over 300 ms, or 81 pulses over 800 ms, to the parallel fibers, suggesting that the temporal profile of the conditioned Purkinje cell response is determined by the initial part (less than 20 ms) of the conditional stimulus and therefore is insensitive to any temporally patterned input during the main part of the interstimulus interval and conditioned response (Fig. 2 B–D). If the granule cell network were necessary for the adaptive timing, the unlikely implication is that three such different stimuli would elicit the same temporal activity pattern in the parallel fibers.
Instead, the data strongly suggest that the main timing mechanism is within the Purkinje cell and that its nature is cellular rather than a network property. Parallel fiber input lacking any temporal pattern can elicit Purkinje cell responses timed to intervals at least as long as 300 ms. Other mechanisms likely contribute to cerebellar motor learning and response timing (19). However, our data demonstrate that one important associative memory trace, exemplified by eyeblink conditioning, resides in the Purkinje cell. In addition, the data show that a main part of the timing of the conditioned response relies on intrinsic cellular mechanisms rather than on a temporal pattern in the input signal.
Materials and Methods
Surgery and Stimulation Sites.
Animal experiments were approved by the Malmö–Lund animal experimentation ethics committee. Twenty-six male 1-y-old ferrets were surgically prepared with electrical stimulation sites as previously described (7). Parallel fibers were stimulated with platinum-tungsten electrodes (pulled and ground tips, 25-μm core diameter). Eliciting or suppressing Purkinje cell simple spikes confirmed on-beam and off-beam location, respectively.
Training Protocol.
For the conditional stimulus, 100- or 50-Hz stimulus trains (230–800 ms, 2–20 μA, 0.1-ms pulse duration) were applied to parallel fibers, or 50-Hz stimulus trains (230–400 ms, 0.6–1.2 mA, 1-ms pulse duration) were applied to the ipsilateral forelimb. For the unconditional stimulus, two five-pulse 500-Hz stimulus trains (30–400 μA, 0.1-ms pulse duration) separated by 10 ms were applied to ipsilateral climbing fibers 150–350 ms after the onset of the conditional stimulus onset. The intertrial interval was 15 ±1 s (randomized). Acquisition sessions with paired conditional stimulus–unconditional stimulus or conditional stimulus-alone stimulation lasted 1–5 h.
Recordings and Data Analysis.
Recording technique and analysis software were as previously described (7). Training effect was defined by a significant reduction in spike frequency in the last third of the interstimulus interval after training (paired sample t test, spikes averaged over 20 or 10 trials and normalized to activity 600 ms pretrial). Data were quantified in 10-ms bins. The first and last bins in a series of consecutive bins with spike activity below the spontaneous activity defined response onset and offset. The last bin in the block of bins with the lowest activity during the interstimulus interval defined response maximum (7). This procedure was motivated by the expected postsynaptic effect on nuclear cells (maximal response at the end of maximal disinhibition). Traces of cell activity in all figures are smoothed using a five-point moving average.
Pharmacology.
Gabazine (Tocris Bioscience) (10 μm–8.97 mM) was injected ∼0.1–1.0 mm away from the recording electrode in steps until stimulation of interneurons no longer caused inhibition of Purkinje cells.
Acknowledgments
This work was supported by Swedish Research Council Grants 349-2007-8695 to the Linnaeus Centre for Cognition, Communication and Learning and 09899 (to G.H.) and by the Krapperup Foundation, the Söderberg Foundation, and the Åhlén Foundation.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a Prearranged Editor.
References
- 1.Mauk MD, Buonomano DV. The neural basis of temporal processing. Annu Rev Neurosci. 2004;27:307–340. doi: 10.1146/annurev.neuro.27.070203.144247. [DOI] [PubMed] [Google Scholar]
- 2.Kehoe EJ, Macrae M. In: A Neuroscientist's Guide to Classical Conditioning. Moore JW, editor. Springer; New York: 2002. pp. 171–231. [Google Scholar]
- 3.Gruart A, Yeo CH. Cerebellar cortex and eyeblink conditioning: bilateral regulation of conditioned responses. Exp Brain Res. 1995;104(3):431–448. doi: 10.1007/BF00231978. [DOI] [PubMed] [Google Scholar]
- 4.Longley M, Yeo CH. Distribution of neural plasticity in cerebellum-dependent motor learning. Prog Brain Res. 2014;210:79–101. doi: 10.1016/B978-0-444-63356-9.00004-2. [DOI] [PubMed] [Google Scholar]
- 5.Hesslow G, Yeo CH. In: A Neuroscientist's Guide to Classical Conditioning. Moore JW, editor. Springer; New York: pp. 86–146. [Google Scholar]
- 6.Hesslow G, Ivarsson M. Suppression of cerebellar Purkinje cells during conditioned responses in ferrets. Neuroreport. 1994;5(5):649–652. doi: 10.1097/00001756-199401000-00030. [DOI] [PubMed] [Google Scholar]
- 7.Jirenhed DA, Bengtsson F, Hesslow G. Acquisition, extinction, and reacquisition of a cerebellar cortical memory trace. J Neurosci. 2007;27(10):2493–2502. doi: 10.1523/JNEUROSCI.4202-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jirenhed DA, Hesslow G. Learning stimulus intervals—adaptive timing of conditioned Purkinje cell responses. Cerebellum. 2011;10(3):523–535. doi: 10.1007/s12311-011-0264-3. [DOI] [PubMed] [Google Scholar]
- 9.Svensson P, Ivarsson M, Hesslow G. Effect of varying the intensity and train frequency of forelimb and cerebellar mossy fiber conditioned stimuli on the latency of conditioned eye-blink responses in decerebrate ferrets. Learn Mem. 1997;4(1):105–115. doi: 10.1101/lm.4.1.105. [DOI] [PubMed] [Google Scholar]
- 10.Svensson P, Jirenhed DA, Bengtsson F, Hesslow G. Effect of conditioned stimulus parameters on timing of conditioned Purkinje cell responses. J Neurophysiol. 2010;103(3):1329–1336. doi: 10.1152/jn.00524.2009. [DOI] [PubMed] [Google Scholar]
- 11.Wetmore DZ, et al. Bidirectional plasticity of Purkinje cells matches temporal features of learning. J Neurosci. 2014;34(5):1731–1737. doi: 10.1523/JNEUROSCI.2883-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hesslow G, Jirenhed DA, Rasmussen A, Johansson F. Classical conditioning of motor responses: what is the learning mechanism? Neural Netw. 2013;47:81–87. doi: 10.1016/j.neunet.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Yamazaki T, Tanaka S. Computational models of timing mechanisms in the cerebellar granular layer. Cerebellum. 2009;8(4):423–432. doi: 10.1007/s12311-009-0115-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hesslow G. Inhibition of classically conditioned eyeblink responses by stimulation of the cerebellar cortex in the decerebrate cat. J Physiol. 1994;476(2):245–256. doi: 10.1113/jphysiol.1994.sp020127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hesslow G. Correspondence between climbing fibre input and motor output in eyeblink-related areas in cat cerebellar cortex. J Physiol. 1994;476(2):229–244. doi: 10.1113/jphysiol.1994.sp020126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jirenhed DA, Hesslow G. Time course of classically conditioned Purkinje cell response is determined by initial part of conditioned stimulus. J Neurosci. 2011;31(25):9070–9074. doi: 10.1523/JNEUROSCI.1653-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rasmussen A, et al. Number of spikes in climbing fibers determines the direction of cerebellar learning. J Neurosci. 2013;33(33):13436–13440. doi: 10.1523/JNEUROSCI.1527-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dutar P, Vu HM, Perkel DJ. Pharmacological characterization of an unusual mGluR-evoked neuronal hyperpolarization mediated by activation of GIRK channels. Neuropharmacology. 1999;38(4):467–475. doi: 10.1016/s0028-3908(98)00206-8. [DOI] [PubMed] [Google Scholar]
- 19.Gao Z, van Beugen BJ, De Zeeuw CI. Distributed synergistic plasticity and cerebellar learning. Nat Rev Neurosci. 2012;13(9):619–635. doi: 10.1038/nrn3312. [DOI] [PubMed] [Google Scholar]
- 20.Jörntell H, Bengtsson F, Schonewille M, De Zeeuw CI. Cerebellar molecular layer interneurons - computational properties and roles in learning. Trends Neurosci. 2010;33(11):524–532. doi: 10.1016/j.tins.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 21.Ito M, Sakurai M, Tongroach P. Climbing fibre induced depression of both mossy fibre responsiveness and glutamate sensitivity of cerebellar Purkinje cells. J Physiol. 1982;324:113–134. doi: 10.1113/jphysiol.1982.sp014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Linden DJ. The return of the spike: postsynaptic action potentials and the induction of LTP and LTD. Neuron. 1999;22(4):661–666. doi: 10.1016/s0896-6273(00)80726-6. [DOI] [PubMed] [Google Scholar]
- 23.Jörntell H, Ekerot CF. Reciprocal bidirectional plasticity of parallel fiber receptive fields in cerebellar Purkinje cells and their afferent interneurons. Neuron. 2002;34(5):797–806. doi: 10.1016/s0896-6273(02)00713-4. [DOI] [PubMed] [Google Scholar]
- 24.Gallistel CR, Craig AR, Shahan TA. Temporal contingency. Behav Processes. 2014;101:89–96. doi: 10.1016/j.beproc.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jörntell H, Ekerot CF. Properties of somatosensory synaptic integration in cerebellar granule cells in vivo. J Neurosci. 2006;26(45):11786–11797. doi: 10.1523/JNEUROSCI.2939-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bengtsson F, Geborek P, Jörntell H. Cross-correlations between pairs of neurons in cerebellar cortex in vivo. Neural Netw. 2013;47:88–94. doi: 10.1016/j.neunet.2012.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Svensson P, Ivarsson M. Short-lasting conditioned stimulus applied to the middle cerebellar peduncle elicits delayed conditioned eye blink responses in the decerebrate ferret. Eur J Neurosci. 1999;11(12):4333–4340. doi: 10.1046/j.1460-9568.1999.00862.x. [DOI] [PubMed] [Google Scholar]