Significance
The release of cytochrome c from its normal intermembrane space in mitochondria marks the initiation of apoptosis in mammalian cells. The process is triggered by the aggregation of B-cell leukemia/lymphoma 2 (BCL2)-associated X (Bax) and BCL2-antagonist/killer (Bak) proteins on the surface of mitochondria. We found that a mitochondrial inner membrane protease, OMA1 (overlapping activity with m-AAA protease), is specifically activated and is responsible for cleaving another inner membrane protein, optical nerve atrophy 1 (OPA1), upon Bax/Bak aggregation. The cleavage of OPA1 triggers the remodeling of mitochondrial cristae, allowing the majority of cytochrome c inside the cristae to be released. This finding provided a more comprehensive understanding of this critical molecular event during apoptosis.
Keywords: Smac, permeability, membrane potential, caspase
Abstract
Intrinsic apoptotic stimuli initiate mammalian cells’ apoptotic program by first activating the proteins that have only Bcl-2 homology domain 3 (BH3), such as Bcl-2 interacting mediator of cell death (Bim) and truncated BH3 interacting death domain agonist (tBid), which in turn trigger conformational changes in BCL2-associated X (Bax) and BCL2-antagonist/killer (Bak) proteins that enable oligomer formation on the mitochondria, causing cytochrome c and other apoptogenic proteins in the intermembrane space to leak out. Leaked cytochrome c then initiates apoptotic caspase activation through a well-defined biochemical pathway. However, how oligomerized Bax and Bak cause cytochrome c release from mitochondria remains unknown. We report here the establishment of cell lines in which Bim or tBid can be inducibly expressed to initiate apoptosis in a controlled, quantitative manner. We used these cell lines to examine apoptotic events after Bax and Bak oligomerization but before cytochrome c release. The mitochondrial metalloprotease OMA1 was activated in this system in a Bax- and Bak-dependent fashion. Activated OMA1 cleaved the dynamin-like GTPase, optical nerve atrophy 1, an event that is critical for remodeling of mitochondrial cristae. Knockdown or knockout of OMA1 in these cells attenuated cytochrome c release. Thus it is clear that oligomerized Bax and Bak trigger apoptosis by causing both the permeabilization of the mitochondrial outer membrane and activation OMA1.
Mitochondria in mammalian cells fulfill multiple functions. They are cells’ bio-energetic center, where reducing agents generated through the Krebs cycle transfer their electrons to oxygen in a manner mediated by the electron transfer chain, a process that builds a proton gradient across the inner membrane of mitochondria. The energy of this gradient is transferred into the high-energy bond of ATP by oxidative phosphorylation of ADP through the F1/F0 ATP synthase. During apoptosis, the sole water-soluble component of the electron transfer chain, cytochrome c, is released from the intermembrane space of mitochondria to the cytosol (1). Cytosolic cytochrome c binds to the Apaf-1 protein to promote the assembly of a heptamer complex named an “apoptosome”; this complex subsequently recruits procaspase-9, which autoactivates once on the apoptosome. The activated caspase-9 then cleaves and activates downstream caspase-3 and caspase-7, which subsequently cleave many intracellular substrates for apoptosis execution (2).
In addition to cytochrome c, other proteins that normally are located in the mitochondrial intermembrane space also function in apoptosis. One such protein is second mitochondria-derived activator of caspase (Smac). When Smac is released, it binds to the BIR domain of inhibitors of apoptosis proteins to relieve their inhibition of the caspases directly or to cause their degradation (3, 4). Thus controlling the permeability of mitochondria for these apoptogenic proteins constitutes a key regulatory step for apoptosis.
The B-cell leukemia/lymphoma 2 (Bcl-2) family of proteins constitutes a protein network that regulates the release of proteins such as cytochrome c and Smac (5, 6). BCL2-associated X (Bax) and BCL2-antagonist/killer (Bak), the proapoptotic members of the family with multiple Bcl-2 homology (BH) domains, form the core of the mitochondrial membrane permeability machinery that is activated by the proapoptotic proteins that have only the BH3 domain, a process that is inhibited by the proteins whose function is similar to that of Bcl-2 itself (7, 8). In response to apoptotic stimuli, BH3-only proteins, such as Bcl-2 interacting mediator of cell death (Bim), Puma, and truncated BH3 interacting death domain agonist (tBid) directly activate Bax/Bak and lift the inhibition of Bcl-2/Bcl-xl by forming stable heterodimers to sequester them from binding Bak/Bak (7, 9–13). Activated Bax and Bak initially form homodimers and then oligomers on the mitochondrial membrane (14–18). Bax/Bak oligomers are believed to form proteinaceous or lipidic pores on the mitochondrial outer membrane that allow the passage of proteins such as cytochrome c and Smac. Although the results of in vitro liposome leakage experiments support this model, there is no direct in vivo evidence to validate such a straightforward model (19–23).
Moreover, increasing evidence indicates that the majority of cytochrome c in the mitochondrial intermembrane space is locked inside cristae by the protein complex containing optical nerve atrophy 1 (OPA1). The cristae must undergo reconfiguration to open up the neck of cristae for the bulk of cytochrome c to be released from the mitochondria after the outer membrane becomes permeable (24–26). The mitochondrial inner membrane fusion factor OPA1, a dynamin-like GTPase, plays a critical role in the remodeling of cristae. OPA1 presents in several spliced and proteolytic forms in mitochondria, and the maintenance of the relative amounts of each of these forms is known to be critical for stabilizing the cristae (27, 28). The longest form, L-OPA1, is cleaved in response to a variety of mitochondrial stresses, leading to the disassembly of OPA1-containing complexes and remodeling of the cristae (24, 26). It has been proposed that several different proteins cleave OPA1; these include the mitochondrial AAA proteases, presenilin-associated rhomboid-like protein (PARL), and the zinc metalloprotease OMA1 (overlapping activity with m-AAA protease) (26, 27, 29–31). However, the relationship between the pore formation on the mitochondrial outer membrane and OPA1 cleavage-mediated cristae remodeling during apoptosis, as well as the precise roles of those mitochondrial proteases in apoptosis, remain to be clarified.
To dissect the molecular details of cytochrome c release induced by BH3-only proteins, we generated cell lines in which Bim or tBid can be inducibly expressed by adding doxycycline (Dox) into the culture medium. The expression of these proteins triggers apoptosis in a controlled and synchronized fashion. We used this cell-based system to characterize the mitochondrial response to the induction of Bim and tBid and found that OMA1 activation is an important step for apoptosis induction.
Results
Inducible Expression of Bim Triggers Mitochondria-Mediated Apoptosis.
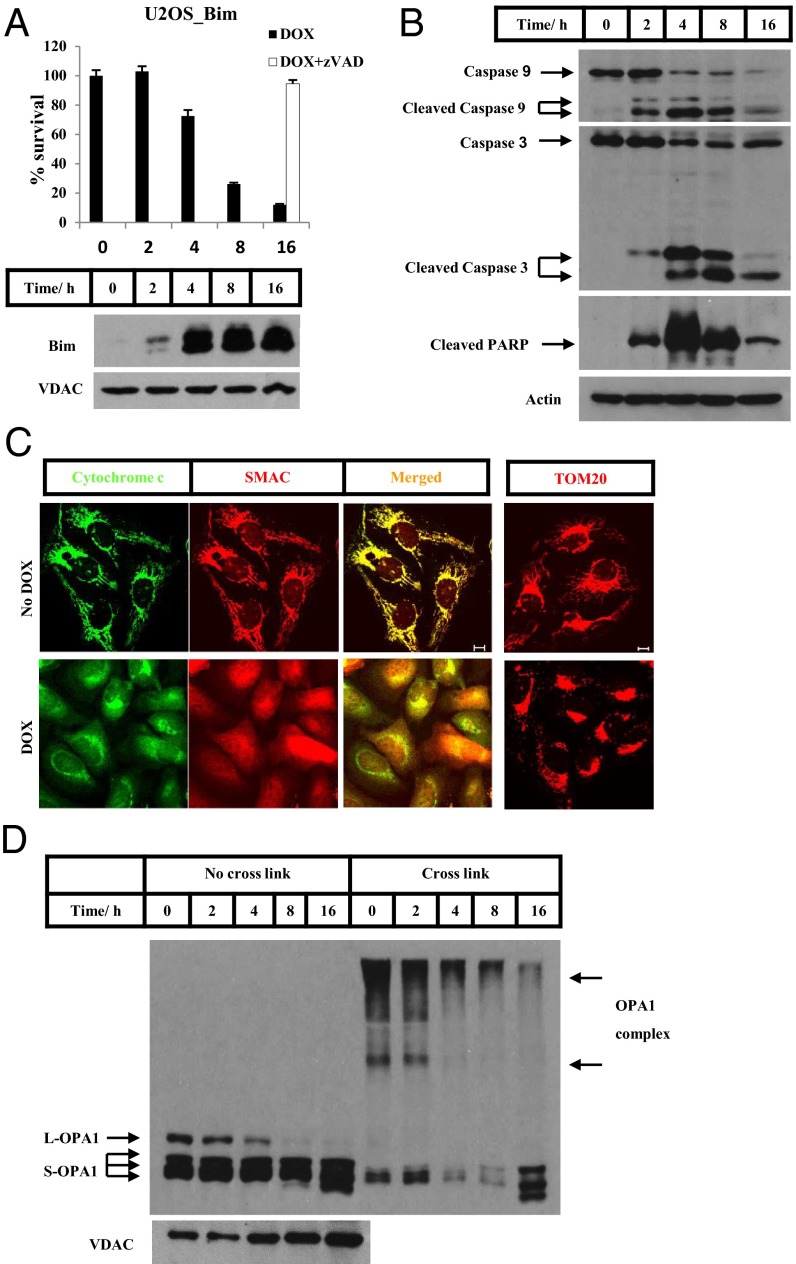
In an effort to dissect the detailed processes of the apoptosis pathway inside mitochondria, we engineered a U2OS human osteosarcoma cancer cell line in which the BH3-only protein Bim is inducibly expressed when Dox is added to the culture medium. As shown in Fig. 1A, there was no detectable Bim expression before the addition of Dox. Bim started to appear 2 h after the addition of Dox and reached an expression plateau at 4 h (Fig. 1A, Lower). Consistently, the cells’ vitality as measured by their intracellular ATP level started to drop at the 4-h time point. The ATP level was preserved at 90% when the pan-caspase inhibitor z-Val-Ala-Asp-fluoromethylketone (z-VAD-fmk) was included in the cultures (Fig. 1A, Upper). The activation of caspase-9 and caspase-3 seemed to follow the kinetics of Bim induction, with a peak of activation at 4 h (Fig. 1B, Top and Middle). Further, most of the poly(ADP ribose) polymerase (PARP), a caspase-3 substrate, was cleaved at 4 h (Fig. 1B).
Fig. 1.
Inducible expression of Bim triggers mitochondria-mediated apoptosis. (A) A U2OS cell line with a stably transfected Bim transgene (U2OS_Bim) was treated with Dox (0.1 µg/mL) in the presence or absence of z-VAD (20 µM) for the times indicated. The same concentrations were used in all experiments. Cell viability was determined by measuring ATP levels using the Cell-Titer Glo kit. Following Dox treatment for the indicated times, the P15 fractions of U2OS_Bim cells were analyzed by Western blotting. (B) U2OS_Bim cells were treated with or without Dox for the times indicated. The S15 fractions of the cells were analyzed by Western blotting. (C) U2OS_Bim cells in the presence of z-VAD were treated with or without Dox for 4 h. Immunostaining was performed using anti-cytochrome c (green) and anti-Smac (red) antibodies or anti-translocase of the outer membrane 20 (TOM20) antibody. (Scale bars: 10 µm.) (D) U2OS_Bim cells were treated with Dox for the times indicated. The P15 fractions were cross-linked with 10 mM 1,6-bis(maleimido)hexane (BMH) where indicated and were analyzed by Western blotting.
The induction of Bim caused a dramatic release of cytochrome c and Smac from mitochondria as measured by immunofluorescent staining (Fig. 1C) and cell fractionation followed by Western blotting analysis (Fig. S1). Both proteins were located exclusively within mitochondria before Dox addition, and the mitochondria were in the healthy tubular form. After Bim induction, the majority of the cytochrome c and Smac was released into the cytosol, and the residual protein remaining in the mitochondria showed a fragmented and aggregated form around the nuclei.
Consistent with previous reports, the expression of Bim also caused concurrent cleavage of the long form of OPA1 (L-OPA1) (Fig. 1D, lanes 3–5) and disassembly of the OPA1 complex, which was measured by adding a protein cross-linker before analysis with Western blotting (Fig. 1D, lanes 8–10).
OPA1 Cleavage and Disassembly of the OPA1-Containing Complex Require OMA1.
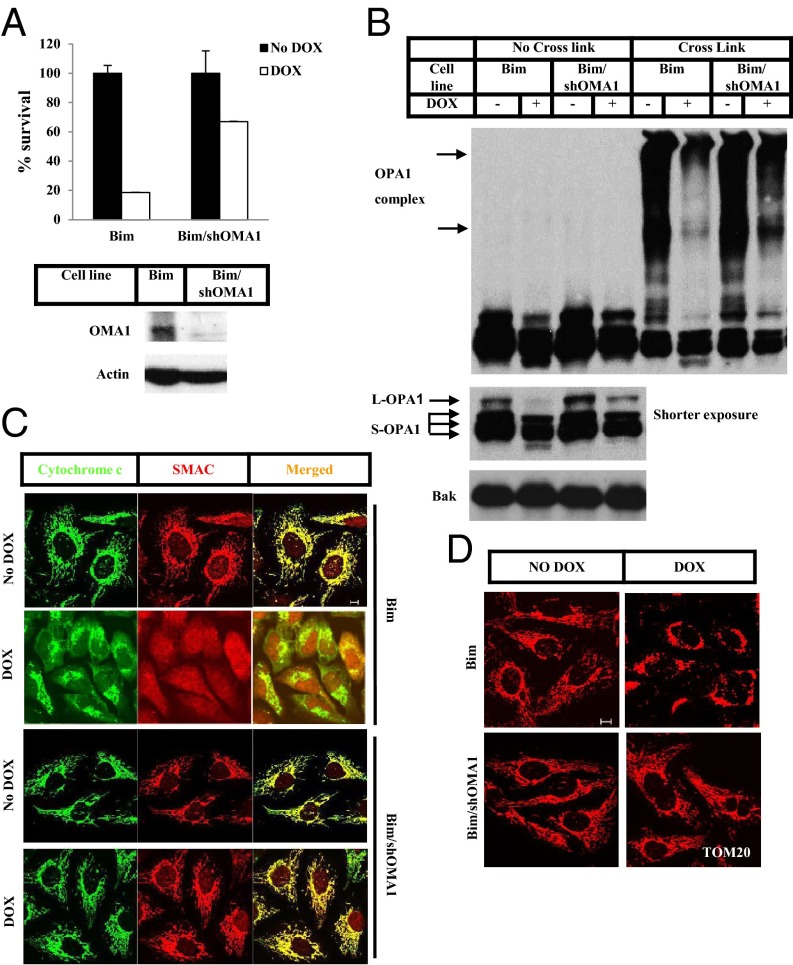
Several proteases have been shown to cleave OPA1 under different stress conditions; these include the i-AAA and m-AAA proteases YME1L, ATPase family gene 3-like 2 (AFG3L2), and paraplegin, the mitochondrial inner membrane zinc protease OMA1, and PARL. Therefore we tested the role of these proteases in Bim-induced apoptosis and OPA1 cleavage (Fig. S2). We found that OMA1 was critically important for apoptosis induction by Bim or tBid expression, whereas knockdown of other mitochondrial proteases had little effect. As shown in Fig. 2, knockdown of OMA1 by stable expression of an shRNA against OMA1 blocked cell death induced by Bim expression (Fig. 2A) and prevented disassembly of the OPA1-containing complex and cleavage of L-OPA1 (Fig. 2B, Top and Bottom). Additionally, knockdown of OMA1 prevented the release of cytochrome c and Smac from the mitochondria (Fig. 2C) and the fragmentation of mitochondria (Fig. 2D).
Fig. 2.
OMA1 controls Bim-induced OPA1 cleavage and disassembly of the OPA1-containing complex. (A) U2OS_Bim (Bim) cells and U2OS_Bim cells stably expressing OMA1 shRNA (Bim/shOMA1) were treated with or without Dox for 12 h. Cell viability was determined using the Cell-Titer Glo kit. Whole-cell extracts of Bim and Bim/shOMA1 cells were analyzed by Western blotting. (B) Bim and Bim/shOMA1 cells were treated with or without Dox for 8 h. The P15 fractions of the cells were cross-linked with 10 mM BMH where indicated. Western blotting was performed using the indicated antibodies. (C and D) Bim and Bim/shOMA1 cells were treated with or without Dox for 8 h. z-VAD was included during the treatment of Bim cells. Immunostaining was performed using anti-cytochrome c (green) and anti-Smac (red) antibodies (C) or anti-TOM20 antibody (D). (Scale bars: 10 µm.)
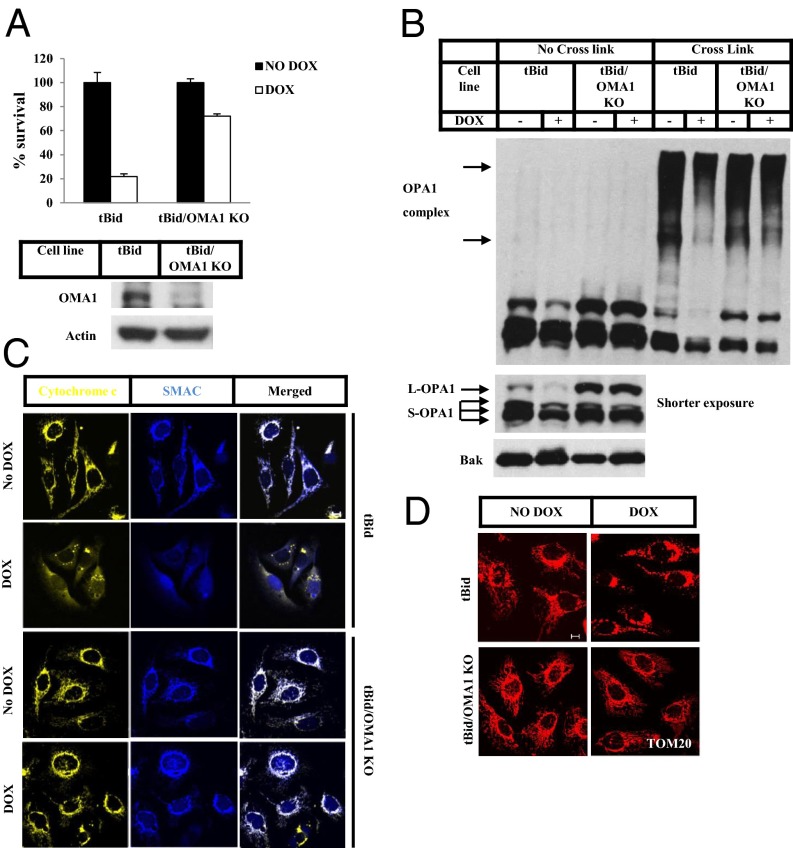
To confirm that OMA1 is required in apoptosis, we generated another U2OS cell line in which tBid, another BH3-only protein, could be induced with Dox. These cells underwent apoptosis when Dox was added to the medium (Fig. 3A and Fig. S3). We used clustered regularly interspaced short palindromic repeats (CRISPR) technology to delete the OMA1 gene from the cell line (Fig. 3A, Lower and Fig. S4); as a consequence, cell death was inhibited upon the addition of Dox (Fig. 3A, Upper). Disassembly of the OPA1-containing complex and cleavage of L-OPA1 were prevented also (Fig. 3B). As in the Bim-expressing cells in which OMA1 was knocked down, we did not detect cytochrome c or Smac release or mitochondrial fragmentation, even when tBid was induced in these cells (Fig. 3 C and D). Knockdown of OMA1 also blocked UV-induced OPA1 cleavage and cytochrome c/Smac release in HeLa cells, indicating that OMA1 plays a general role in promoting apoptosis (Fig. S5).
Fig. 3.
OMA1 controls tBid-induced OPA1 cleavage and disassembly of the OPA1-containing complex. (A) A U2OS cell line with a stably transfected tBid transgene (tBid) and tBid cells with stable knockout of OMA1 (tBid/OMA1 KO) were treated with or without Dox for 12 h. Cell viability was determined using the Cell-Titer Glo kit. Whole-cell extracts of tBid and tBid/OMA1 KO cells were analyzed by Western blotting. (B) tBid and tBid/OMA1 KO cells were treated with or without Dox for 8 h. The P15 fractions of the cells were cross-linked with 10 mM BMH where indicated. Western blotting was performed using the indicated antibodies. (C and D) tBid and tBid/OMA1 KO cells were treated with or without Dox for 8 h. z-VAD was included during the treatment for tBid cells. Immunostaining was performed using anti-cytochrome c (fluorescent secondary antibody with excitation at 633 nm, shown in yellow) and anti-Smac (blue) antibodies (C) or anti-TOM20 antibody (D). (Scale bar: 10 µm.)
Cleavage of L-OPA1 and Cytochrome c Release Require OMA1 Protease Activity.
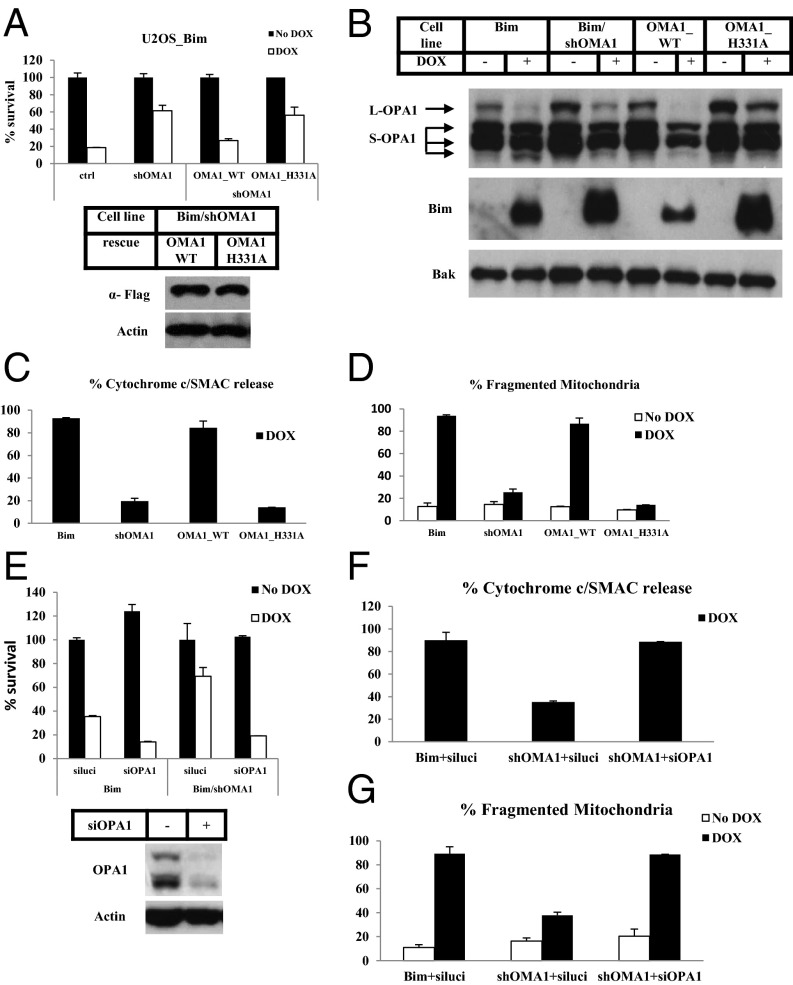
To characterize the function of OMA1 further, we reintroduced the shRNA-resistant wild-type or a protease active site mutant (H331A) version of OMA1 back into the U2OS-Bim cell line in which OMA1 was knocked down and induced apoptosis by adding Dox to the culture medium. As shown in Fig. 4A, reintroduction of the wild-type OMA1, but not the inactive H331A mutant, restored the apoptosis response, even when both forms were expressed at similar levels. Consistently, the attenuation of L-OPA1 cleavage upon Bim induction was completely reversed by expression of the wild-type OMA1 transgene, but not by expression of the H331A mutant (Fig. 4B). Of note, Bim accumulated to a higher level when apoptosis was blocked by disabling OMA1 (compare lanes in Fig. 4B), even though the Bak level remained constant.
Fig. 4.
OMA1 promotes cytochrome c release through cleavage of OPA1. (A) Bim cells (ctrl), Bim/shOMA1 cells, Bim/shOMA1 cells rescued with wild-type OMA1 (OMA1_WT), and Bim/shOMA1 cells rescued with the protease active site mutant of OMA1 (OMA1_H331A) were treated with or without Dox for 16 h. Cell viability was determined using the Cell-Titer Glo kit. Whole-cell extracts of the indicated cell lines were analyzed by Western blotting. (B) The indicated cell lines were treated with or without Dox for 16 h. The P15 fractions of the cells were analyzed by Western blotting. (C and D) The indicated cell lines were treated with or without Dox for 16 h. z-VAD was included during the treatment for Bim and OMA1_WT cells. Percentages of cells with cytochrome c and Smac release into cytosol (C) or percentages of cells with fragmented mitochondria (D) as examined by immunostaining were calculated. Mitochondria were visualized with immunostaining of TOM20. (E) Bim and Bim/shOMA1 cells were transfected with siRNA against luciferase (luci) or OPA1. Forty-eight hours later, cells were treated with or without Dox for 12 h. Cell viability was determined using the Cell-Titer Glo kit. Whole-cell extracts of Bim cells before and after 48 h of OPA1 siRNA transfection were analyzed by Western blotting. (F and G) Bim and Bim/shOMA1 cells were transfected with the indicated siRNAs and were treated with or without Dox for 8 h. z-VAD was included during the treatment for Bim cells and Bim/shOMA1 cells with OPA1 siRNA transfection. Percentages of cells with cytochrome c and Smac release into cytosol (F) and percentages of cells with fragmented mitochondria (G) as determined by immunostaining were calculated.
The ectopic expression of the shRNA-resistant wild-type OMA1, but not the H331A mutant, also restored cytochrome c and Smac release from mitochondria (Fig. 4C and Fig. S6A) and mitochondrial fragmentation (Fig. 4D and Fig. S6B) upon Bim induction.
The OMA1 Effect on Apoptosis Can Be Bypassed by OPA1 Knockdown.
Although OMA1 seems to be the protease that cleaves L-OPA1 upon Bim/tBid expression and seems to promote disassembly of the OPA1-containing complex, we sought to confirm whether OPA1 is the downstream effector of OMA1 during apoptosis. To do so, we knocked down OPA1 with siRNA in cells in which apoptosis was blocked by the lack of OMA1. As shown in Fig. 4E, knockdown of OPA1 in cells expressing OMA1 shRNA restored the apoptosis response when Bim was induced by Dox. Both cytochrome c/Smac release and mitochondrial fragmentation were normal when OPA1 was knocked down (Fig. 4 F and G and Fig. S7 A and B). Similar results were obtained with the U2OS-tBid OMA1 knockout cells (Fig. S7 C and D). These results indicate that OPA1 is indeed the downstream effector of OMA1.
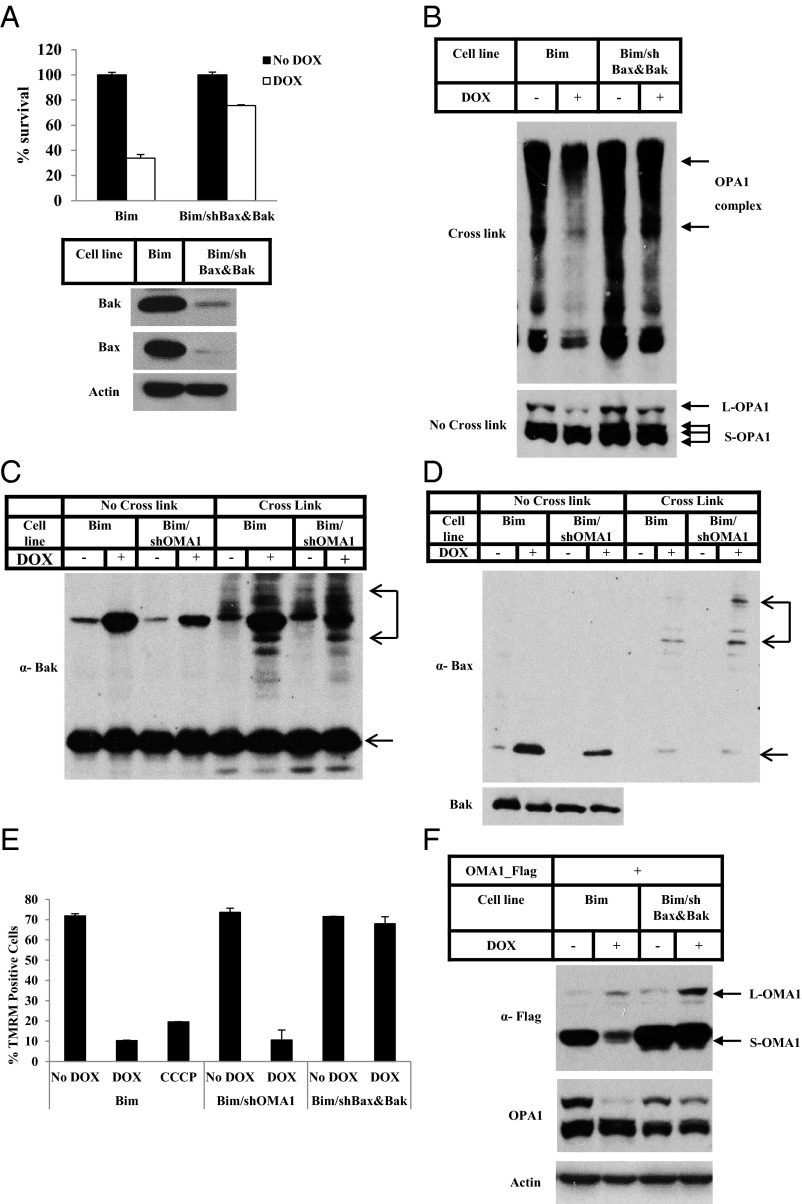
Bax and Bak Function Upstream of OMA1 Activation.
To test if Bax/Bak activation is upstream or independent of OMA1 function in apoptosis, we knocked down both Bax and Bak in the cell line in which Bim was inducibly expressed. The knockdown effect of Bak and Bax was nearly complete (Fig. 5A, Lower). The apoptosis response in those cells was largely eliminated following induction of Bim (Fig. 5B, Upper). Interestingly, although knockdown of OMA1 did not affect Bak and Bax oligomerization upon Bim induction (Fig. 5 C and D), the cleavage of L-OPA1 and the disassembly of the OPA1-containing complex were attenuated after Bak/Bax knockdown (Fig. 5B). Therefore it is quite clear that Bax and Bak function upstream of OMA1.
Fig. 5.
Bax and Bak function upstream of OMA1 activation. (A) Bim cells and Bim cells stably expressing both Bax and Bak shRNAs (Bim/shBax&Bak) were treated with or without Dox for 12 h. Cell viability was determined using the Cell-Titer Glo kit. Whole-cell extracts of Bim and Bim/shBax&Bak cells were analyzed by Western blotting. (B–D) Bim and Bim/shBax&Bak cells (B) or Bim and Bim/shOMA1 cells (C and D) were treated with or without Dox for 8 h. The P15 fractions were cross-linked with 10 mM BMH where indicated. Western blotting was performed using the indicated antibodies. (E) The indicated cell lines were treated with or without Dox for 8 h or were treated with 10 µM CCCP for 90 min. TMRM staining followed by flow cytometry analysis was performed. TMRM+ cells were quantified. (F) Bim and Bim/shBax&Bak cells were transfected with Flag-tagged OMA1 for 48 h. Cells then were treated with Dox for 8 h or were left untreated. Whole-cell extracts were analyzed by Western blotting. L-OMA1, full-length precursor OMA1; S-OMA1, shorter version of OMA1.
Bim and tBid Induction Stabilizes the OMA1 Precursor and Activates OMA1 Activity.
OMA1 constantly undergoes autocleavage in healthy mitochondria (Fig. S8A). Upon Bim and tBid induction by Dox, the full-length precursor of OMA1 accumulated on the mitochondria, and the level of the shorter mature version of OMA1 decreased (Fig. S8 B and C); L-OPA1 was concurrently cleaved. The accumulation of full-length OMA1 and the decreased abundance of shorter OMA1 are similar to the results observed when cells were treated with the mitochondrial uncoupler carbonylcyanide m-chlorophenylhydrazone (CCCP) (Fig. S8D and ref. 31). Interestingly, CCCP treatment caused a similar accumulation of full-length OMA1 but led to the total disappearance of the shorter OMA1.
These results suggested that induction of Bim or tBid might stabilize precursor OMA1 by disrupting mitochondrial membrane potential and destabilizing mature OMA1 by enhancing its autocleavage activity. Indeed, the addition of Dox caused a drop in the mitochondrial membrane potential as measured by a fluorescent dye, tetramethylrhodamine (TMRM) (Fig. S9 A and B). This result was similar to that in cells treated with CCCP. However, unlike cells treated with CCCP, the loss of membrane potential could be rescued if Bax and Bak were knocked down (Fig. 5E and Fig. S9 A–C). Furthermore, knockdown of Bax and Bak blocked Bim-triggered, but not CCCP-triggered, OMA1 destabilization (Fig. 5F and Fig. S9D).
Discussion
Bak and Bax Oligomerization Can Cause both Outer Membrane Permeabilization and Activation of OMA1 to Accommodate Cytochrome c Release.
The current model for cytochrome c release during apoptosis centers on the formation of pores by oligomerized Bak and Bax, which are induced by the BH3-only proteins such as Bim and tBid (16, 19–23, 32). Such pores allow apoptogenic proteins in the intermembrane space of mitochondria to leak out to the cytosol passively. Such a model does not take into account that most of the cytochrome c in mitochondria actually is locked inside cristae by the OPA1-containing complexes at the neck of mitochondrial cristae. The disassembly of such a complex thus is critical for the majority of cytochrome c to gain access to the inner boundary of the mitochondrial outer membrane (24–26, 33).
The results presented here demonstrate that OMA1 is another downstream target for Bax/Bak activation, in addition to its functional role in Bax and Bak oligomerization during outer membrane pore formation. Elimination of OMA1 by either shRNA knockdown or CRISPR-mediated gene knockout significantly attenuated cytochrome c release without affecting Bax/Bak oligomerization. However, knockdown of OPA1 circumvented knockdown of OMA1, indicating that the role of OMA1 in cytochrome c release is to cleave OPA1, leading to the disassembly of the OPA1-containing complex.
OMA1 can be activated by a variety of mitochondrial stress signals (34). Both CCCP, which dissipates mitochondrial membrane potential, and oligomycin, which inhibits F0 ATP synthase and thus increases mitochondrial membrane potential, have been shown to activate OMA1, leading to the cleavage of OPA1 and mitochondrial fragmentation (31, 34). However, simply treating cells with CCCP or oligomycin does not lead to cytochrome c release. Thus, OMA1 activation is necessary, but not sufficient, for cytochrome c release. In contrast, Bax and Bak oligomerization induced by either Bim or tBid expression can cause both outer membrane permeabilization and activation of OMA1.
Thus, oligomerized Bax- and Bak-induced cytochrome c release requires two steps. One is to permeabilize the outer membrane, allowing the cytochrome c that has free access to the outer membrane to leak out; the second, concurrent step is the activation of OMA1, which cleaves L-OPA1 and causes disassembly of the OPA1-containing protein complexes that hold most of the cytochrome c within cristae. The second step may not be critical for apoptosis initiation but clearly is able to accelerate caspase-9 activation and change the dynamics of apoptosis progression.
OMA1 Activation Manifested in Accelerated Autocleavage and Cleavage of Its Substrate OPA1.
It is apparent that loss of mitochondrial membrane potential stalls the import process of precursor OMA1 at the outer membrane, resulting in its accumulation (Fig. S8 B–D). Interestingly, treatment with CCCP or induction of Bim or tBid also decreased the level of the active 40-kDa OMA1. Because 40-kDa OMA1 undergoes autocleavage under normal conditions (Fig. S8A), it is likely that OMA1 activation also accelerates its own degradation. The rapid elimination of OMA1 following treatment with CCCP or Bax/Bak oligomerization (Fig. 5F and Fig. S8 B–D) is caused by the blockage of OMA1 import into the mitochondrial inner membrane and accelerated autocleavage-mediated degradation of the existing OMA1. Accelerated OMA1 activity also resulted in more cleavage of its substrate, L-OPA1.
How Does Oligomerized Bax/Bak Activate OMA1?
Because OMA1 is an inner mitochondrial membrane protease, Bax and Bak oligomers must affect the inner membrane in some way. Indeed, loss of mitochondrial inner membrane potential was observed after Bim or tBid induction (Fig. 5E and Fig. S9 A and B). However, neither the proteinaceous nor lipidic pores that have been proposed for oligomer Bax and Bak can account for such a function if such pores are located solely on the outer membrane of mitochondria. It also is hard to imagine that the permeability of the outer membrane and the activation of OMA1 on the inner membrane result from two separate modes of action mediated by the same protein. In addition, treatment of mitochondria with tBid completely eliminated ADP-stimulated oxygen consumption (stage III respiration) (35), suggesting that the disruption of the coupling of F1/F0 ATP synthase and mitochondrial membrane potential is similar to that seen with oligomycin treatment.
We thus propose that oligomerized Bax and Bak must interact with the contact sites of the outer and inner membranes so that they can affect outer membrane permeability, OMA1 activation, and F0/F1 ATP synthase coupling, thus accounting for all the observed effects. Such interactions cause the enhancement of OMA1 protease activity, resulting in accelerated autodegradation and more cleavage of L-OPA1. The molecular mechanism by which oligomer Bax and Bak control OMA1 activity should be an interesting topic of future studies. The resulting disassociation of cytochrome c from its normal functional sites, the dissipation of mitochondrial membrane potential, and the decoupling of electron transfer chain and oxidative phosphorylation even might be used normally to synchronize electron transfer chain activity with the required supply and the demand for cellular energy. This hypothesis is in consistent with the low energy expenditure and heat generation observed in the OMA1-knockout mice (36). However, because this disassociation also leads to oxidative damage in cells, the ensuing apoptosis might be a clean exit strategy for cells in which Bax and Bak oligomerization is too strong.
Materials and Methods
Reagents, plasmids, siRNA oligos, and methods for cell viability assay, transfection, lentiviral packaging and viral infection, cell culture and stable cell lines, cellular fractionation, and the protein cross-linking assay are described in SI Materials and Methods. Also see SI Materials and Methods for details of the preparation of whole-cell extract, UV irradiation, immunostaining, and TMRM staining. Data are presented as means ± SD of duplicate experiments.
Supplementary Material
Acknowledgments
We thank Mr. Le Yin and Ms. Jie Chen for technical assistance and the Imaging Center and the Biological Resource Center at the National Institute of Biological Sciences, Beijing for technical support. This work was supported by National Basic Science 973 Grant 2010CB835400 from the Chinese Ministry of Science and Technology.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1417253111/-/DCSupplemental.
References
- 1.Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: Requirement for dATP and cytochrome c. Cell. 1996;86(1):147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 2.Li P, et al. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91(4):479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 3.Du C, Fang M, Li Y, Li L, Wang X. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell. 2000;102(1):33–42. doi: 10.1016/s0092-8674(00)00008-8. [DOI] [PubMed] [Google Scholar]
- 4.Verhagen AM, et al. Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell. 2000;102(1):43–53. doi: 10.1016/s0092-8674(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 5.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: A primary site for Bcl-2 regulation of apoptosis. Science. 1997;275(5303):1132–1136. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 6.Yang J, et al. Prevention of apoptosis by Bcl-2: Release of cytochrome c from mitochondria blocked. Science. 1997;275(5303):1129–1132. doi: 10.1126/science.275.5303.1129. [DOI] [PubMed] [Google Scholar]
- 7.Wei MC, et al. Proapoptotic BAX and BAK: A requisite gateway to mitochondrial dysfunction and death. Science. 2001;292(5517):727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nechushtan A, Smith CL, Lamensdorf I, Yoon SH, Youle RJ. Bax and Bak coalesce into novel mitochondria-associated clusters during apoptosis. J Cell Biol. 2001;153(6):1265–1276. doi: 10.1083/jcb.153.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei MC, et al. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000;14(16):2060–2071. [PMC free article] [PubMed] [Google Scholar]
- 10.Kuwana T, et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17(4):525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Willis SN, et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19(11):1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peng J, et al. tBid elicits a conformational alteration in membrane-bound Bcl-2 such that it inhibits Bax pore formation. J Biol Chem. 2006;281(47):35802–35811. doi: 10.1074/jbc.M608303200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren D, et al. BID, BIM, and PUMA are essential for activation of the BAX- and BAK-dependent cell death program. Science. 2010;330(6009):1390–1393. doi: 10.1126/science.1190217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eskes R, Desagher S, Antonsson B, Martinou JC. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol. 2000;20(3):929–935. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antonsson B, Montessuit S, Sanchez B, Martinou JC. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J Biol Chem. 2001;276(15):11615–11623. doi: 10.1074/jbc.M010810200. [DOI] [PubMed] [Google Scholar]
- 16.Annis MG, et al. Bax forms multispanning monomers that oligomerize to permeabilize membranes during apoptosis. EMBO J. 2005;24(12):2096–2103. doi: 10.1038/sj.emboj.7600675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Czabotar PE, et al. Bax crystal structures reveal how BH3 domains activate Bax and nucleate its oligomerization to induce apoptosis. Cell. 2013;152(3):519–531. doi: 10.1016/j.cell.2012.12.031. [DOI] [PubMed] [Google Scholar]
- 18.Lovell JF, et al. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell. 2008;135(6):1074–1084. doi: 10.1016/j.cell.2008.11.010. [DOI] [PubMed] [Google Scholar]
- 19.Korsmeyer SJ, et al. Pro-apoptotic cascade activates BID, which oligomerizes BAK or BAX into pores that result in the release of cytochrome c. Cell Death Differ. 2000;7(12):1166–1173. doi: 10.1038/sj.cdd.4400783. [DOI] [PubMed] [Google Scholar]
- 20.Basañez G, et al. Bax-type apoptotic proteins porate pure lipid bilayers through a mechanism sensitive to intrinsic monolayer curvature. J Biol Chem. 2002;277(51):49360–49365. doi: 10.1074/jbc.M206069200. [DOI] [PubMed] [Google Scholar]
- 21.Kuwana T, et al. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111(3):331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- 22.Martinez-Caballero S, et al. Assembly of the mitochondrial apoptosis-induced channel, MAC. J Biol Chem. 2009;284(18):12235–12245. doi: 10.1074/jbc.M806610200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schafer B, et al. Mitochondrial outer membrane proteins assist Bid in Bax-mediated lipidic pore formation. Mol Biol Cell. 2009;20(8):2276–2285. doi: 10.1091/mbc.E08-10-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frezza C, et al. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006;126(1):177–189. doi: 10.1016/j.cell.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 25.Olichon A, et al. Loss of OPA1 perturbates the mitochondrial inner membrane structure and integrity, leading to cytochrome c release and apoptosis. J Biol Chem. 2003;278(10):7743–7746. doi: 10.1074/jbc.C200677200. [DOI] [PubMed] [Google Scholar]
- 26.Yamaguchi R, et al. Opa1-mediated cristae opening is Bax/Bak and BH3 dependent, required for apoptosis, and independent of Bak oligomerization. Mol Cell. 2008;31(4):557–569. doi: 10.1016/j.molcel.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Song Z, Chen H, Fiket M, Alexander C, Chan DC. OPA1 processing controls mitochondrial fusion and is regulated by mRNA splicing, membrane potential, and Yme1L. J Cell Biol. 2007;178(5):749–755. doi: 10.1083/jcb.200704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cipolat S, et al. Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell. 2006;126(1):163–175. doi: 10.1016/j.cell.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 29.Ehses S, et al. Regulation of OPA1 processing and mitochondrial fusion by m-AAA protease isoenzymes and OMA1. J Cell Biol. 2009;187(7):1023–1036. doi: 10.1083/jcb.200906084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stiburek L, et al. YME1L controls the accumulation of respiratory chain subunits and is required for apoptotic resistance, cristae morphogenesis, and cell proliferation. Mol Biol Cell. 2012;23(6):1010–1023. doi: 10.1091/mbc.E11-08-0674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Head B, Griparic L, Amiri M, Gandre-Babbe S, van der Bliek AM. Inducible proteolytic inactivation of OPA1 mediated by the OMA1 protease in mammalian cells. J Cell Biol. 2009;187(7):959–966. doi: 10.1083/jcb.200906083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dejean LM, et al. Oligomeric Bax is a component of the putative cytochrome c release channel MAC, mitochondrial apoptosis-induced channel. Mol Biol Cell. 2005;16(5):2424–2432. doi: 10.1091/mbc.E04-12-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arnoult D, Grodet A, Lee YJ, Estaquier J, Blackstone C. Release of OPA1 during apoptosis participates in the rapid and complete release of cytochrome c and subsequent mitochondrial fragmentation. J Biol Chem. 2005;280(42):35742–35750. doi: 10.1074/jbc.M505970200. [DOI] [PubMed] [Google Scholar]
- 34.Baker MJ, et al. Stress-induced OMA1 activation and autocatalytic turnover regulate OPA1-dependent mitochondrial dynamics. EMBO J. 2014;33(6):578–593. doi: 10.1002/embj.201386474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gonzalvez F, et al. tBid interaction with cardiolipin primarily orchestrates mitochondrial dysfunctions and subsequently activates Bax and Bak. Cell Death Differ. 2005;12(6):614–626. doi: 10.1038/sj.cdd.4401571. [DOI] [PubMed] [Google Scholar]
- 36.Quirós PM, et al. Loss of mitochondrial protease OMA1 alters processing of the GTPase OPA1 and causes obesity and defective thermogenesis in mice. EMBO J. 2012;31(9):2117–2133. doi: 10.1038/emboj.2012.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.