Abstract
Purpose
Plasma lipoprotein-associated phospholipase A2 (Lp-PLA2) binds to low-density lipoprotein. The levels of Lp-PLA2 reflect the plaque burden, and are upregulated in acute coronary syndrome (ACS). We investigated the diagnostic value of Lp-PLA2 levels and found that it might be a potential biomarker for ACS.
Materials and Methods
We classified 226 study participants into three groups: patients without significant stenosis (control group), patients with significant stenosis with stable angina (SA group), and patients with ACS (ACS group).
Results
Lp-PLA2 and high-sensitivity C-reactive protein (hs-CRP) levels were significantly greater in the ACS group than in the SA group (p=0.044 and p=0.029, respectively). Multivariate logistic regression analysis revealed that Lp-PLA2 levels are significantly associated with ACS (odds ratio=1.047, p=0.013). The addition of Lp-PLA2 to the ACS model significantly increased the global χ2 value over traditional risk factors (28.14 to 35.602, p=0.006). The area under the receiver operating characteristic curve for Lp-PLA2 was 0.624 (p=0.004). The addition of Lp-PLA2 level to serum hs-CRP concentration yielded an integrated discrimination improvement of 0.0368 (p=0.0093, standard error: 0.0142) and improved the ability to diagnose ACS.
Conclusion
Lp-PLA2 levels are related to plaque stability and might be a diagnostic biomarker for ACS.
Keywords: Lp-PLA2, acute coronary syndrome, biomarker, coronary atherosclerotic plaque instability
INTRODUCTION
Cardiovascular disease is the major cause of death in most industrialized nations. Coronary heart disease (CHD) appears either as a stable disease, progressing slowly over several years, or as an unstable disease, progressing rapidly and resulting in acute coronary syndrome (ACS), such as myocardial infarction, which may cause sudden death. Progression to ACS seems to depend most on exaggerated inflammatory changes within atherosclerotic plaques.1,2,3
In recent studies, lipoprotein-associated phospholipase A2 (Lp-PLA2) was found to play a crucial role in the incidence of CHD in people whose low-density lipoprotein (LDL) concentrations were not predictive of high CHD risk.4 Earlier reports suggested a relation between Lp-PLA2 and CHD risk;4,5,6,7 however, recent studies have shown that Lp-PLA2 is an independent predictor of CHD.5,8 As a biomarker for coronary artery disease (CAD), Lp-PLA2 levels are notably increased in ACS in particular, because Lp-PLA2 activity is associated with vulnerable atherosclerotic plaques.8 Liu, et al.9 demonstrated that Lp-PLA2 level is associated with vulnerable coronary atherosclerotic plaques and that Lp-PLA2 could be a strong predictive biomarker for ACS10 In addition, recent studies have shown that Lp-PLA2 levels are elevated in ACS, which suggests that Lp-PLA2 may be a clinical biomarker for ACS. The clinical diagnostic value of Lp-PLA2 for ACS; however, has yet to be fully evaluated in comparison with other biomarkers, such as high-sensitivity C-reactive protein (hs-CRP) or lipoprotein concentration. hs-CRP is an accepted biomarker of inflammation and can be used as a potential biomarker of plaque instability; nevertheless, a question of tissue specificity has arisen for hs-CRP. Specifically, it is unclear as to whether hs-CRP is a plaque-specific inflammatory marker or a systemic inflammatory marker. Lp-PLA2 is specific for vascular inflammation because it is made by macrophages in the plaque, in contrast to other markers of inflammation like CRP, which are made in the liver. Lp-PLA2 may hold the potential to be a useful, vascular tissue-specific biomarker, if its measurement could be standardized.11,12 Therefore, this study was conducted to evaluate the diagnostic value of Lp-PLA2 as an independent biomarker of ACS by comparing it to hs-CRP, which is widely used as a pro-inflammatory marker for coronary plaques.
MATERIALS AND METHODS
Subjects & clinical analysis
We studied 226 consecutive patients scheduled to undergo coronary angiography at Gangnam Severance Hospital in Seoul, Korea, from 2010 to 2012. Demographic data and clinical history were obtained for each patient through chart review, with attention to clinical presentation, CAD risk factors, and medication, including lipid-lowering agents. Study participants were classified into three groups on the basis of their clinical presentation: 1) 53 cases with no significant stenotic lesions of the coronary arteries (control group); 2) 57 cases of stable angina (SA group); and 3) 116 cases of ACS including 60 cases of unstable angina (UA) and 56 cases of acute myocardial infarction (AMI) (ACS group). The age range was 36-92 years (mean age 62.69±10.59 years); 139 subjects were male and 87 were female. For all study groups, the exclusion criteria were malignant tumors, inflammatory disorders, severe liver disease (plasma alanine aminotransferase level ≥100 IU/L), renal disease (plasma creatinine ≥2.2 mg/dL), and hypersensitivity to contrast dye.
The study subjects were divided into ACS, SA, and control groups. In this study, the non-ACS group included both the control and SA groups. The control group was composed of patients who did not have significant coronary artery stenosis despite chest pain-likely atypical pattern. SA was defined as no changes in angina symptoms in the preceding 6 weeks, with no biomarkers of necrosis. Patients who underwent follow-up coronary angiography 9-12 months after the previous procedure, due either to residual stenotic lesions or to a history of stenting, were also included in the SA group. In contrast, ACS was characterized by typical chest pain, generally occurring at rest or with minimal exertion. The subtypes of ACS included UA and AMI. We defined UA as the presence of 1) angina at rest, 2) new onset accelerated angina within the past two months, or 3) chronic stable angina in patients who developed accelerated angina but had not experienced angina at rest during the preceding two months (according to the Braunwald classification).13 Subjects with elevated cardiac biomarkers [e.g., troponins, creatine kinase (CK), and CK-MB] were categorized as AMI patients, and the remaining patients were regarded as UA patients.14,15 In addition, we defined heart failure as the presence of New York Heart Association (NYHA) class II-IV symptoms accompanying cardiomegaly with pulmonary congestion on chest X-ray, decreased left ventricular (LV) systolic function (EF <50%), LV diastolic dysfunction suggestive of increased LV filling pressure, or cardiogenic shock. We defined metabolic syndrome using the National Cholesterol Education Program-Third Adult Treatment Panel (NCEP ATP III) criteria. The definition of central obesity was modified to a body mass index ≥25 kg/m2 because waist circumference was not measured in most patients. Other criteria included high blood pressure (≥130/85 mm Hg or taking antihypertensive medication), hyperglycemia (≥110 mg/dL or being on hypoglycemic medication or insulin therapy), hypertriglyceridemia (≥150 mg/dL or taking a lipid-lowering agent), and low high-density lipoprotein (HDL) cholesterol (<40 mg/dL in men, <50 mg/dL in women or taking a lipid-lowering agent). We classified patients as having metabolic syndrome when they met three or more of these five criteria.
Biochemical tests
For measurement of Lp-PLA2 concentration in plasma, 5 mL of fasting blood samples were drawn before coronary angiography was performed. The blood samples, collected in EDTA, were centrifuged at 1000×g for 15 minutes at -20℃ within 30 minutes of collection, and the separated samples were stored at -80℃. Plasma concentrations of Lp-PLA2, which was the mass level of Lp-PLA2, was determined using a commercially available Lp-PLA2 enzyme-linked immunosorbent assay (ELISA) kit (CUSABIO Biotech, Wuhan, China), an ELISA test with two specific monoclonal antibodies.16,17 Each sample was measured twice and the mean of the two values was calculated to minimize error. The interassay coefficient of variation was 16.11%. hs-CRP levels were measured using the latex-enhanced immunoturbidimetric method (Denka Seiken Co., Ltd., Tokyo, Japan) with a detection limit of 0.09 mg/L in serum, using a Hitachi 7600-110 automatic analyzer (Hitachi Co., Tokyo, Japan). Total cholesterol, LDL cholesterol, HDL cholesterol, and triglyceride levels in serum were measured using the standard enzymatic method (Daiichi Sankyo Co., Ltd., Tokyo, Japan for total, LDL and HDL cholesterol; Roche Diagnostics, Indianapolis, IN, USA for triglycerides) with a Hitachi 7600-110 analyzer (Hitachi Co., Tokyo, Japan).
Statistical analyses
Data are presented as a percentage or mean value±standard error. Two group comparisons were made using the Student's t-test for continuous variables or the χ2 test for categorical variables. Multiple group comparisons were performed by analysis of variance, followed by Tukey-Kramer post-hoc analysis. To assess the relation between Lp-PLA2 and serum hs-CRP levels, we conducted bivariate correlation analysis. Univariate and multivariate logistic regression analyses were performed to identify the risk factors for ACS. In addition, univariate and multivariate Cox regression analyses were used to identify diagnostic tools of ACS. To identify the incremental value of Lp-PLA2 in diagnosis of ACS, three models were designed: 1) Model I: traditional risk factors, including male gender, age, hypertension, and smoking, which were identified as meaningful diagnostic tools of ACS in our study using multivariate logistic regression analysis; 2) Model II: traditional risk factors+elevated CRP; and 3) Model III: traditional risk factors+elevated CRP+elevated Lp-PLA2 level. Receiver operating characteristic (ROC) analysis was performed to identify the optimal cut-off values for Lp-PLA2 level and hs-CRP level for diagnosis of ACS. We also constructed an ROC curve for the combination of hs-CRP and Lp-PLA2 levels to evaluate whether it showed an improved ability to diagnose ACS. Additionally, we used new statistical analysis methods, calculation of integrated discrimination improvement (IDI) and net reclassification improvement (NRI) indices, to increase the discriminative value of the new biomarker, Lp-PLA2 levels. The NRI index is calculated from the net of differences between the "upward" movement in categories for event subjects and the "downward" movement in those for non-event subjects. The IDI index is the difference in Yates discrimination slopes between the new and old models.18,19 Analyses were performed using the statistical software SPSS version 18.0 for Windows (IBM Canada Ltd., Markham, Ontario, Canada) and SAS version 9.2 (SAS Institute Inc., Cary, NC, USA). Statistical significance was assumed for values of p<0.05.
RESULTS
Baseline characteristics
The baseline characteristics and selected laboratory values of the study patients are presented in Table 1. There were significant differences among the three groups in age, history of hypertension, smoking, metabolic scores, heart failure, statin users (%), and serum HDL cholesterol levels. Mean Lp-PLA2 mass level was 15.96±10.88 IU/mL.
Table 1.
Baseline Characteristics
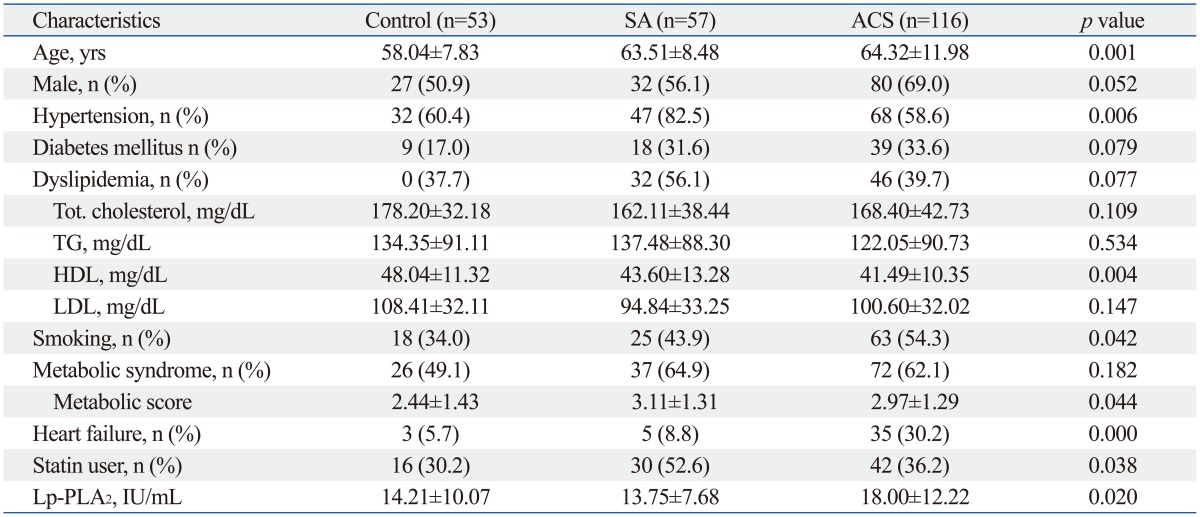
SA, stable angina; ACS, acute coronary syndrome; Lp-PLA2, lipoprotein-associated phospholipase A2.
Association with acute coronary syndrome
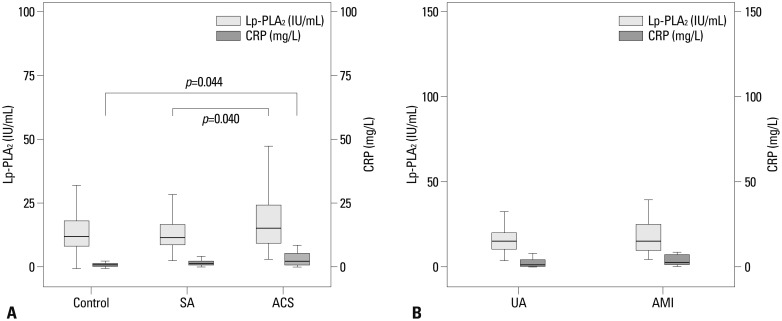
The level of Lp-PLA2 was significantly greater in the ACS group than in the SA group (18.00±12.22 IU/mL vs. 13.75±7.68 IU/mL, p=0.040). Lp-PLA2 level was also higher in the ACS group than in the control group, but this difference was not statistically significant (18.00±12.22 IU/mL vs. 14.21±10.07 IU/mL, p=0.087) (Fig. 1A). Within the ACS group, Lp-PLA2 levels tended to be higher in the AMI group (19.86±14.19 IU/mL in the AMI group vs. 16.27±9.85 IU/mL in the UA group, p=0.090) (Fig. 1B). Lp-PLA2 level was insignificantly higher in the UA group than in the SA group (16.27±9.85 IU/mL in the UA group vs. 13.75±7.68 IU/mL in the SA group, p=0.124). In addition, serum hs-CRP concentration was significantly higher in the ACS group than in the control group (7.57±18.03 IU/mL vs. 1.86±3.20 IU/mL, p=0.044) and higher in the ACS group than in the SA group, but this latter difference was not statistically significant (7.57±18.03 IU/mL vs. 2.73±5.54 IU/mL, p=0.087) (Fig. 1A). The relation between Lp-PLA2 and hs-CRP levels was not statistically significant either within the group of ACS patients (r=0.056, p=0.607) or within the population of patients as a whole (r=0.065, p=0.387).
Fig. 1.
Lp-PLA2 and hs-CRP levels in each group. (A) Levels of Lp-PLA2 and CRP in control, SA, and ACS groups. In Lp-PLA2, p=0.040, compared with SA group. In CRP, p=0.044, compared with control group. (B) Levels of Lp-PLA2 and CRP in ACS group including UA and AMI groups. Lp-PLA2, lipoprotein-associated phospholipase A2; SA, stable angina; ACS, acute coronary syndrome; hs-CRP, high-sensitivity C-reactive protein; UA, unstable; AMI, acute myocardial infarction.
Using univariate logistic regression analysis, male sex, old age, Lp-PLA2 level, hs-CRP level, smoking, and heart failure were significantly associated with ACS (Table 2). Multivariate logistic regression analysis showed that old age and Lp-PLA2 level were significantly associated with ACS. Lp-PLA2 level was associated with the prevalence of ACS with an odds ratio (OR) of 1.047 [95% confidence interval (CI): 1.010-1.086, p=0.013], and this association was statistically significant (Table 2).
Table 2.
Odds Ratio (OR) as a Biomarker for Diagnosis of ACS Using Univariate Logistic Analysis and Multivariate Logistic Analysis
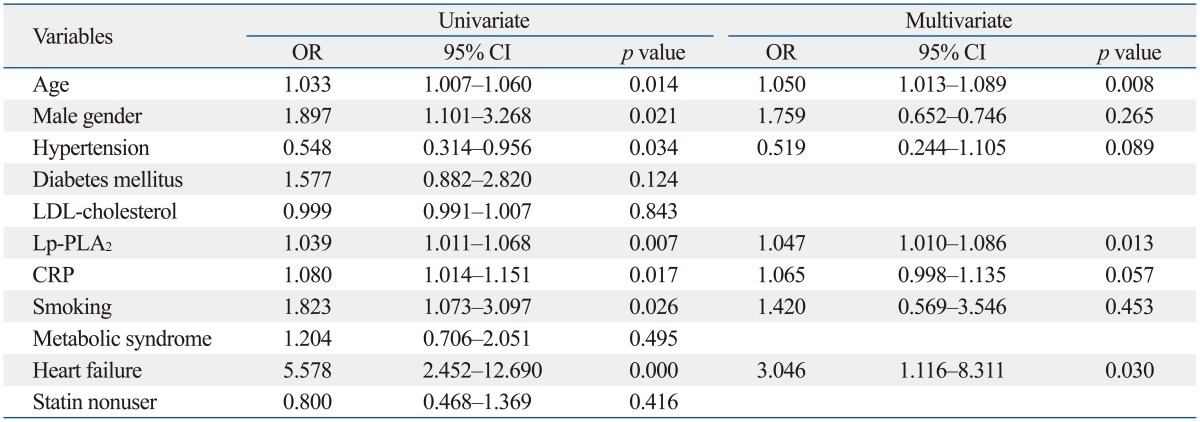
ACS, acute coronary syndrome; CI, confidence interval; CRP, C-reactive protein.
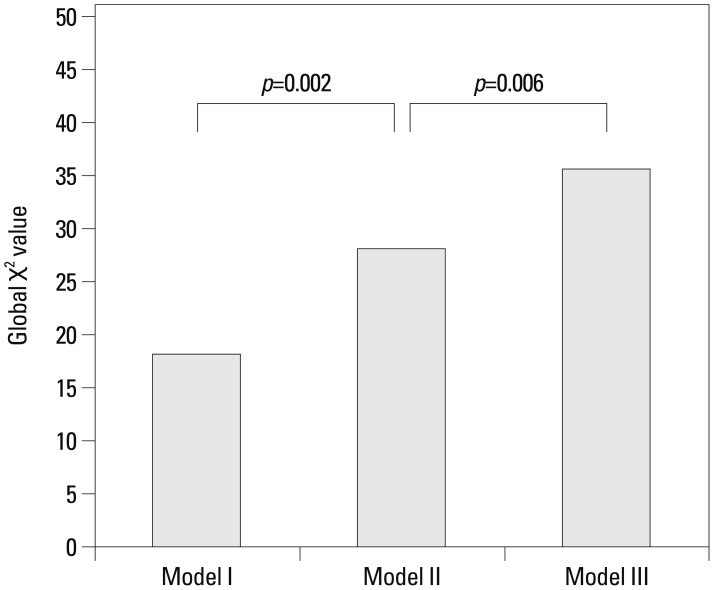
To evaluate the incremental value of Lp-PLA2 as a biomarker for diagnosis of ACS over traditional risk factors and CRP, we measured global χ2 values. Significant increases in global χ2 values were observed with elevated hs-CRP level over traditional risk factors (male sex, age, hypertension, and smoking) for diagnosis of ACS (Model I vs. Model II: 18.413 vs. 28.14, incremental global χ2 value: 9.727, p=0.002) and with elevated Lp-PLA2 level over traditional risk factors and elevated hs-CRP level (Model II vs. Model III: 28.14 vs. 35.602, incremental global χ2 value: 7.462, p=0.006) (Fig. 2).
Fig. 2.
Bar chart comparing global χ2 values with three models for diagnosis of ACS. CRP had incremental value over traditional risk factors (Model I vs. Model II: 18.413 vs. 28.14, incremental global χ2 value: 9.727, p=0.002), and Lp-PLA2 level had incremental value over traditional risk factors and CRP (Model II vs. Model III: 28.14 vs. 35.602, incremental global χ2 value: 7.462, p=0.006). ACS, acute coronary syndrome; CRP, C-reactive protein; Lp-PLA2, lipoprotein-associated phospholipase A2.
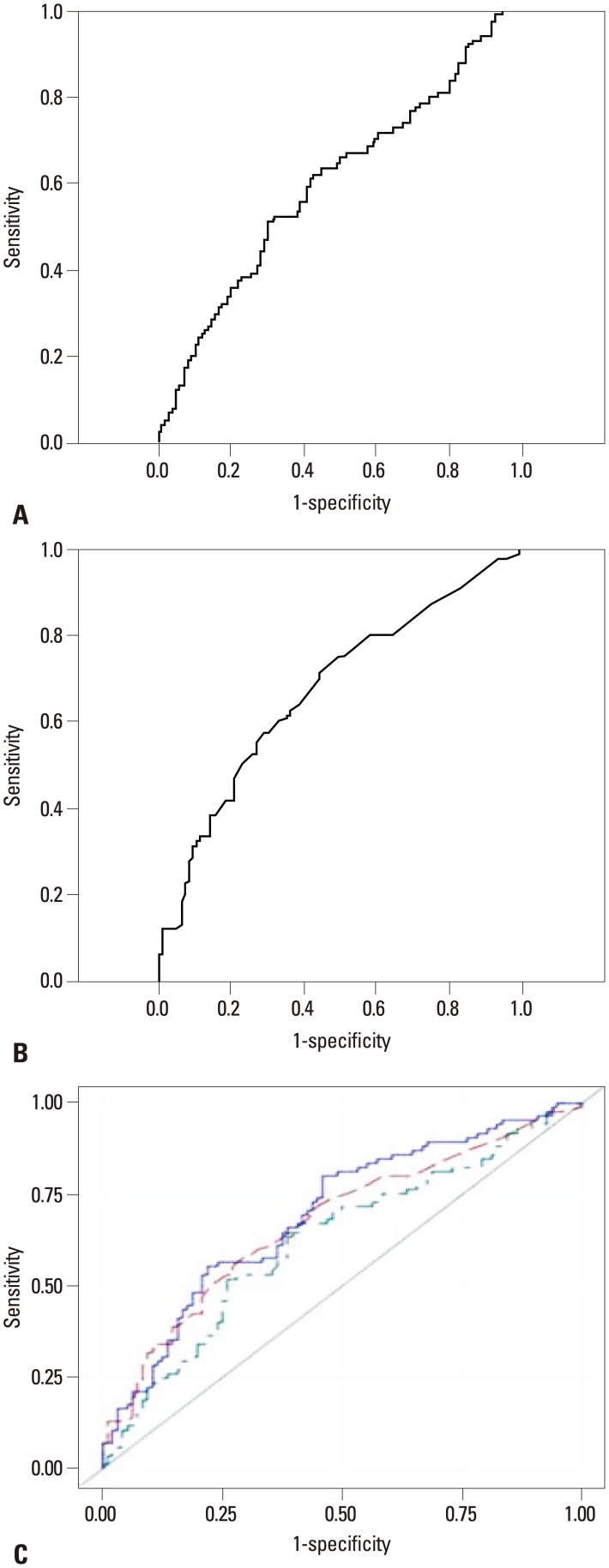
To determine the sensitivity and specificity of Lp-PLA2 levels for diagnosis of ACS, we performed ROC analysis. The area under the ROC curve (AUC) of Lp-PLA2 levels for ACS was 0.624 (95% CI: 0.542-0.706, p=0.004). ROC analysis showed that the optimal cut-off value for Lp-PLA2 level was 15.41 IU/mL, and the sensitivity and specificity for diagnosis of ACS were 51.8% and 74%, respectively. We also evaluated the optimal cut-off value of hs-CRP levels: the AUC for ACS was 0.673 (95% CI: 0.594-0.752, p=0.000), the optimal cut-off value of hs-CRP was 1.85 IU/mL, and the sensitivity and specificity for diagnosis of ACS were 57.6% and 70.8%, respectively (Fig. 3). As compared with hs-CRP level, combination of Lp-PLA2 with serum hs-CRP levels tended to increase the AUC (0.695, 95% CI: 0.618-0.772, p=0.017) for diagnosis of ACS (Fig. 3). However, when using new statistical analysis methods (NRI and IDI indices), we found that the addition of Lp-PLA2 level to hs-CRP level improved the ability to diagnose ACS. The addition of Lp-PLA2 level to hs-CRP level yielded an IDI of 0.0368% (p=0.0093, SE: 0.0142) and an NRI of 0.0854% (p=0.2046, SE: 0.0673).
Fig. 3.
Receiver operating characteristic (ROC) curves. ROC curve for Lp-PLA2. (A) Area under the ROC curve for Lp-PLA2 was 0.624 [95% confidence interval (CI): 0.542-0.706, p=0.004]. ROC curve for hs-CRP. (B) Area under the ROC curve for hs-CRP was 0.673 (95% CI: 0.594-0.752, p=0.000). (C) ROC curves for Lp-PLA2 (green line), hs-CRP (red line), and Lp-PLA2+hs-CRP (blue line). Area under the ROC curve for Lp-PLA2+hs-CRP was 0.695 (95% CI: 0.618-0.772). Lp-PLA2, lipoprotein-associated phospholipase A2; hs-CRP, high-sensitivity C-reactive protein.
Effect of statins (statin users vs. statin non-users)
We analyzed the levels of Lp-PLA2 according to statin use at the time of coronary angiography. The study patients were treated with atorvastatin, simvastatin, or rosuvastatin. Although the differences were not statistically significant, Lp-PLA2 levels tended to be lower in statin users than in statin non-users in each group (14.19±12.70 vs. 14.21±8.91, 12.43±6.87 vs. 15.22±8.38, and 15.78±10.22 vs. 19.26±13.12 IU/mL in the control, SA, and ACS groups, respectively) (p=0.996, 0.178, 0.116, respectively).
DISCUSSION
The main pathophysiological mechanism of ACS is erosion and rupture of the atherosclerotic plaque, which results in myocardial hypoperfusion.20 Lp-PLA2 is an enzyme found in atherosclerotic plaques, and it exists in the bloodstream bound to LDL.11,21,22 Numerous studies have shown that ruptured plaques contain more immune and inflammatory effector cells than do intact plaques and that Lp-PLA2 may be an essential factor in the association between lipoproteins and inflammation.23 Lp-PLA2 is abundantly expressed within the atherosclerotic plaques,9,24,25 and its enzymatic products take part in the progression of inflammation and cell death, rendering the plaque vulnerable to rupture.23,26 Therefore, the amount of plasma Lp-PLA2 reflects the plaque burden and inflammatory activity of atherosclerosis and enables the prediction of cardiovascular outcomes in patients with CHD. Herein, we measured plasma Lp-PLA2 mass levels, which have been shown to have a strong positive correlation with plasma Lp-PLA2 activity.16,27 Plasma Lp-PLA2 activity is related to plaque rupture, and it may be a marker for vulnerable plaques.28 In this study, we investigated the possible role of Lp-PLA2 levels as a diagnostic biomarker for ACS with vulnerable plaques. Our analysis of 226 participants showed that Lp-PLA2 levels are associated with risk of ACS. We found that Lp-PLA2 levels were significantly increased in the ACS group, and within this group, Lp-PLA2 levels were greater in the AMI group than in the UA group. This finding may be a result of the relation between vulnerable plaque rupture and Lp-PLA2 level.14 To exclude the effect of the myocardial injury, we compared Lp-PLA2 levels between the SA group and UA group, omitting the AMI group. Lp-PLA2 levels tended to be higher in the UA group than in the SA group, which suggest Lp-PLA2 is associated with plaque vulnerability, regardless of myocardial injury. There was no significant difference in Lp-PLA2 levels between the ACS group and the control group, which might have resulted from the small number of enrolled patients.
Achieving target LDL cholesterol concentrations according to personal cardiovascular disease risk, based on the Framingham risk criteria, is very important for the prevention of primary and secondary atherosclerotic vascular diseases, including CHD. For those with moderate risk, a target LDL cholesterol concentration of <130 mg/dL is recommended, based on the Framingham risk calculator. The ATP III 2004 guidelines, however, recommend measurement of pro-inflammatory biomarkers, specifically Lp-PLA2, as an adjustment to classical risk factor assessment. For those patients classified as having moderate risk, Lp-PLA2 should be measured to allow for reclassification and to identify those patients in relatively high-risk groups.29
CRP is a representative risk factor for atherosclerotic inflammation, and its concentrations are known to be significantly greater in patients with ACS than in those without ACS.30,31 Similarly, because the concentration of Lp-PLA2 is greater in patients with ACS, Lp-PLA2 may be a good biomarker for diagnosis of ACS, as is CRP. In this study, we used more sensitive tests to evaluate improvement in model performance and usefulness of the new biomarker.18 We evaluated the ability of Lp-PLA2 in combination with CRP to diagnose ACS by calculation of net reclassification improvement and integrated discrimination improvement indices. Although the diagnostic power was lower, as compared with serum hs-CRP concentration, the addition of Lp-PLA2 level to serum hs-CRP concentration could significantly improve the ability to diagnose ACS, as shown by IDI indices.
Traditional risk factors such as LDL cholesterol or HDL cholesterol cannot be used to directly assess plaque-rupturing tendency, but greater amounts of Lp-PLA2 signify plaque instability. Interventions to decrease Lp-PLA2 activity, such as statin administration, might be useful for stabilizing vulnerable plaques in patients with ACS; thus, intensified lipid-lowering therapy might be emphasized. Lp-PLA2 level tended to be lower in statin users than in statin non-users in each group in our study, although this result was not statistically significant. Patients with hypercholesterolemia have elevated levels of oxidized LDL (OxLDL) in the plasma. OxLDL can induce the expression of the procoagulant protein tissue factor (TF) in human monocytes and can also activate the coagulation system. Statins reduce the levels of OxLDL and thus inhibit inducible TF expression in the aorta and in atherosclerotic lesions.32 Reduced OxLDL levels and reduced TF expression lead to the inactivation of the coagulation system and should inhibit the development of a prothrombotic state. Therefore, statins can reduce inflammation and thrombogenicity in patients with hypercholesterolemia, and it is suggested that statins may lower the risk of advanced cardiovascular disease, including ACS. Indeed, more evidence of a statin effect was shown in the Myocardial Ischemia Reduction with Aggressive Cholesterol Lowering (MIRACL) trial. A substudy with analysis of the MIRACL trial cohort revealed that high-dose atorvastatin significantly decreases Lp-PLA2 mass and activity after ACS.33 Currently, Lp-PLA2 inhibition is thought to lead to reduced phospholipid oxidation and reduced expression of adhesion molecules that accelerate atherogenesis. Therefore, specific Lp-PLA2 inhibitors, such as darapladib, have been developed.8,34 Darapladib was suggested that it might produce sustained inhibition of circulating Lp-PLA2 activity in dyslipidemic patients, and be associated with reduction in plaque burden in CHD.8,35,36 Darapladib has undergone evaluation in the coronary atherosclerosis imaging trial, in which it prevented necrotic core expansion, a crucial determinant of plaque vulnerability.8 Now, phase III clinical trials, investigating the effects of darapladib on cardiovascular outcome in CHD, are ongoing.37,38,39 In our study, as mentioned above, although the finding was not statistically significant, Lp-PLA2 level tended to be lower in statin users than in statin non-users in each of the groups. Among ACS patients, there were patients with low Lp-PLA2 levels who had taken statins previously. The previous statin users had taken normal doses of various statins, and Lp-PLA2 levels were measured only at the time of coronary angiography in this study. Therefore, we could not measure Lp-PLA2 after ACS, but we could guess that statin usage would reduce Lp-PLA2 levels, as garnered from simultaneous comparisons between statin users and non-users in each group. Because some people take statins for primary prevention, assessing ACS risk solely on the basis of Lp-PLA2 would not be appropriate. Indeed, patient usage of various medications that may interfere with cholesterol production should also be considered.
The limitations of this study merit consideration. First, because this was not a prospective study, we could not compare sequential measurements of Lp-PLA2 level in the same patient. Furthermore, there may be differences in baseline Lp-PLA2 levels among individuals. Therefore, we cannot be sure that statins reduce Lp-PLA2 levels. In addition, Lp-PLA2 levels were slightly greater in the control group. Such an unexpected result might be attributable to the small number of enrolled patients in this study. Also, although coronary angiography is one of the most commonly performed procedures for illustrating luminal narrowing and arterial anatomy and is considered the gold standard, it only offers information about the vessel lumen and does not provide information on plaque components or on plaque burden within the vessel wall.40 Additionally, coronary angiography was performed because the patients in the control group might have had chest pain or a positive treadmill test. Therefore, microvascular angina or syndrome X is a possible diagnosis for those patients, even though their coronary arteries did not have significant narrowed lesions. Finally, it is known that myocardial damage or death induces inflammatory cells from the bone marrow, which could also affect Lp-PLA2 level. To discriminate which factor was related to increased Lp-PLA2 levels, identification of tissue characteristics, such as by MRI, might be needed.
In conclusion, Lp-PLA2 may be of use as a meaningful biomarker to diagnose ACS independent of hs-CRP and lipid profiles. Although Lp-PLA2 measurement is not widely used in the clinic, use of this biomarker could help tailor an individualized treatment approach to patients with ACS. Specifically, patients with higher levels of Lp-PLA2 at baseline could appear to have an increased risk of cardiovascular events. Thus, we could select, at an early stage, patients with high levels of Lp-PLA2 who might benefit from aggressive lifestyle modification and more robust risk reduction strategies, such as intensified lipid-lowering therapy, with the overall effect of reducing the morbidity and mortality of cardiovascular disease.
ACKNOWLEDGEMENTS
This study was supported by the Brain Korea 21 Project for Medical Science, Yonsei University and the Korean Institute of Medicine.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.WRITING GROUP MEMBERS. Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Hamirani YS, Pandey S, Rivera JJ, Ndumele C, Budoff MJ, Blumenthal RS, et al. Markers of inflammation and coronary artery calcification: a systematic review. Atherosclerosis. 2008;201:1–7. doi: 10.1016/j.atherosclerosis.2008.04.045. [DOI] [PubMed] [Google Scholar]
- 3.Epps KC, Wilensky RL. Lp-PLA2- a novel risk factor for high-risk coronary and carotid artery disease. J Intern Med. 2011;269:94–106. doi: 10.1111/j.1365-2796.2010.02297.x. [DOI] [PubMed] [Google Scholar]
- 4.Caslake MJ, Packard CJ, Robertson M, Cooney J, Nelson JJ, Ford I, et al. Lipoprotein-associated phospholipase A(2), inflammatory biomarkers, and risk of cardiovascular disease in the Prospective Study of Pravastatin in the Elderly at Risk (PROSPER) Atherosclerosis. 2010;210:28–34. doi: 10.1016/j.atherosclerosis.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 5.Packard CJ. Lipoprotein-associated phospholipase A2 as a biomarker of coronary heart disease and a therapeutic target. Curr Opin Cardiol. 2009;24:358–363. doi: 10.1097/HCO.0b013e32832bcb22. [DOI] [PubMed] [Google Scholar]
- 6.Ballantyne CM, Hoogeveen RC, Bang H, Coresh J, Folsom AR, Heiss G, et al. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident coronary heart disease in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Circulation. 2004;109:837–842. doi: 10.1161/01.CIR.0000116763.91992.F1. [DOI] [PubMed] [Google Scholar]
- 7.Koenig W, Khuseyinova N, Löwel H, Trischler G, Meisinger C. Lipoprotein-associated phospholipase A2 adds to risk prediction of incident coronary events by C-reactive protein in apparently healthy middle-aged men from the general population: results from the 14-year follow-up of a large cohort from southern Germany. Circulation. 2004;110:1903–1908. doi: 10.1161/01.CIR.0000143377.53389.C8. [DOI] [PubMed] [Google Scholar]
- 8.Serruys PW, García-García HM, Buszman P, Erne P, Verheye S, Aschermann M, et al. Effects of the direct lipoprotein-associated phospholipase A(2) inhibitor darapladib on human coronary atherosclerotic plaque. Circulation. 2008;118:1172–1182. doi: 10.1161/CIRCULATIONAHA.108.771899. [DOI] [PubMed] [Google Scholar]
- 9.Liu YS, Hu XB, Li HZ, Jiang WD, Wang X, Lin H, et al. Association of lipoprotein-associated phospholipase A2 with characteristics of vulnerable coronary atherosclerotic plaques. Yonsei Med J. 2011;52:914–922. doi: 10.3349/ymj.2011.52.6.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gotsman I, Stabholz A, Planer D, Pugatsch T, Lapidus L, Novikov Y, et al. Serum cytokine tumor necrosis factor-alpha and interleukin-6 associated with the severity of coronary artery disease: indicators of an active inflammatory burden? Isr Med Assoc J. 2008;10:494–498. [PubMed] [Google Scholar]
- 11.Kolodgie FD, Burke AP, Skorija KS, Ladich E, Kutys R, Makuria AT, et al. Lipoprotein-associated phospholipase A2 protein expression in the natural progression of human coronary atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:2523–2529. doi: 10.1161/01.ATV.0000244681.72738.bc. [DOI] [PubMed] [Google Scholar]
- 12.Wilensky RL, Macphee CH. Lipoprotein-associated phospholipase A(2) and atherosclerosis. Curr Opin Lipidol. 2009;20:415–420. doi: 10.1097/MOL.0b013e3283307c16. [DOI] [PubMed] [Google Scholar]
- 13.Braunwald E. Unstable angina. A classification. Circulation. 1989;80:410–414. doi: 10.1161/01.cir.80.2.410. [DOI] [PubMed] [Google Scholar]
- 14.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non ST-Elevation Myocardial Infarction): developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons: endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. Circulation. 2007;116:e148–e304. doi: 10.1161/CIRCULATIONAHA.107.181940. [DOI] [PubMed] [Google Scholar]
- 15.Hamm CW, Braunwald E. A classification of unstable angina revisited. Circulation. 2000;102:118–122. doi: 10.1161/01.cir.102.1.118. [DOI] [PubMed] [Google Scholar]
- 16.Dada N, Kim NW, Wolfert RL. Lp-PLA2: an emerging biomarker of coronary heart disease. Expert Rev Mol Diagn. 2002;2:17–22. doi: 10.1586/14737159.2.1.17. [DOI] [PubMed] [Google Scholar]
- 17.Caslake MJ, Packard CJ. Lipoprotein-associated phospholipase A2 (platelet-activating factor acetylhydrolase) and cardiovascular disease. Curr Opin Lipidol. 2003;14:347–352. doi: 10.1097/00041433-200308000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Pencina MJ, D'Agostino RB, Sr, D'Agostino RB, Jr, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 19.Cook NR, Ridker PM. Advances in measuring the effect of individual predictors of cardiovascular risk: the role of reclassification measures. Ann Intern Med. 2009;150:795–802. doi: 10.7326/0003-4819-150-11-200906020-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, et al. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: The Task Force for the management of acute coronary syndromes (ACS) in patients presenting without persistent ST-segment elevation of the European Society of Cardiology (ESC) Eur Heart J. 2011;32:2999–3054. doi: 10.1093/eurheartj/ehr236. [DOI] [PubMed] [Google Scholar]
- 21.Mannheim D, Herrmann J, Versari D, Gössl M, Meyer FB, McConnell JP, et al. Enhanced expression of Lp-PLA2 and lysophosphatidylcholine in symptomatic carotid atherosclerotic plaques. Stroke. 2008;39:1448–1455. doi: 10.1161/STROKEAHA.107.503193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stafforini DM, Tjoelker LW, McCormick SP, Vaitkus D, McIntyre TM, Gray PW, et al. Molecular basis of the interaction between plasma platelet-activating factor acetylhydrolase and low density lipoprotein. J Biol Chem. 1999;274:7018–7024. doi: 10.1074/jbc.274.11.7018. [DOI] [PubMed] [Google Scholar]
- 23.Zalewski A, Macphee C. Role of lipoprotein-associated phospholipase A2 in atherosclerosis: biology, epidemiology, and possible therapeutic target. Arterioscler Thromb Vasc Biol. 2005;25:923–931. doi: 10.1161/01.ATV.0000160551.21962.a7. [DOI] [PubMed] [Google Scholar]
- 24.Lp-PLA(2) Studies Collaboration. Thompson A, Gao P, Orfei L, Watson S, Di Angelantonio E, et al. Lipoprotein-associated phospholipase A(2) and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet. 2010;375:1536–1544. doi: 10.1016/S0140-6736(10)60319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tellis CC, Tselepis AD. The role of lipoprotein-associated phospholipase A2 in atherosclerosis may depend on its lipoprotein carrier in plasma. Biochim Biophys Acta. 2009;1791:327–338. doi: 10.1016/j.bbalip.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 26.Häkkinen T, Luoma JS, Hiltunen MO, Macphee CH, Milliner KJ, Patel L, et al. Lipoprotein-associated phospholipase A(2), platelet-activating factor acetylhydrolase, is expressed by macrophages in human and rabbit atherosclerotic lesions. Arterioscler Thromb Vasc Biol. 1999;19:2909–2917. doi: 10.1161/01.atv.19.12.2909. [DOI] [PubMed] [Google Scholar]
- 27.Tew DG, Southan C, Rice SQ, Lawrence MP, Li H, Boyd HF, et al. Purification, properties, sequencing, and cloning of a lipoprotein-associated, serine-dependent phospholipase involved in the oxidative modification of low-density lipoproteins. Arterioscler Thromb Vasc Biol. 1996;16:591–599. doi: 10.1161/01.atv.16.4.591. [DOI] [PubMed] [Google Scholar]
- 28.Liu CF, Qin L, Ren JY, Chen H, Wang WM, Liu J, et al. Elevated plasma lipoprotein-associated phospholipase A2 activity is associated with plaque rupture in patients with coronary artery disease. Chin Med J (Engl) 2011;124:2469–2473. [PubMed] [Google Scholar]
- 29.Davidson MH, Corson MA, Alberts MJ, Anderson JL, Gorelick PB, Jones PH, et al. Consensus panel recommendation for incorporating lipoprotein-associated phospholipase A2 testing into cardiovascular disease risk assessment guidelines. Am J Cardiol. 2008;101:51F–57F. doi: 10.1016/j.amjcard.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 30.Jung CH, Lee WY, Kim BY, Park SE, Rhee EJ, Park CY, et al. The risk of metabolic syndrome according to the white blood cell count in apparently healthy Korean adults. Yonsei Med J. 2013;54:615–620. doi: 10.3349/ymj.2013.54.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seo HS. The role and clinical significance of high-sensitivity C-reactive protein in cardiovascular disease. Korean Circ J. 2012;42:151–153. doi: 10.4070/kcj.2012.42.3.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Owens AP, 3rd, Passam FH, Antoniak S, Marshall SM, McDaniel AL, Rudel L, et al. Monocyte tissue factor-dependent activation of coagulation in hypercholesterolemic mice and monkeys is inhibited by simvastatin. J Clin Invest. 2012;122:558–568. doi: 10.1172/JCI58969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ryu SK, Mallat Z, Benessiano J, Tedgui A, Olsson AG, Bao W, et al. Phospholipase A2 enzymes, high-dose atorvastatin, and prediction of ischemic events after acute coronary syndromes. Circulation. 2012;125:757–766. doi: 10.1161/CIRCULATIONAHA.111.063487. [DOI] [PubMed] [Google Scholar]
- 34.Wilensky RL, Shi Y, Mohler ER, 3rd, Hamamdzic D, Burgert ME, Li J, et al. Inhibition of lipoprotein-associated phospholipase A2 reduces complex coronary atherosclerotic plaque development. Nat Med. 2008;14:1059–1066. doi: 10.1038/nm.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohler ER, 3rd, Ballantyne CM, Davidson MH, Hanefeld M, Ruilope LM, Johnson JL, et al. The effect of darapladib on plasma lipoprotein-associated phospholipase A2 activity and cardiovascular biomarkers in patients with stable coronary heart disease or coronary heart disease risk equivalent: the results of a multicenter, randomized, double-blind, placebo-controlled study. J Am Coll Cardiol. 2008;51:1632–1641. doi: 10.1016/j.jacc.2007.11.079. [DOI] [PubMed] [Google Scholar]
- 36.Daida H, Iwase T, Yagi S, Ando H, Nakajima H. Effect of darapladib on plasma lipoprotein-associated phospholipase A2 activity in Japanese dyslipidemic patients, with exploratory analysis of a PLA2G7 gene polymorphism of Val279Phe. Circ J. 2013;77:1518–1525. doi: 10.1253/circj.cj-12-0813. [DOI] [PubMed] [Google Scholar]
- 37.White H, Held C, Stewart R, Watson D, Harrington R, Budaj A, et al. Study design and rationale for the clinical outcomes of the STABILITY Trial (STabilization of Atherosclerotic plaque By Initiation of darapLadIb TherapY) comparing darapladib versus placebo in patients with coronary heart disease. Am Heart J. 2010;160:655–661. doi: 10.1016/j.ahj.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 38.Rosenson RS, Hurt-Camejo E. Phospholipase A2 enzymes and the risk of atherosclerosis. Eur Heart J. 2012;33:2899–2909. doi: 10.1093/eurheartj/ehs148. [DOI] [PubMed] [Google Scholar]
- 39.O'Donoghue ML, Braunwald E, White HD, Serruys P, Steg PG, Hochman J, et al. Study design and rationale for the Stabilization of pLaques usIng Darapladib-Thrombolysis in Myocardial Infarction (SOLID-TIMI 52) trial in patients after an acute coronary syndrome. Am Heart J. 2011;162:613–619. doi: 10.1016/j.ahj.2011.07.018. [DOI] [PubMed] [Google Scholar]
- 40.Drakopoulou M, Toutouzas K, Stefanadi E, Tsiamis E, Tousoulis D, Stefanadis C. Association of inflammatory markers with angiographic severity and extent of coronary artery disease. Atherosclerosis. 2009;206:335–339. doi: 10.1016/j.atherosclerosis.2009.01.041. [DOI] [PubMed] [Google Scholar]