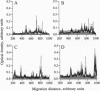
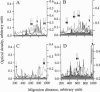
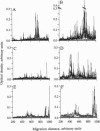
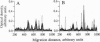Abstract
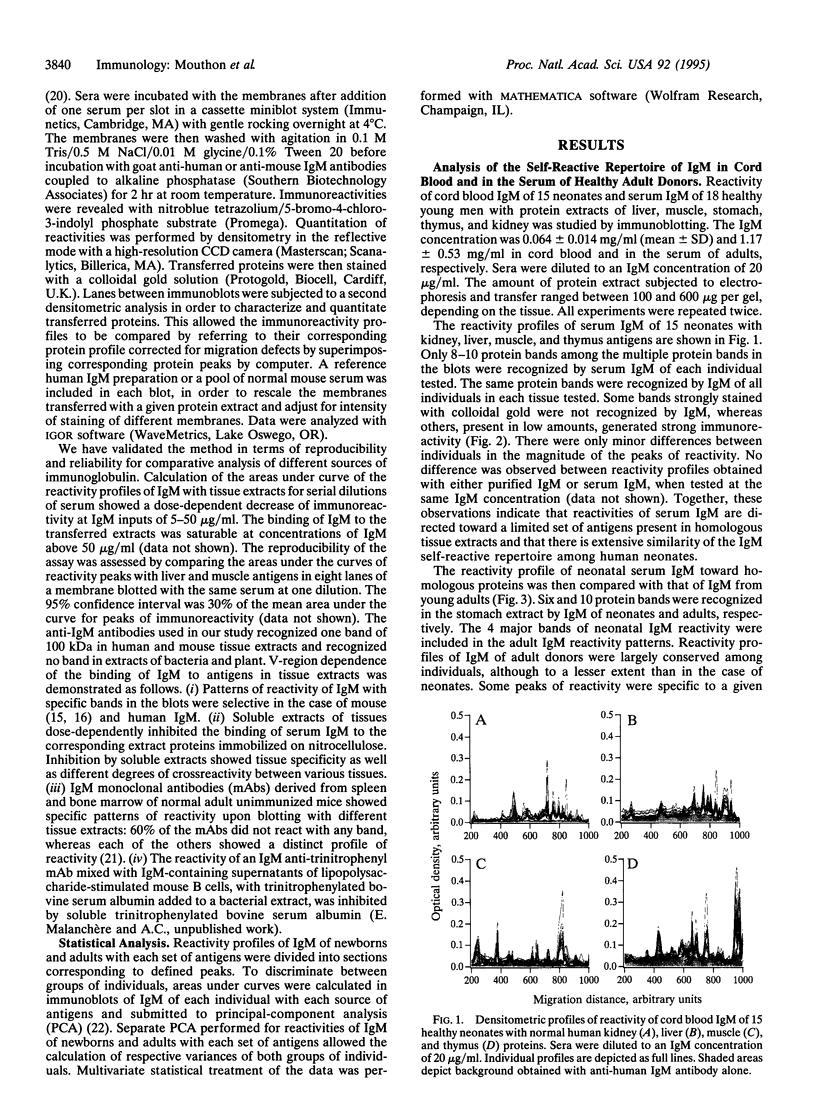
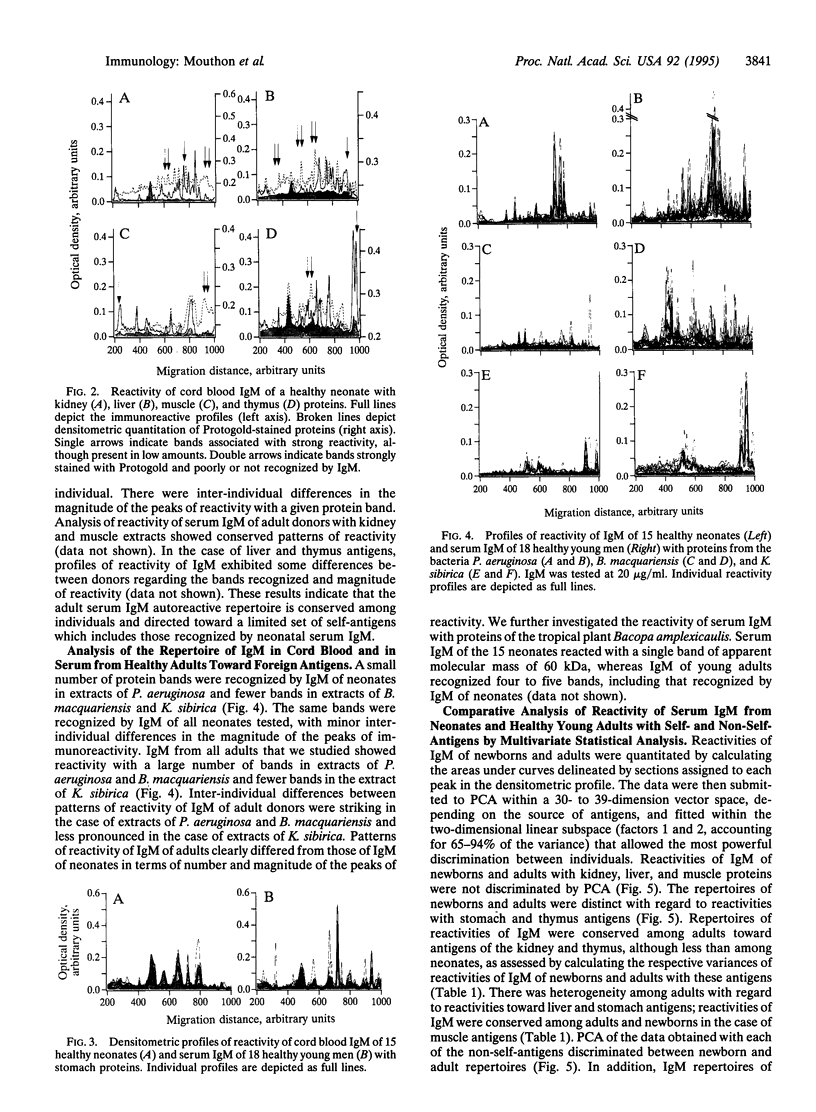
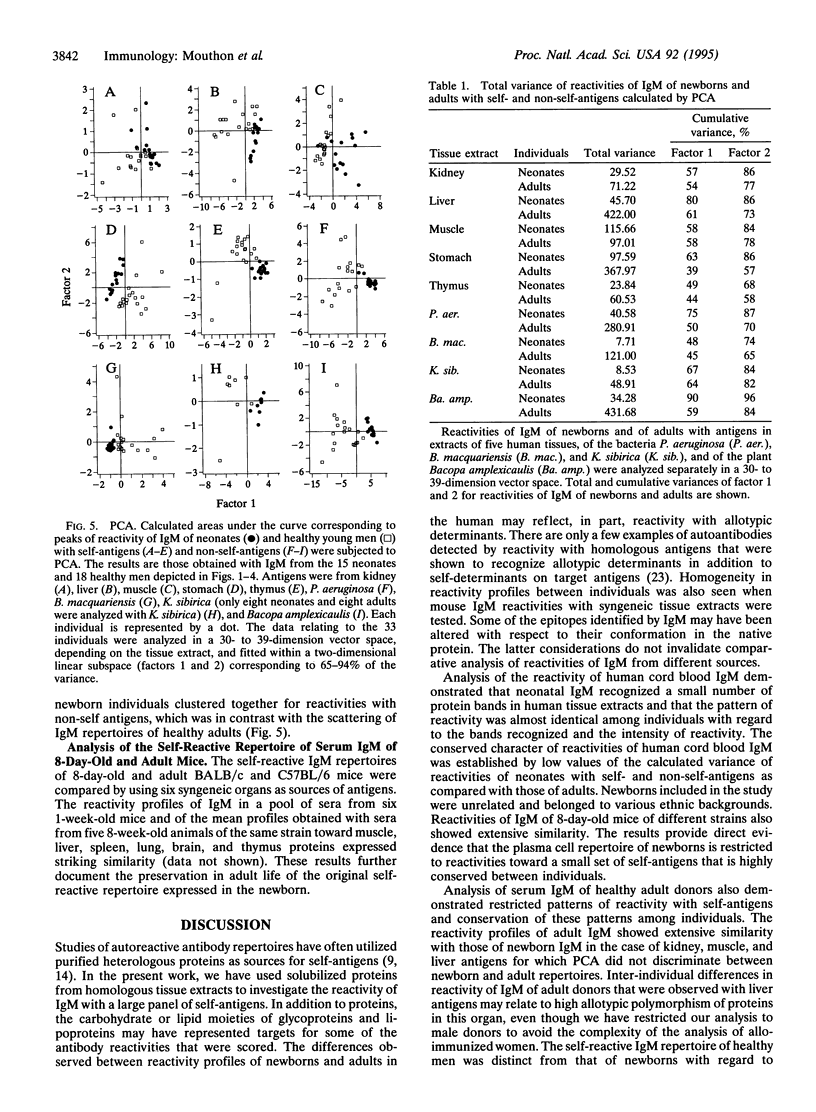
Analysis of the reactivity of IgM with self-antigens in tissues by a quantitative immunoblotting technique showed striking invariance among newborns in the human and in the mouse. The self-reactive repertoire of IgM of adults was also markedly conserved; it comprised most anti-self reactivities that prevailed among neonates. Multivariate analysis confirmed the homogeneity of IgM repertoires of neonates toward self- and non-self-antigens. Multivariate analysis discriminated between newborn and adult repertoires for reactivity with two of five sources of self-proteins and with non-self-antigens. Our observations support the concept that naturally activated B lymphocytes are selected early in development and throughout life for reactivity with a restricted set of self-antigens.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Algiman M., Dietrich G., Nydegger U. E., Boieldieu D., Sultan Y., Kazatchkine M. D. Natural antibodies to factor VIII (anti-hemophilic factor) in healthy individuals. Proc Natl Acad Sci U S A. 1992 May 1;89(9):3795–3799. doi: 10.1073/pnas.89.9.3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avrameas S. Natural autoantibodies: from 'horror autotoxicus' to 'gnothi seauton'. Immunol Today. 1991 May;12(5):154–159. doi: 10.1016/S0167-5699(05)80045-3. [DOI] [PubMed] [Google Scholar]
- Batteiger B., Newhall W. J., 5th, Jones R. B. The use of Tween 20 as a blocking agent in the immunological detection of proteins transferred to nitrocellulose membranes. J Immunol Methods. 1982 Dec 30;55(3):297–307. doi: 10.1016/0022-1759(82)90089-8. [DOI] [PubMed] [Google Scholar]
- Belikova V. A., Cherevach N. V., Kalakutskii L. V. Novyi vid bakterii roda Kurthia--Kurthia sibirica sp. nov. Mikrobiologiia. 1986 Sep-Oct;55(5):831–835. [PubMed] [Google Scholar]
- Bosma M. J., Carroll A. M. The SCID mouse mutant: definition, characterization, and potential uses. Annu Rev Immunol. 1991;9:323–350. doi: 10.1146/annurev.iy.09.040191.001543. [DOI] [PubMed] [Google Scholar]
- Cohen I. R. The cognitive paradigm and the immunological homunculus. Immunol Today. 1992 Dec;13(12):490–494. doi: 10.1016/0167-5699(92)90024-2. [DOI] [PubMed] [Google Scholar]
- Dighiero G., Lymberi P., Holmberg D., Lundquist I., Coutinho A., Avrameas S. High frequency of natural autoantibodies in normal newborn mice. J Immunol. 1985 Feb;134(2):765–771. [PubMed] [Google Scholar]
- Feeney A. J. Lack of N regions in fetal and neonatal mouse immunoglobulin V-D-J junctional sequences. J Exp Med. 1990 Nov 1;172(5):1377–1390. doi: 10.1084/jem.172.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay D., Saunders T., Camper S., Weigert M. Receptor editing: an approach by autoreactive B cells to escape tolerance. J Exp Med. 1993 Apr 1;177(4):999–1008. doi: 10.1084/jem.177.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandien A., Fucs R., Nobrega A., Andersson J., Coutinho A. Negative selection of multireactive B cell clones in normal adult mice. Eur J Immunol. 1994 Jun;24(6):1345–1352. doi: 10.1002/eji.1830240616. [DOI] [PubMed] [Google Scholar]
- Grandien A., Modigliani Y., Freitas A., Andersson J., Coutinho A. Positive and negative selection of antibody repertoires during B-cell differentiation. Immunol Rev. 1994 Feb;137:53–89. doi: 10.1111/j.1600-065x.1994.tb00659.x. [DOI] [PubMed] [Google Scholar]
- Guigou V., Guilbert B., Moinier D., Tonnelle C., Boubli L., Avrameas S., Fougereau M., Fumoux F. Ig repertoire of human polyspecific antibodies and B cell ontogeny. J Immunol. 1991 Feb 15;146(4):1368–1374. [PubMed] [Google Scholar]
- Haury M., Grandien A., Sundblad A., Coutinho A., Nobrega A. Global analysis of antibody repertoires. 1. An immunoblot method for the quantitative screening of a large number of reactivities. Scand J Immunol. 1994 Jan;39(1):79–87. doi: 10.1111/j.1365-3083.1994.tb03343.x. [DOI] [PubMed] [Google Scholar]
- Hillson J. L., Oppliger I. R., Sasso E. H., Milner E. C., Wener M. H. Emerging human B cell repertoire. Influence of developmental stage and interindividual variation. J Immunol. 1992 Dec 1;149(11):3741–3752. [PubMed] [Google Scholar]
- Huetz F., Carlsson L., Tornberg U. C., Holmberg D. V-region directed selection in differentiating B lymphocytes. EMBO J. 1993 May;12(5):1819–1826. doi: 10.1002/j.1460-2075.1993.tb05830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurez V., Kaveri S. V., Kazatchkine M. D. Expression and control of the natural autoreactive IgG repertoire in normal human serum. Eur J Immunol. 1993 Apr;23(4):783–789. doi: 10.1002/eji.1830230402. [DOI] [PubMed] [Google Scholar]
- Jakobovits A., Vergara G. J., Kennedy J. L., Hales J. F., McGuinness R. P., Casentini-Borocz D. E., Brenner D. G., Otten G. R. Analysis of homozygous mutant chimeric mice: deletion of the immunoglobulin heavy-chain joining region blocks B-cell development and antibody production. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2551–2555. doi: 10.1073/pnas.90.6.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong H. D., Teale J. M. Comparison of the fetal and adult functional B cell repertoires by analysis of VH gene family expression. J Exp Med. 1988 Aug 1;168(2):589–603. doi: 10.1084/jem.168.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura D., Roes J., Kühn R., Rajewsky K. A B cell-deficient mouse by targeted disruption of the membrane exon of the immunoglobulin mu chain gene. Nature. 1991 Apr 4;350(6317):423–426. doi: 10.1038/350423a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Marshall B. J., Ohye D. F. Bacillus macquariensis n.sp., a psychrotrophic bacterium from sub-antarctic soil. J Gen Microbiol. 1966 Jul;44(1):41–46. doi: 10.1099/00221287-44-1-41. [DOI] [PubMed] [Google Scholar]
- Nobrega A., Haury M., Grandien A., Malanchère E., Sundblad A., Coutinho A. Global analysis of antibody repertoires. II. Evidence for specificity, self-selection and the immunological "homunculus" of antibodies in normal serum. Eur J Immunol. 1993 Nov;23(11):2851–2859. doi: 10.1002/eji.1830231119. [DOI] [PubMed] [Google Scholar]
- Pascual V., Verkruyse L., Casey M. L., Capra J. D. Analysis of Ig H chain gene segment utilization in human fetal liver. Revisiting the "proximal utilization hypothesis". J Immunol. 1993 Oct 15;151(8):4164–4172. [PubMed] [Google Scholar]
- Perlmutter R. M., Kearney J. F., Chang S. P., Hood L. E. Developmentally controlled expression of immunoglobulin VH genes. Science. 1985 Mar 29;227(4694):1597–1601. doi: 10.1126/science.3975629. [DOI] [PubMed] [Google Scholar]
- Portnoï D., Freitas A., Holmberg D., Bandeira A., Coutinho A. Immunocompetent autoreactive B lymphocytes are activated cycling cells in normal mice. J Exp Med. 1986 Jul 1;164(1):25–35. doi: 10.1084/jem.164.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raaphorst F. M., Timmers E., Kenter M. J., Van Tol M. J., Vossen J. M., Schuurman R. K. Restricted utilization of germ-line VH3 genes and short diverse third complementarity-determining regions (CDR3) in human fetal B lymphocyte immunoglobulin heavy chain rearrangements. Eur J Immunol. 1992 Jan;22(1):247–251. doi: 10.1002/eji.1830220136. [DOI] [PubMed] [Google Scholar]
- Schroeder H. W., Jr, Wang J. Y. Preferential utilization of conserved immunoglobulin heavy chain variable gene segments during human fetal life. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6146–6150. doi: 10.1073/pnas.87.16.6146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. S., Stollar B. D. Heavy-chain directed B-cell maturation: continuous clonal selection beginning at the pre-B cell stage. Immunol Today. 1994 Jan;15(1):27–32. doi: 10.1016/0167-5699(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Tiegs S. L., Russell D. M., Nemazee D. Receptor editing in self-reactive bone marrow B cells. J Exp Med. 1993 Apr 1;177(4):1009–1020. doi: 10.1084/jem.177.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakil M., Kearney J. F. Functional characterization of monoclonal auto-anti-idiotype antibodies isolated from the early B cell repertoire of BALB/c mice. Eur J Immunol. 1986 Sep;16(9):1151–1158. doi: 10.1002/eji.1830160920. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Desiderio S. V., Paskind M., Kearney J. F., Baltimore D., Alt F. W. Preferential utilization of the most JH-proximal VH gene segments in pre-B-cell lines. Nature. 1984 Oct 25;311(5988):727–733. doi: 10.1038/311727a0. [DOI] [PubMed] [Google Scholar]
- Yancopoulos G. D., Malynn B. A., Alt F. W. Developmentally regulated and strain-specific expression of murine VH gene families. J Exp Med. 1988 Jul 1;168(1):417–435. doi: 10.1084/jem.168.1.417. [DOI] [PMC free article] [PubMed] [Google Scholar]