Abstract
Purpose
Transient receptor potential vanilloid 1 (TRPV1) is a ligand-gated nonselective cation channel, which can be activated by capsaicin and other noxious stimuli. Recently, an association between bone pain and TRPV1 has been reported. However, the influence of osteoporosis on TRPV1 in the sensory system innervating the femur has not been reported.
Materials and Methods
TRPV1-immunoreactive (ir) in dorsal root ganglia (DRG) neurons labeled with neurotracer [Fluoro-Gold (FG)] innervating the femurs of Sprague Dawley rats were examined in control, sham, and ovariectomized (OVX) rats. We evaluated osteoporosis in the femurs and compared the proportion of TRPV1-ir DRG neurons innervating femur between the 3 groups of rats.
Results
OVX rats showed osteoporotic cancellous bone in the femur. FG labeled neurons were distributed from L1 to L6 DRG, but there was no significant difference in the proportion of labeled neurons between the 3 groups (p>0.05). The proportions of FG labeled TRPV1-ir DRG neurons were 1.7%, 1.7%, and 2.8% of DRG neurons innervating the femur, in control, sham-operated, and OVX rats, respectively. The proportion of TRPV1-ir neurons in DRG innervating the femur in OVX rats was significantly higher than that in control and sham-operated rats (p<0.05).
Conclusion
Under physiological conditions, DRG neurons innervating femurs in rats contain TRPV1. Osteoporosis increases the numbers of TRPV1-ir neurons in DRG innervating osteoporotic femurs in rats. These findings suggest that TRPV1 may have a role in sensory perception of osteoporotic femurs.
Keywords: Osteoporosis, pain, transient receptor potential vanilloid 1, femur
INTRODUCTION
Although skeletal pain plays a major role in reducing the quality of life for patients suffering from osteoporosis, osteoarthritis, Paget's disease, sickle cell anemia, and bone cancer, little is known about the mechanisms that generate and maintain this pain. Elderly patients with thoracic and lumbar vertebral fractures caused by osteoporosis often experience back pain. On the other hand, Paget's disease of bone is a common disease characterized by focal areas of increased and disorganized bone turnover.1 Some patients are asymptomatic, whereas others develop complications such as pain.1 In this regard, high turnover of mineralized bone in such patients has the possibility to induce bone pain. However, whether osteoporosis causes pain in the absence of acute or chronic fractures is unknown.
Leg bones including the femur or tibia from animals have often been used to explore the mechanism of pain from bone.2,3,4,5,6,7 The mechanism of pain from normal bone, aging bone, inflammation, fracture, and bone cancer has been explored.2,3,4,5,6 For the normal mouse femur, the sensory and sympathetic innervation of mineralized bone, bone marrow, and periosteum have been reported.2 Thinly myelinated and unmyelinated peptidergic sensory fibers from nociceptive neurons are observed in the femur.2 Nerve growth factor-dependent sensory and sympathetic nerve fibers undergo profuse sprouting and form neuroma-like structures as tumor growth progresses within bone during bone cancer.3 This pathological remodeling of the peripheral nervous system participates in driving cancer pain.3 During aging, bone quality shows a significant age-related decline, but the density of peptide containing sensory nerve fibers that innervate the bone remain remarkably unchanged.6 Thus, while bone mass, quality, and strength undergo a significant decline with age, the density of nociceptive sensory nerve fibers remains largely intact.6
Transient receptor potential vanilloid 1 (TRPV1) is a ligand-gated nonselective cation channel, which can be activated by capsaicin and other stimuli such as noxious heat and low pH.7 TRPV1s have attracted attention in acute inflammatory nociception research, and their role in chronic inflammatory pain has begun to attract more interest.8,9 A recent study showed that TRPV1s are involved in bone pain.10 A TRPV1 antagonist significantly reduced pain and improved weight-bearing in a murine model of bone pain.11 Examination of TRPV1 expression is therefore warranted in investigations of nociceptive innervation. Whether osteoporosis influences TRPV1s in the sensory system innervating bone has not been reported.
The purpose of the current study was to investigate TRPV1-immunoreactive (ir) Fluoro-Gold (FG)-labeled dorsal root ganglion (DRG) neurons innervating the femur of control, sham, and ovariectomized (OVX) Sprague Dawley (SD) rats with osteoporosis.
MATERIALS AND METHODS
Osteoporosis model
All rats were housed in a vivarium at 21℃ on a 12 h light/dark cycle (08:30-20:30) and allowed access to food pellets and to tap water ad libitum. Experiments were performed with permission of the ethics committee of the Graduate School of Medicine, Chiba University, following the National Institutes of Health guidelines for the care and use of laboratory animals (1996 revision). As a model of osteoporosis, female SD rats weighing from 300 to 500 g at 35 weeks of age, that had been OVX at 5 weeks, were prepared (OVX group; CLEA, Tokyo, Japan) (n=10). The other 2 groups were a sham surgery group that had only their ovary exposed, and a control group with no ovarian surgery (n=10, each). We confirmed an osteoporotic state by hematoxylin and eosin (HE) staining of the right femur. HE staining of lumbar specimens was performed in an orthodox manner.
Retrograde Fluoro-Gold labeling for detection of DRG neurons innervating the left femur
Thirty female SD rats were used in this study. Rats were anesthetized with sodium pentobarbital (40 mg/kg, i.p.) and treated aseptically throughout the experiments. For each rat, a longitudinal incision 1 cm long and 5 mm depth was made using a surgical knife, and the left femur was exposed under a microscope. The neurotracer FG (Fluorochrome, Denver, CO, USA) was applied to the inside of left femurs (mineralized bone) in all 30 rats to label DRG neurons innervating the left femur. The incision was immediately sealed with cyanoacrylate adhesive to prevent leakage of FG, and the skin was closed.
Immunohistochemistry for TRPV1
Fourteen days after the surgery, rats were anesthetized with sodium pentobarbital (40 mg/kg, i.p.) and transcardially perfused with 0.9% saline, followed by 500 mL of fixative solution comprising 4% paraformaldehyde in phosphate buffer (0.1 M, pH 7.4). Left DRG from L1 to L6 were resected and the specimens immersed in the same fixative solution overnight at 4℃. After storing in 0.01 M phosphate buffered saline (PBS) containing 20% sucrose for 20 hours at 4℃, each ganglion was sectioned at 10 µm thickness on a cryostat, and mounted on poly-L-lysine-coated slides. Endogenous tissue peroxidase activity was quenched by soaking the sections in 0.3% hydrogen peroxide solution in 0.01M PBS for 30 minutes. The specimens were then treated for 90 minutes at room temperature in blocking solution consisting of 0.01 M PBS containing 0.3% Triton X-100 and 3% skim milk. To stain TRPV1, the DRG sections were processed for TRPV1 immunohistochemistry using a rabbit antibody to TRPV1 (1:500; Calbiochem, San Diego, CA, USA) diluted in blocking solution, and incubated for 20 hours at 4℃. Sections were incubated with goat anti-rabbit Alexa 488 fluorescent antibody conjugate for visualization (1:400; Molecular Probes, Eugene, OR, USA). After each step, the sections were rinsed three times in 0.01 M PBS, and were examined using a fluorescence microscope. The total number of DRG neurons, FG-labeled neurons, and FG-labeled and TRPV1-ir neurons were counted. The number of neurons per 0.45 mm2 was counted at 400× magnification using a counting grid.
Statistical analysis
The proportions of FG-labeled and FG-labeled TRPV1-ir neurons of total neurons in each DRG were compared using Welch's unpaired t test. The distribution of the FG-labeled DRG neurons and FG-labeled TRPV1-ir DRG neurons was analyzed using a nonrepeated measures ANOVA with Bonferroni post hoc correction. p<0.05 was considered statistically significant. Results are reported as means±SEMs.
RESULTS
Osteoporotic femur
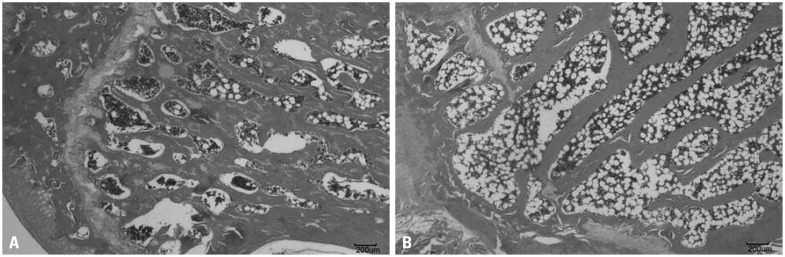
HE staining of the OVX femur showed thinner bone cortex and more widely separated bone trabeculae with decreased density compared with those in the control and sham groups (Fig. 1).
Fig. 1.
HE staining of control and OVX femurs. OVX femur samples showed thinner cortex and more widely separated bone trabeculae with decreased density compared with samples from the control group. (A) Control. (B) OVX. HE, hematoxylin and eosin; OVX, ovariectomized.
FG-labeled DRG neurons innervating the left femur
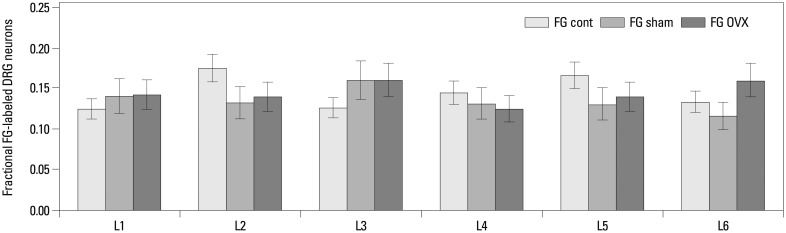
FG-labeled DRG neurons, indicating where FG was transported from the femur, were present in DRG from L1 through L6 (Figs. 2 and 3). There was no significant difference in the proportion of FG-labeled neurons in the DRG from L1 to L6 among the three groups (Fig. 3).
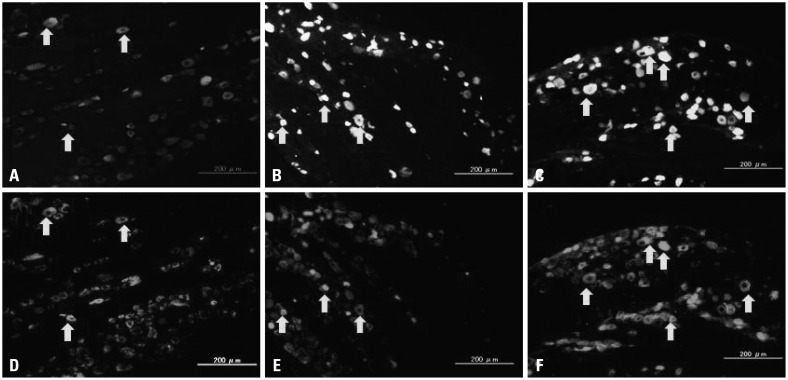
Fig. 2.
Innervation of the left femur. FG-labeled (A, B, and C) and TRPV1 immunoreactive (ir) DRG neurons (D, E, and F) [A and D (control), B and E (sham), and C and F (OVX) are same sections. Arrows indicate double labeled neurons]. FG, Fluoro-Gold; TRPV1, transient receptor potential vanilloid 1; DRG, dorsal root ganglia; OVX, ovariectomized.
Fig. 3.
Distribution and proportion of FG-labeled DRG neurons innervating the left femur. FG-labeled DRG neurons were present in DRG from L1 through L6, and there was no significant difference in the proportion of FG-labeled neurons between the 3 groups. FG, Fluoro-Gold; DRG, dorsal root ganglia; OVX, ovariectomized.
FG-labeled and TRPV1-ir DRG neurons innervating the left femur
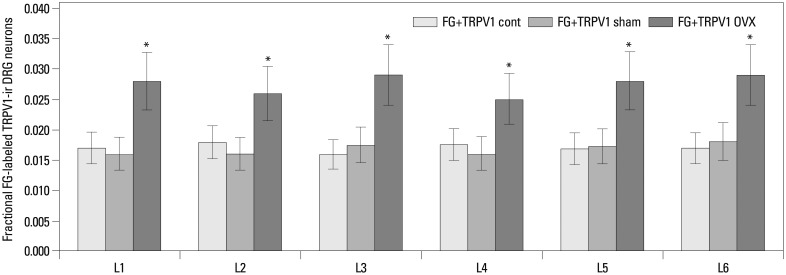
FG-labeled and TRPV1-ir DRG neurons innervating the left femur were present in DRG from L1 through L6 (Figs. 2 and 4). There was no significant difference in the proportion of FG-labeled and TRPV1-ir DRG neurons in the DRG from L1 to L6 in each group. Of the DRG neurons, the percentage of FG-labeled TRPV1-ir neurons was 1.7±0.2%, 1.7±0.3%, and 2.8±0.6% (means±SEMs) in control, sham-operated, and OVX rats, respectively. The proportion of TRPV1-ir neurons in DRG innervating femur in OVX rats was significantly higher than those in control and sham-operated rats (p<0.05) (Fig. 4).
Fig. 4.
Distribution and proportion of FG-labeled and TRPV1-ir DRG neurons innervating the left femur. FG-labeled and TRPV1-ir DRG neurons innervating the left femur were present in DRG from L1 through L6. The proportion of FG-labeled and TRPV1-ir DRG neurons in OVX rats was significantly higher than those in control and sham-operated rats (*p<0.05). FG, Fluoro-Gold; TRPV1, transient receptor potential vanilloid 1; DRG, dorsal root ganglia; OVX, ovariectomized; ir, immunoreactive.
DISCUSSION
In this study, the proportion of FG labeled TRPV1-ir DRG neurons was 1.7%, 1.7%, and 2.8% of total DRG neurons innervating the femur in control, sham-operated, and OVX rats, respectively. The proportion of TRPV1-ir neurons in DRG innervating femur in OVX rats was significantly higher than those in control and sham-operated rats. Thus, we speculate that TRPV1 is important in sensory innervation from the osteoporotic femur.
It is not known whether osteoporosis itself is the origin of pain because patients do not show acute fracture. In clinical reports, some authors have reported an association between osteoporosis and pain.12,13 Iwamoto, et al.12 reported that patients who had osteoporosis without vertebral fractures, and who were receiving bisphosphonate, showed a significant increase in the lumbar bone mineral density (BMD) and a decrease in the urinary N-terminal telopeptide of type I collagen (NTx) levels with alleviation of back pain. Ohtori, et al.13 have reported that osteoporotic patients without fracture treated with bisphosphonate showed a significant increase in their lumbar BMD and a decrease of their urinary NTx levels, and alleviation of back pain. They suggested that despite the absence of vertebral fractures, bone resorption may cause pain because of osteoporosis. However, in the current model, we evaluated the rat femu, and found that osteoporotic patients generally have no pain in the limbs without fracture. Further study is needed to explore the relationship between pain and osteoporosis.
In the current study, the proportion of TRPV1-ir DRG neurons innervating osteoporotic femurs in rat, increased compared with normal femur. In pain studies, it is important to consider sensory innervation and neuropeptides, and inducers of pain such as cytokines. In vertebral bodies harvested from patients with back pain, many sensory nerves show immunoreactivity for neuropeptides involved in pain transmission, such as substance P and calcitonin gene related peptide (CGRP), and substance P-ir and CGRP-ir nociceptors have also been observed.14 In the mouse femur, CGRP-ir fibers associated with inflammatory pain are present throughout the bone marrow, mineralized bone and periosteum, and dominantly in bone marrow.2 Orita, et al.15 have reported elevated TRPV1 and CGRP expression in osteoporotic vertebrae in rat. TRPV1 has been reported to modulate the synthesis and release of CGRP in sensory nerves by its activation.16
On the other hand, inducers of pain such as cytokines are important producers of pain originating from osteoporotic bone. Generally, bisphosphonates are used for osteoporosis and they affect pain from osteoporosis. Possible mechanisms for the improvement of pain through the use of bisphosphonates are the inhibition of osteoclast activity and an anti-inflammatory effect.17,18 The inflammatory factors associated with tumor-related osteolysis include tumor necrosis factor-alpha (TNF-α), interleukin-1, transforming growth factor (TGF)-α and TGF-β.19,20 In vitro and in vivo studies have shown that bisphosphonates inhibit the synthesis of proinflammatory cytokines.19,20
In a mouse model of cancer, exogenous TNF-α is shown to evoke heat hyperalgesia in vivo and sensitize nociceptive nerve fibers to heat in vitro.21 TNF-α enhances the expression of the nociceptor-specific heat transducer TRPV1,21 and it enhances functional thermal and chemical responses of TRPV1 channels in human synoviocytes.22 The density of TRPV1-positive nerve fibers is higher in ovaries from women with complaints of pain from endometriosis than in ovaries from control patients.23 TRPV1 expression was found to be elevated significantly when stimulated with inflammatory mediators such as prostaglandin E2 and TNF-α.23 This suggests that inducers of pain such as cytokines and TRPV1 may contribute to pain transmission in osteoporosis.
There are some limitations in the current study. First, we demonstrated that TRPV1-ir DRG neurons innervated the femur. However, we did not examine the function of TRPV1 or perform a quantitative analysis of TRPV1. Second, we did not evaluate pain from normal or osteoporotic femurs because pain is difficult to measure accurately. Third, we did not evaluate BMD, but only used histological measures to evaluate low bone mass. We previously reported that female rats ovariectomized at 5 weeks of age, the model used in the current study, histologically showed osteoporosis and lower BMD than controls, demonstrated using quantitative computed tomography by 35 weeks of age.15,24 Therefore, we used the same model in the current study. Fourth, there was a possibility that microfractures occurred in the mineralized bone. Microfractures were not evaluated by HE staining of the OVX femur, and further study is necessary to rule out that microfractures occurred.
In conclusion, the present study demonstrated that the proportion of TRPV1-ir neurons was higher in DRG neurons innervating osteoporotic femurs than in DRG neurons innervating normal femurs, suggesting that TRPV1 may modulate sensory transmission from osteoporotic bone.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Ralston SH, Layfield R. Pathogenesis of Paget disease of bone. Calcif Tissue Int. 2012;91:97–113. doi: 10.1007/s00223-012-9599-0. [DOI] [PubMed] [Google Scholar]
- 2.Mach DB, Rogers SD, Sabino MC, Luger NM, Schwei MJ, Pomonis JD, et al. Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience. 2002;113:155–166. doi: 10.1016/s0306-4522(02)00165-3. [DOI] [PubMed] [Google Scholar]
- 3.Mantyh WG, Jimenez-Andrade JM, Stake JI, Bloom AP, Kaczmarska MJ, Taylor RN, et al. Blockade of nerve sprouting and neuroma formation markedly attenuates the development of late stage cancer pain. Neuroscience. 2010;171:588–598. doi: 10.1016/j.neuroscience.2010.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Freeman KT, Koewler NJ, Jimenez-Andrade JM, Buus RJ, Herrera MB, Martin CD, et al. A fracture pain model in the rat: adaptation of a closed femur fracture model to study skeletal pain. Anesthesiology. 2008;108:473–483. doi: 10.1097/ALN.0b013e3181649351. [DOI] [PubMed] [Google Scholar]
- 5.Yang CJ, Wang XW, Li X, Wu GC, Wang YQ, Mao-Ying QL. A rat model of bone inflammation-induced pain by intra-tibial complete Freund's adjuvant injection. Neurosci Lett. 2011;490:175–179. doi: 10.1016/j.neulet.2010.12.027. [DOI] [PubMed] [Google Scholar]
- 6.Jimenez-Andrade JM, Mantyh WG, Bloom AP, Freeman KT, Ghilardi JR, Kuskowski MA, et al. The effect of aging on the density of the sensory nerve fiber innervation of bone and acute skeletal pain. Neurobiol Aging. 2012;33:921–932. doi: 10.1016/j.neurobiolaging.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 8.Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- 9.Yu L, Yang F, Luo H, Liu FY, Han JS, Xing GG, et al. The role of TRPV1 in different subtypes of dorsal root ganglion neurons in rat chronic inflammatory nociception induced by complete Freund's adjuvant. Mol Pain. 2008;4:61. doi: 10.1186/1744-8069-4-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Niiyama Y, Kawamata T, Yamamoto J, Omote K, Namiki A. Bone cancer increases transient receptor potential vanilloid subfamily 1 expression within distinct subpopulations of dorsal root ganglion neurons. Neuroscience. 2007;148:560–572. doi: 10.1016/j.neuroscience.2007.05.049. [DOI] [PubMed] [Google Scholar]
- 11.Niiyama Y, Kawamata T, Yamamoto J, Furuse S, Namiki A. SB366791, a TRPV1 antagonist, potentiates analgesic effects of systemic morphine in a murine model of bone cancer pain. Br J Anaesth. 2009;102:251–258. doi: 10.1093/bja/aen347. [DOI] [PubMed] [Google Scholar]
- 12.Iwamoto J, Takeda T, Ichimura S, Matsu K, Uzawa M. Effects of cyclical etidronate with alfacalcidol on lumbar bone mineral density, bone resorption, and back pain in postmenopausal women with osteoporosis. J Orthop Sci. 2003;8:532–537. doi: 10.1007/s00776-003-0655-5. [DOI] [PubMed] [Google Scholar]
- 13.Ohtori S, Akazawa T, Murata Y, Kinoshita T, Yamashita M, Nakagawa K, et al. Risedronate decreases bone resorption and improves low back pain in postmenopausal osteoporosis patients without vertebral fractures. J Clin Neurosci. 2010;17:209–213. doi: 10.1016/j.jocn.2009.06.013. [DOI] [PubMed] [Google Scholar]
- 14.Brown MF, Hukkanen MV, McCarthy ID, Redfern DR, Batten JJ, Crock HV, et al. Sensory and sympathetic innervation of the vertebral endplate in patients with degenerative disc disease. J Bone Joint Surg Br. 1997;79:147–153. doi: 10.1302/0301-620x.79b1.6814. [DOI] [PubMed] [Google Scholar]
- 15.Orita S, Ohtori S, Koshi T, Yamashita M, Yamauchi K, Inoue G, et al. The effects of risedronate and exercise on osteoporotic lumbar rat vertebrae and their sensory innervation. Spine (Phila Pa 1976) 2010;35:1974–1982. doi: 10.1097/BRS.0b013e3181d5959e. [DOI] [PubMed] [Google Scholar]
- 16.Luo D, Zhang YW, Peng WJ, Peng J, Chen QQ, Li D, et al. Transient receptor potential vanilloid 1-mediated expression and secretion of endothelial cell-derived calcitonin gene-related peptide. Regul Pept. 2008;150:66–72. doi: 10.1016/j.regpep.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Bonabello A, Galmozzi MR, Bruzzese T, Zara GP. Analgesic effect of bisphosphonates in mice. Pain. 2001;91:269–275. doi: 10.1016/S0304-3959(00)00447-4. [DOI] [PubMed] [Google Scholar]
- 18.Fulfaro F, Casuccio A, Ticozzi C, Ripamonti C. The role of bisphosphonates in the treatment of painful metastatic bone disease: a review of phase III trials. Pain. 1998;78:157–169. doi: 10.1016/S0304-3959(98)00135-3. [DOI] [PubMed] [Google Scholar]
- 19.Van Offel JF, Schuerwegh AJ, Bridts CH, Bracke PG, Stevens WJ, De Clerck LS. Influence of cyclic intravenous pamidronate on proinflammatory monocytic cytokine profiles and bone density in rheumatoid arthritis treated with low dose prednisolone and methotrexate. Clin Exp Rheumatol. 2001;19:13–20. [PubMed] [Google Scholar]
- 20.Varenna M, Zucchi F, Ghiringhelli D, Binelli L, Bevilacqua M, Bettica P, et al. Intravenous clodronate in the treatment of reflex sympathetic dystrophy syndrome. A randomized, double blind, placebo controlled study. J Rheumatol. 2000;27:1477–1483. [PubMed] [Google Scholar]
- 21.Constantin CE, Mair N, Sailer CA, Andratsch M, Xu ZZ, Blumer MJ, et al. Endogenous tumor necrosis factor alpha (TNFalpha) requires TNF receptor type 2 to generate heat hyperalgesia in a mouse cancer model. J Neurosci. 2008;28:5072–5081. doi: 10.1523/JNEUROSCI.4476-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kochukov MY, McNearney TA, Yin H, Zhang L, Ma F, Ponomareva L, et al. Tumor necrosis factor-alpha (TNF-alpha) enhances functional thermal and chemical responses of TRP cation channels in human synoviocytes. Mol Pain. 2009;5:49. doi: 10.1186/1744-8069-5-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu J, Liu X, Duan K, Zhang Y, Guo SW. The expression and functionality of transient receptor potential vanilloid 1 in ovarian endometriomas. Reprod Sci. 2012;19:1110–1124. doi: 10.1177/1933719112443876. [DOI] [PubMed] [Google Scholar]
- 24.Suzuki M, Orita S, Miyagi M, Ishikawa T, Kamoda H, Eguchi Y, et al. Vertebral compression exacerbates osteoporotic pain in an ovariectomy-induced osteoporosis rat model. Spine (Phila Pa 1976) 2013;38:2085–2091. doi: 10.1097/BRS.0000000000000001. [DOI] [PubMed] [Google Scholar]