Abstract
Purpose
This study investigated the possible relationship between viral infection and first trimester pregnancy loss.
Materials and Methods
A prospective study was performed on 51 gravidas with missed abortion, fetal anomaly, pre-term delivery, and full-tem delivery at Hanyang University Hospital. Enteroviruses were detected by semi-nested reverse transcription-polymerase chain reaction (RT-PCR) and immunohistochemistry in abortive tissues and placentas. Enterovirus serotypes were confirmed by genome sequencing. Herpesviruses were detected by PCR.
Results
Coxsackievirus B3 (CVB3) was detected in 8 of 14 missed abortion cases, 1 of 27 full-term cases, and none of the 9 pre-term cases. Coxsackievirus B1 (CVB1) was detected in an encephalocele case. Herpes simplex virus type 1 was found in 4 full-term cases, 3 pre-term cases, and none of the missed abortion cases.
Conclusion
The prevalence of CVB3 was significantly higher in missed abortion cases compared to full-term or pre-term delivery cases. CVB infection may therefore be an important etiological agent of missed abortion.
Keywords: Coxsackievirus, missed abortion, fetal anomaly
INTRODUCTION
Viral infections during pregnancy can cause significant effects on the fetus. For example, rubella, cytomegalovirus (CMV), and herpes simplex virus (HSV) are well-known causes of congenital malformations with high morbidity and mortality. Parvovirus B19, enteroviruses, the hepatitis B virus, and the human immunodeficiency virus are also considered as important etiologic agents causing adverse outcomes on pregnancy. Coxsackievirus B (CVB) is a positive-sense single stranded RNA virus in a member of enterovirus genus, Picornaviridae. CVB has 6 serotypes of CVB1 to 6. CVB infection during late gestation or the perinatal period leads to life-threatening diseases. These diseases include neonatal myocarditis, meningitis, hepatitis, encephalitis, long-term neurological deficits, and sudden death.1,2,3,4,5,6,7 However, clinical outcomes following CVB infection during early gestation are unclear.8,9
The etiology of miscarriage is diverse, but may include maternal age, chromosomal abnormalities, endocrine disorders, thrombophilia, infection, and environmental factors.10 More than 30% of all pregnancies in healthy women are spontaneously aborted during the early gestational period and chromosomal anomalies are present in approximately 50% of early abortion cases. However, the causes of these spontaneous abortions in many cases are still unknown.11 In this study, we evaluated whether coxsackievirus infection is related to missed abortion cases in Korea.
MATERIALS AND METHODS
This work was approved by the Hanyang University Seoul Hospital Institutional Review Board. Patient consent was obtained prior to sample collection, which was conducted based on the protocol HYUH IRB 2011-R-07. A total of 51 gravidas, ranging in age from 17-44 years, who gave birth to live infants with full-term or pre-term delivery or underwent an abortion, were included in this study. The placental specimens and abortive tissues were collected prospectively and consecutively at the time of delivery, or dilatation and curettage, from October 2010 to April 2012 at Hanyang University Hospital in Seoul, Korea. The placental samples, including part of the chorionic plate and chorionic villi, were immediately fixed for histology or frozen at -80℃ until use. Abortive tissues were stored in the same fashion as tissues from other delivery cases.
Viral genome detection
Two methods were used to detect viral genomes. Semi-nested reverse transcription-polymerase chain reaction (RT-PCR) was performed for enteroviruses with primers described in Table 1. PCR was performed for herpesviruses with primers described in Table 2. Total RNA from each tissue was obtained using an Easy Blue™ total RNA extraction kit (INtRON Biotechnology, Seoul, Korea) for the detection of enteroviruses. RT-PCR was performed using the Maxime RT-PCR Premix kit (1st PCR) (INtRON Biotechnology). RT-PCR amplification for enteroviruses were carried out using Thermo hybrid PCR express thermal cycler as follows: 45 min at 42℃, 5 min at 95℃, 40 cycles of 30 sec at 95℃, 30 sec at 55℃, and 40 sec at 72℃. Two microliters of RT-PCR product was added to PCR mixture for semi-nested amplification. The PCR cycling condition was 5 min at 95℃, 40 cycles of 30 sec at 95℃, 30 sec at 55℃, and 40 sec at 72℃ (2nd PCR).
Table 1.
Primer Sequences for Semi-Nested RT-PCR Detection of Enteroviruses
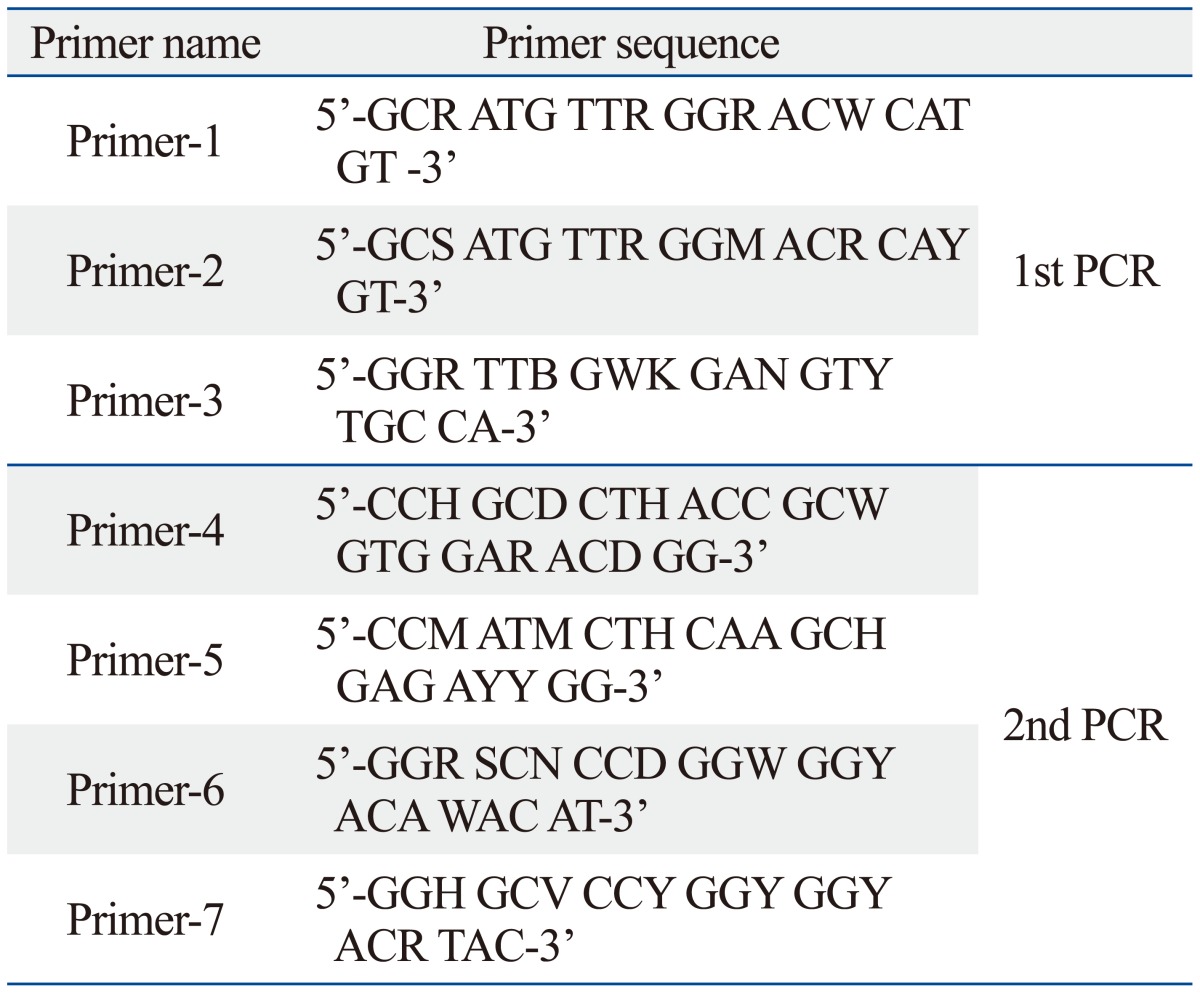
RT-PCR, reverse transcription-polymerase chain reaction.
Table 2.
Primer Sequences for PCR Detection of Herpesviruses
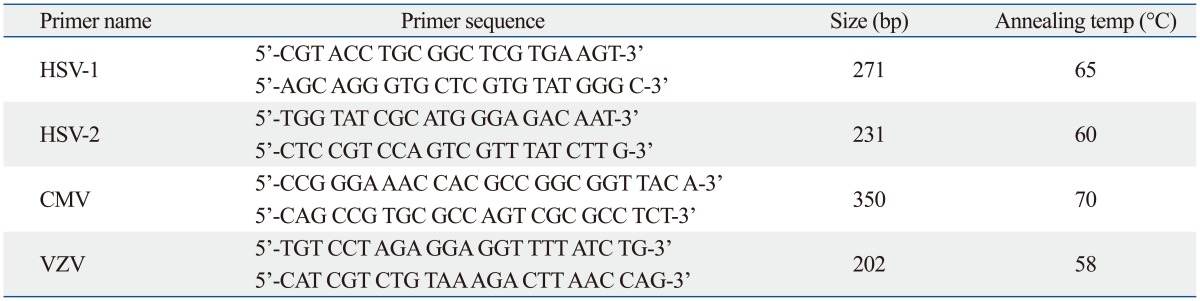
HSV, herpes simplex virus type; CMV, cytomegalovirus; VZV, varicella-zoster virus; PCR, polymerase chain reaction.
DNA was extracted using the Solg™ Genomic DNA Prep Kit (SolGent S&C, Daejeon, Korea) according to the manufacturer's instructions for the detection of herpesviruses. After amplification, aliquots of the PCR reactions were separated on a 1.2% agarose gel containing ethidium bromide. The products of semi-nested RT-PCR were then purified and sequenced through the Macrogen sequencing service (Macrogen Inc., Seoul, Korea) for determining the enterovirus serotype.
Immunohistochemistry
Tissues were fixed in 4% formalin and then embedded in paraffin. Four micrometer-thick sections were placed on a glass slide for immunohistochemistry (IHC). IHC was performed as previously described.12 Briefly, the paraffin sections were de-paraffinized and heat-induced epitope retrieval was performed at 120℃ for 10 min in citrate buffer, pH 6.0. The endogenous peroxidase activity was blocked by incubation with 3% hydrogen peroxide for 10 min, followed by washing the sections in Tris-buffered saline with Tween20. The sections were then incubated with a 1:500 dilution of mouse anti-CVB3 (Chemicon, Temecula, CA, USA) or 1:100 dilution of mouse anti-enterovirus (DAKO, Glostrup, Denmark), for 60 min at room temperature, and then treated with an EnVision detection kit (DAKO, Glostrup, Denmark) according to the manufacturer's instructions. The slides were imaged using a ScanScope® scanner (Aperio Technologies, Vista, CA, USA).
Statistical analysis
Fisher's exact test was performed to compare the incidence of viral infection between groups. SPSS software version 19.0 (SPSS Inc., Chicago, IL, USA) was used for the analysis. The significance level for the p-value was set as <0.05.
RESULTS
Sample characteristics
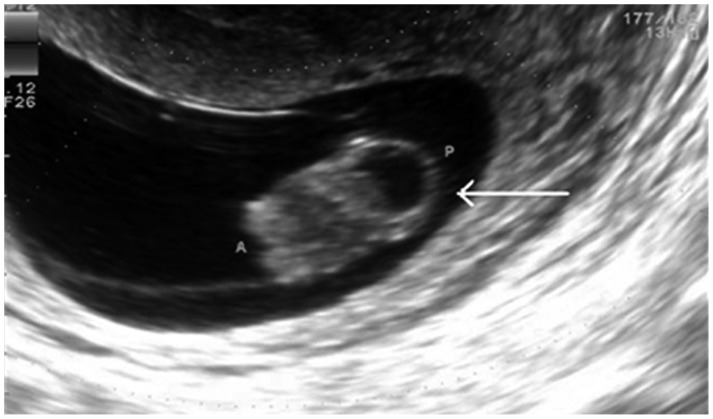
A total of 51 patients were enrolled (27 full term, 9 preterm, 14 missed abortion, and 1 fetal anomaly). Mean maternal ages were 33.1±5.2, 31±2.1, 34.4±4.4, and 34 years in full term, preterm, missed abortion, and fetal anomaly cases, respectively (Table 3). The fetal anomaly was a case of a 34-year-old gravida with an ultrasonographic diagnosis of fetal encephalocele at 14 gestational weeks. Ultrasound examination of a fetal anomaly case revealed a single female fetus, with an irregular and predominantly cystic heterogeneous mass protruding externally from the occiput, measuring 2.0×1.3 cm, and causing disfigurement of the fetal head (Fig. 1).
Table 3.
Characteristics of Enrolled Gravidas

Fig. 1.
Ultrasound examination of a malformation case revealed a single female fetus with an irregular, predominantly cystic, heterogeneous mass. The mass, measuring 2.0×1.3 cm, protruded externally from the occiput, disfiguring the head of the fetus.
Prevalence of enteroviruses
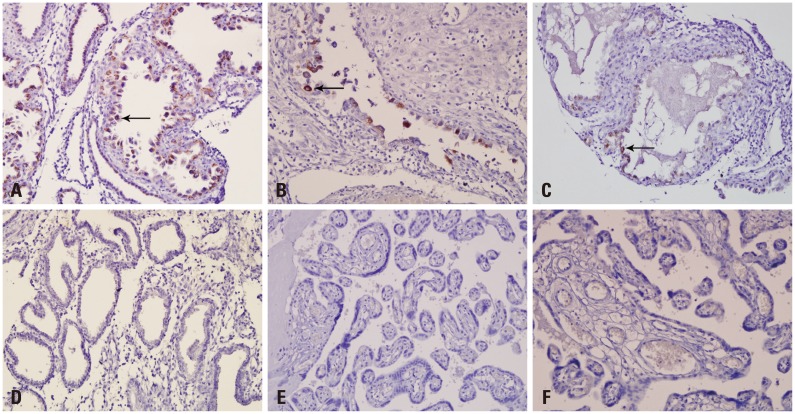
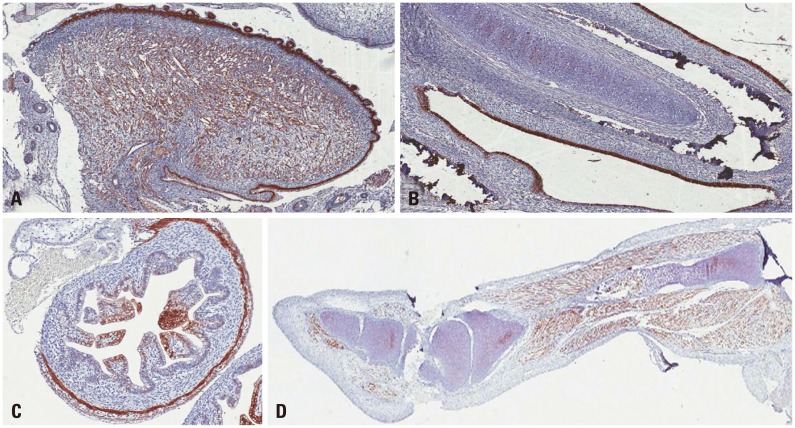
Samples which showed positive results for either genome sequencing or IHC were considered as positive for enterovirus. Sequencing was performed for enterovirus-positive samples through semi-nested RT-PCR and the serotype was confirmed. Enteroviruses were detected in 10 (19.6%) of the total samples, 1 (3.7%) full-term cases, 8 (57.1%) missed abortion cases, 1 case of malformation, and none of the pre-term cases (Table 4). By sequencing analysis, 1 full-term sample and 5 of missed abortion samples were confirmed as Coxsackievirus B3 (CVB3)-positive and the encephalocele case was confirmed positive for Coxsackievirus B1 (CVB1). Three cases of missed abortion were positive in IHC using CVB3 monoclonal antibody. The cytoplasm of endometrial glandular epithelial cells (Fig. 2A, B, and C) was strongly stained in the three missed abortive tissues. However, there was no reaction in CVB3 semi-nested RT-PCR negative missed abortive tissues (Fig. 2D). None of the full-term and pre-term cases were positive in IHC (Fig. 2E and F). A full-term delivered infant, who revealed CVB3 positive in the placenta by sequencing analysis, did not show any clinical symptoms and there were no sign of chronic villitis or other inflammation in the placenta. Anti-enterovirus antibody which reacts with enterovirus VP1 capsid protein was used for IHC of encephalocele sample. VP1 protein was highly expressed in the epithelium and muscle layer of the tongue (Fig. 3A) and respiratory epithelium (Fig. 3B), mucosal and muscle layer of the small intestine (Fig. 3C), and the leg muscle (Fig. 3D) of the fetus with encephalocele. The incidence of enterovirus infection in the missed abortion group was statistically higher than the full-term or pre-term groups (p<0.05).
Table 4.
Prevalence of Enterovirus in Placentas and Abortive Tissues by Sequencing and IHC
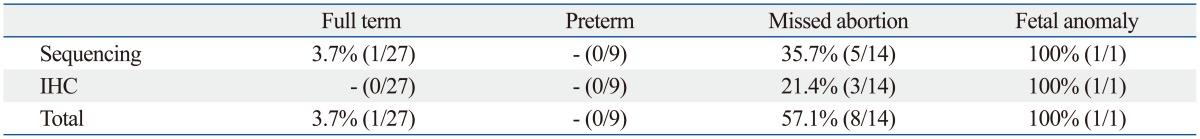
IHC, immunohistochemistry.
Fig. 2.
Immunohistochemistry for CVB3 in tissues using anti-CVB3 monoclonal antibody. The cytoplasms of endometrial glandular epithelial cells are strongly stained in the three CVB3 positive missed abortive tissues with brown color (A, B, and C). There are no specific staining in CVB3 negative missed abortive tissue (D), full-term (E), and pre-term (F) placentas. CVB3, Coxsackievirus B3.
Fig. 3.
The immunohistochemical analysis of the enteroviral VP1 capsid protein from tissues in an encephalocele case. VP1 was highly expressed in the epithelium and muscle layer of the tongue (A) and respiratory epithelium (B), mucosal and muscle layer of the small intestine (C), and leg muscles (D).
Prevalence of herpesviruses
The HSV-1 genome was found in 7 (13.7%) of the total samples, 4 (14.8%) full-term, and 3 (33.3%) pre-term and none of the missed abortion. However, the incidence of HSV-1 was not statistically different between groups. HSV-2, CMV, and VZV were not detected in any samples.
DISCUSSION
The outcomes of viral infection during pregnancy depend on the maternal immune status, type of viral infection, and timing of infection during pregnancy or delivery. There are a few case reports of CVB infection in the human fetus during early pregnancy.6,9,13 Basso, et al.13 isolated CVB2 from spontaneous abortive tissues and Axelsson, et al.9 reported that CVB infection was more frequent in women suffering from a miscarriage than women receiving a voluntary termination before 13 weeks of gestation. The frequency of IgM antibody against CVB1-5 was 42% among miscarriaged women before 13 weeks and it was significantly higher than control women (18%). Moreover, the isolation rate of CVB5 was 26%.9 In addition, Ornoy and Tenenbaum6 reported that CVB may cause spontaneous abortion. Recently, we found that the pregnancy loss rate in CVB3-infected mice during early gestation was 38.3% compared to 4.7% in mock-infected mice.14
In this study, the prevalence of enterovirus infection in cases with early pregnancy loss was evaluated by semi-nested RT-PCR and IHC. However, there were discordant results between the two methods. The reason of discordance was that only a small number of cells in the placenta were infected with CVB3 (as shown in Fig 2B and C) and the regions used for each method were not the same sites in a placenta. Therefore, if either the genome sequencing of the sample following semi-nested RT-PCR matched the specific enteroviruse or the sample was IHC positive, then the sample was regarded as positive. All of the viruses found in missed abortion cases, including the encephalocele case, were CVB and the prevalence rate of CVB3 infection in missed abortion was 57.1%.
The coxsackievirus-adenovirus receptor (CAR) is expressed in the developing heart and is abundant in the developing brain.15,16,17 Therefore, CVB has a tropism at the heart and central nervous system (CNS), especially during fetal or neonatal periods.18 CAR is a critical molecule for early embryonic cardiac development and CAR knockout mice die by 11.5 days postconception.15 In the process of CVB infection, the binding of the virus to host cells leads to the abrogation of CAR expression at the cell surface.19 Therefore, if CVB infection occurs during early pregnancy, organ formation may be hindered by inappropriate CAR adhesion functions, leading to miscarriage. In this regard, Watson, et al.20 have isolated CVB1 from the amniotic fluid of a fetus with a congenital heart anomaly. In this study, the gestational ages of CVB3-positive missed abortion cases were between 7 to 11 weeks, an active period of heart development. However, we could not confirm whether the heart anomalies existed in tissues from missed abortion cases because it was impossible to distinguish the heart from other parts of abortive tissues. One of the full-term cases was CVB3 positive in sequencing analysis. However, CVB3 IHC result was negative and there was no pathology in the placenta. Moreover, the infant was healthy without any symptom and sign of viral infection. Therefore, the viral load might be insufficient to influence the fetus or the delivery in this case. In the encephalocele case occurred at 14 weeks of gestation, CVB1 was detected. Tissues obtained from the encephalocele case did not include the brain and the heart. Therefore, we could not confirm enterovirus in the brain and the heart. Malformation of occipital encephalocele should be considered to be a Meckel syndrome. However, there was no family history and kidney anomaly in ultrasonography. Digits were not well distinguishable in ultrasonography, however, there was no comment of polydactyly in pathology report. Therefore, we concluded this encephalocele case is not related to the Meckel syndrome. Kim, et al.21 reported that the most common clinical manifestation of CVB1 infection in the neonate was meningitis in Korea during 2008-2009. Therefore, the CVB1 serotype appears to have a higher affinity for the CNS.
To rule out other viral infection during pregnancy, we tested herpesviruses as well. It was known in the past that transplacental intrauterine HSV infection is very rare; when it occurs, however, the risk of abortion, pre-term birth, growth retardation, and intrauterine death increases.22 Recently, Kapranos and Kotronias23 reported that the incidence of HSV detection was significantly high (43.2%) in cases with pregnancy loss during the first trimester compared to elective abortion (16.7%) in Greece. Additionally, HSV infection in the amniotic cavity during the mid-trimester was recognized as a risk factor for pre-term birth.24,25 Although HSV-1 was most prevalent in pre-term delivery (33%) cases in this study, there were no statistical differences between other groups due to the small number of samples. Garceau, et al.26 reported that HSV-1 was found in 62.6% of genital herpes patients and was a common cause of genital herpes in New Brunswick in Canada. Therefore, HSV-1 infection still warrants detection during pregnancy. In contrast, we could not detect HSV-2 in any groups. This study has some limitations. First, although the results showed a statistically significant occurrence of CVB3 infection in missed abortion cases, the sample size was small. Second, a cytogenetic evaluation was not performed for the encephalocele case, because chromosomal analysis was not included in the study design. Therefore, we could not know whether any chromosomal anomaly exists or not. Third, sera were not obtained for the detection of antibody or virus.
Still, to our knowledge, this is the first report linking CVB infection to abortion in Korea. Most CVB infections in adults are asymptomatic and are usually unrecognized in gravidas during the first trimester. Therefore, further large-scale epidemiological studies are necessary to elucidate the exact relevance of CVB infection with early pregnancy loss in Korea.
ACKNOWLEDGEMENTS
This work was supported by the Research Fund of Hanyang University (HY-2010-MC).
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Sauerbrei A, Glück B, Jung K, Bittrich H, Wutzler P. Congenital skin lesions caused by intrauterine infection with coxsackievirus B3. Infection. 2000;28:326–328. doi: 10.1007/s150100070029. [DOI] [PubMed] [Google Scholar]
- 2.Euscher E, Davis J, Holzman I, Nuovo GJ. Coxsackie virus infection of the placenta associated with neurodevelopmental delays in the newborn. Obstet Gynecol. 2001;98:1019–1026. doi: 10.1016/s0029-7844(01)01625-8. [DOI] [PubMed] [Google Scholar]
- 3.Bendig JW, Franklin OM, Hebden AK, Backhouse PJ, Clewley JP, Goldman AP, et al. Coxsackievirus B3 sequences in the blood of a neonate with congenital myocarditis, plus serological evidence of maternal infection. J Med Virol. 2003;70:606–609. doi: 10.1002/jmv.10437. [DOI] [PubMed] [Google Scholar]
- 4.Genen L, Nuovo GJ, Krilov L, Davis JM. Correlation of in situ detection of infectious agents in the placenta with neonatal outcome. J Pediatr. 2004;144:316–320. doi: 10.1016/j.jpeds.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 5.Cheng LL, Ng PC, Chan PK, Wong HL, Cheng FW, Tang JW. Probable intrafamilial transmission of coxsackievirus b3 with vertical transmission, severe early-onset neonatal hepatitis, and prolonged viral RNA shedding. Pediatrics. 2006;118:e929–e933. doi: 10.1542/peds.2006-0554. [DOI] [PubMed] [Google Scholar]
- 6.Ornoy A, Tenenbaum A. Pregnancy outcome following infections by coxsackie, echo, measles, mumps, hepatitis, polio and encephalitis viruses. Reprod Toxicol. 2006;21:446–457. doi: 10.1016/j.reprotox.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Konstantinidou A, Anninos H, Spanakis N, Kotsiakis X, Syridou G, Tsakris A, et al. Transplacental infection of Coxsackievirus B3 pathological findings in the fetus. J Med Virol. 2007;79:754–757. doi: 10.1002/jmv.20887. [DOI] [PubMed] [Google Scholar]
- 8.Frisk G, Diderholm H. Increased frequency of coxsackie B virus IgM in women with spontaneous abortion. J Infect. 1992;24:141–145. doi: 10.1016/0163-4453(92)92798-n. [DOI] [PubMed] [Google Scholar]
- 9.Axelsson C, Bondestam K, Frisk G, Bergström S, Diderholm H. Coxsackie B virus infections in women with miscarriage. J Med Virol. 1993;39:282–285. doi: 10.1002/jmv.1890390405. [DOI] [PubMed] [Google Scholar]
- 10.Brown S. Miscarriage and its associations. Semin Reprod Med. 2008;26:391–400. doi: 10.1055/s-0028-1087105. [DOI] [PubMed] [Google Scholar]
- 11.Wilcox AJ, Weinberg CR, O'Connor JF, Baird DD, Schlatterer JP, Canfield RE, et al. Incidence of early loss of pregnancy. N Engl J Med. 1988;319:189–194. doi: 10.1056/NEJM198807283190401. [DOI] [PubMed] [Google Scholar]
- 12.Seok SH, Kang SH, Lee SJ, Hwang TY, Bae YK. Reduced expression of claudin-7 correlates with invasiveness and nuclear grade of breast carcinomas. Korean J Pathol. 2007;41:158–164. [Google Scholar]
- 13.Basso NG, Fonseca ME, Garcia AG, Zuardi JA, Silva MR, Outani H. Enterovirus isolation from foetal and placental tissues. Acta Virol. 1990;34:49–57. [PubMed] [Google Scholar]
- 14.Hwang JY, Lee KM, Kim YH, Shim HM, Bae YK, Hwang JH, et al. Pregnancy loss following coxsackievirus b3 infection in mice during early gestation due to high expression of coxsackievirus-adenovirus receptor (CAR) in uterus and embryo. Exp Anim. 2014;63:63–72. doi: 10.1538/expanim.63.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asher DR, Cerny AM, Weiler SR, Horner JW, Keeler ML, Neptune MA, et al. Coxsackievirus and adenovirus receptor is essential for cardiomyocyte development. Genesis. 2005;42:77–85. doi: 10.1002/gene.20127. [DOI] [PubMed] [Google Scholar]
- 16.Dorner AA, Wegmann F, Butz S, Wolburg-Buchholz K, Wolburg H, Mack A, et al. Coxsackievirus-adenovirus receptor (CAR) is essential for early embryonic cardiac development. J Cell Sci. 2005;118(Pt 15):3509–3521. doi: 10.1242/jcs.02476. [DOI] [PubMed] [Google Scholar]
- 17.Honda T, Saitoh H, Masuko M, Katagiri-Abe T, Tominaga K, Kozakai I, et al. The coxsackievirus-adenovirus receptor protein as a cell adhesion molecule in the developing mouse brain. Brain Res Mol Brain Res. 2000;77:19–28. doi: 10.1016/s0169-328x(00)00036-x. [DOI] [PubMed] [Google Scholar]
- 18.Kamei S, Hersch SM, Kurata T, Takei Y. Coxsackie B antigen in the central nervous system of a patient with fatal acute encephalitis: immunohistochemical studies of formalin-fixed paraffin-embedded tissue. Acta Neuropathol. 1990;80:216–221. doi: 10.1007/BF00308928. [DOI] [PubMed] [Google Scholar]
- 19.Chung SK, Kim JY, Kim IB, Park SI, Paek KH, Nam JH. Internalization and trafficking mechanisms of coxsackievirus B3 in HeLa cells. Virology. 2005;333:31–40. doi: 10.1016/j.virol.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 20.Watson WJ, Awadallah S, Jaqua MJ. Intrauterine infection with coxsackievirus: is it a cause of congenital cardiac malformations? Infect Dis Obstet Gynecol. 1995;3:79–81. doi: 10.1155/S1064744995000366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim H, Kang B, Hwang S, Hong J, Chung J, Kim S, et al. Molecular characteristics of human coxsackievirus B1 infection in Korea, 2008-2009. J Med Virol. 2013;85:110–115. doi: 10.1002/jmv.23359. [DOI] [PubMed] [Google Scholar]
- 22.Avgil M, Ornoy A. Herpes simplex virus and Epstein-Barr virus infections in pregnancy: consequences of neonatal or intrauterine infection. Reprod Toxicol. 2006;21:436–445. doi: 10.1016/j.reprotox.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 23.Kapranos NC, Kotronias DC. Detection of herpes simplex virus in first trimester pregnancy loss using molecular techniques. In Vivo. 2009;23:839–842. [PubMed] [Google Scholar]
- 24.Gervasi MT, Romero R, Bracalente G, Chaiworapongsa T, Erez O, Dong Z, et al. Viral invasion of the amniotic cavity (VIAC) in the midtrimester of pregnancy. J Matern Fetal Neonatal Med. 2012;25:2002–2013. doi: 10.3109/14767058.2012.683899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silasi M. Viral invasion of the amniotic cavity (VIAC) in the midtrimester of pregnancy. Am J Reprod Immunol. 2013;69:195–196. doi: 10.1111/aji.12073. [DOI] [PubMed] [Google Scholar]
- 26.Garceau R, Leblanc D, Thibault L, Girouard G, Mallet M. Herpes simplex virus type 1 is the leading cause of genital herpes in New Brunswick. Can J Infect Dis Med Microbiol. 2012;23:15–18. doi: 10.1155/2012/856759. [DOI] [PMC free article] [PubMed] [Google Scholar]