Abstract
Women who develop gestational diabetes mellitus or impaired glucose tolerance during pregnancy are at substantially increased risk for type 2 diabetes and comorbidities after pregnancy. Little is known about the role of genetic factors and their interactions with environmental factors in determining the transition from gestational diabetes mellitus to overt type 2 diabetes mellitus. These critical data gaps served as the impetus for this Diabetes & Women’s Health study with the overall goal of investigating genetic factors and their interactions with risk factors amenable to clinical or public health interventions in relation to the transition of gestational diabetes mellitus to type 2 diabetes mellitus. To achieve the goal efficiently, we are applying a hybrid design enrolling and collecting data longitudinally from approximately 4000 women with a medical history of gestational diabetes mellitus in two existing prospective cohorts, the Nurses’ Health Study II and the Danish National Birth Cohort. Women who had a medical history of gestational diabetes mellitus in one or more of their pregnancies are eligible for the present study. After enrollment, we follow study participants for an additional 2 years to collect updated information on major clinical and environmental factors that may predict type 2 diabetes mellitus risk as well as with biospecimens to measure genetic and biochemical markers implicated in glucose metabolism. Newly collected data will be appended to the relevant existing data for the creation of a new database inclusive of genetic, epigenetic and environmental data. Findings from the study are critical for the development of targeted and more effective strategies to prevent type 2 diabetes mellitus and its complications in this high-risk population.
Keywords: Diabetes & Women’s Health study, gestational diabetes, impaired glucose tolerance, type 2 diabetes, glucose tolerance test, metabolism, pregnancy, genetic factors, environmental factors
Background and significance
Type 2 diabetes mellitus (T2DM) has become a global epidemic affecting approximately 21 million individuals in the USA alone. Worldwide, it is expected to affect 552 million individuals by the year 2030 (1). More alarmingly, by the time T2DM is diagnosed, most individuals may have developed complications such as peripheral artery disease, coronary heart disease, stroke, potentially vision-threatening retinopathy, abnormal renal function, and/or neuropathy. The vast majority of diabetic patients will eventually die of these complications (2). Of added concern is an emerging trend of earlier age at onset for T2DM, underscoring the importance of identifying populations at risk for targeted interventions (3). Women who developed glucose intolerance during pregnancy are a high risk group for T2DM; it is estimated that up to one-third of parous women with T2DM had a prior history of gestational diabetes mellitus (GDM) (3).
Gestational diabetes mellitus is a common pregnancy complication. Approximately 4–7% of all pregnancies are complicated by GDM, resulting in more than 200 000 cases in the USA annually (4). Importantly, this number is growing as the prevalence of obesity among women of reproductive age increases (5). In European countries, the prevalence of GDM ranges on average from 2 to 6%, with a lower prevalence in Northern or Atlantic seaboard parts of Europe (mostly <4%) compared with the South or Mediterranean seaboard regions (mostly >6%) (6). In Denmark, a prevalence of 2.7% was reported in a prospective multiple center study (7). GDM has been related to short- and long-term adverse health outcomes for both women and their offspring. Offspring of women with GDM are more likely to be obese and have impaired glucose tolerance and diabetes in early adulthood (8,9). Women who develop GDM have an increased risk of preeclampsia and cesarean section delivery during pregnancy and an increased risk of T2DM after pregnancy (4,10), with an estimated 30–70% developing T2DM within 15 years after the index GDM pregnancy (10–12). Reported conversion rates from GDM to T2DM varied by the length of follow-up and cohort retention. Findings from one recent large cohort study suggested a 1.7% cumulative incidence of T2DM in the 12 months following delivery, 16% at 10 years, and 35% at 18 years after the index pregnancy (12). Even among low-risk women who were neither overweight nor obese, a history of GDM was associated with more than a sixfold increased risk of T2DM. These women are worthy of close surveillance so that early intervention may be instituted to mitigate their excess risk for T2DM and complications in both mothers and offspring.
Type 2 diabetes mellitus has a strong genetic component and is highly heritable (13). In fact, at least 50 genetic variants have been associated with T2DM (14,15). Unlike the rare genetic mutations that cause monogenic diseases, genetic factors underlying the individual susceptibility to complex diseases such as T2DM have modest effects, which can be amplified in the presence of certain environmental triggering factors. Thus, a better understanding of the progression from GDM to T2DM requires a careful investigation of gene–environment interactions. However, little is known about the role of genetic factors and their interactions with environmental factors in determining the transition from GDM to overt T2DM. Further, few studies have followed through later adulthood for the development of T2DM and co-morbidities. These critical data gaps serve as the impetus for this Diabetes & Women’s Health (DWH) study.
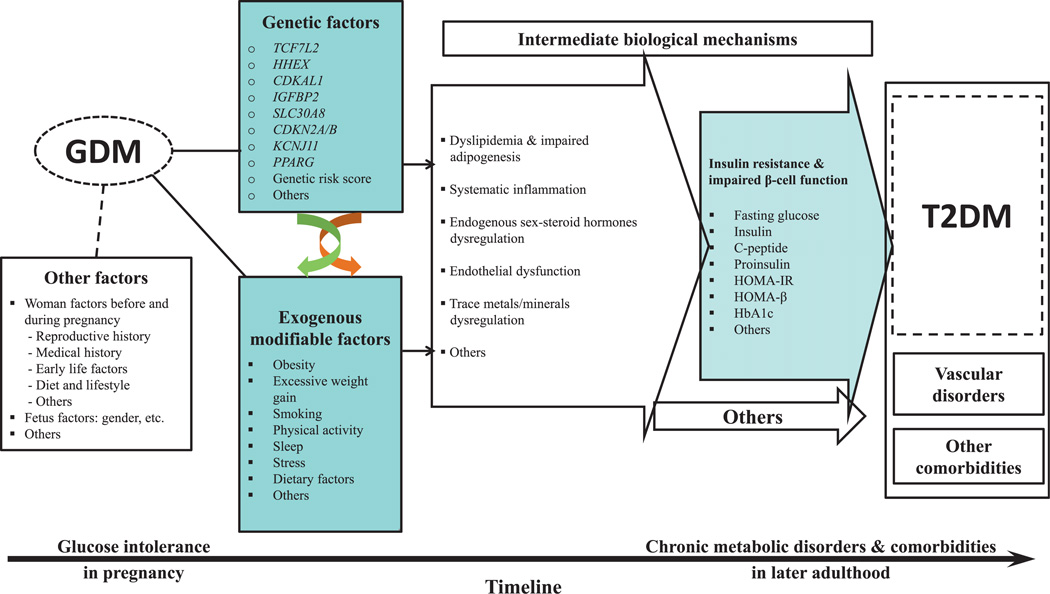
The overarching goal of the study is to identify genomic and environmental determinants underlying the progression from GDM to T2DM and comorbidities and to delineate underlying molecular mechanisms. Figure 1 describes the conceptual framework of the DWH study.
Figure 1.
Diabetes & Women’s Health study conceptual framework. The figure outlines potential major determinants for the progression from gestational diabetes to type 2 diabetes and co-morbidities and potential underlying mechanisms based on a life course perspective. GDM, gestational diabetes mellitus; T2DM, type 2 diabetes mellitus.
Specific scientific aims are as follows:
To investigate whether the composition of diet after a pregnancy affected by GDM is related to the risk of subsequent development of T2DM
-
To investigate whether energy balance, as measured by physical activity, weight gain and total body adiposity after a pregnancy affected by GDM, are related to the risk of subsequent development of T2DM and, if so, to quantify the association.
To investigate the roles of other modifiable factors, such as smoking, alcohol intake, and sleeping duration and quality in the progression from GDM to T2DM.
To investigate the role of genetic variants reported to affect glucose homeostasis in the progression from GDM to T2DM, specifically genetic variants which include at least 50 loci that have been related to glucose homeostasis. This field of research is rapidly evolving and more variants will likely be identified and measured during the conduct of the DWH study. The association of these emerging genetic loci with quantitative metabolic phenotypes related to T2DM (such as adipokines, inflammation markers, hemoglobin A1c, and sex hormones) will be examined as well.
To evaluate the interactions between the above-mentioned genetic loci and environmental factors (such as diet, body weight, and physical activity) in the progression from GDM to T2DM.
In addition, with diet and physical activity data during pregnancy available in the Danish National Birth Cohort (DNBC), we will be able (1) to investigate whether the composition of diet during pregnancy is related to the risk of subsequent development of T2DM and (2) to investigate whether energy balance, as measured by physical activity and weight gain during pregnancy, is related to the risk of subsequent development of glucose intolerance after pregnancy. Lastly, we will collect baseline and feasibility data for studies of pubertal impaired glucose tolerance and metabolic disorders among children born from pregnancies complicated by glucose intolerance.
Study design and population
Study design
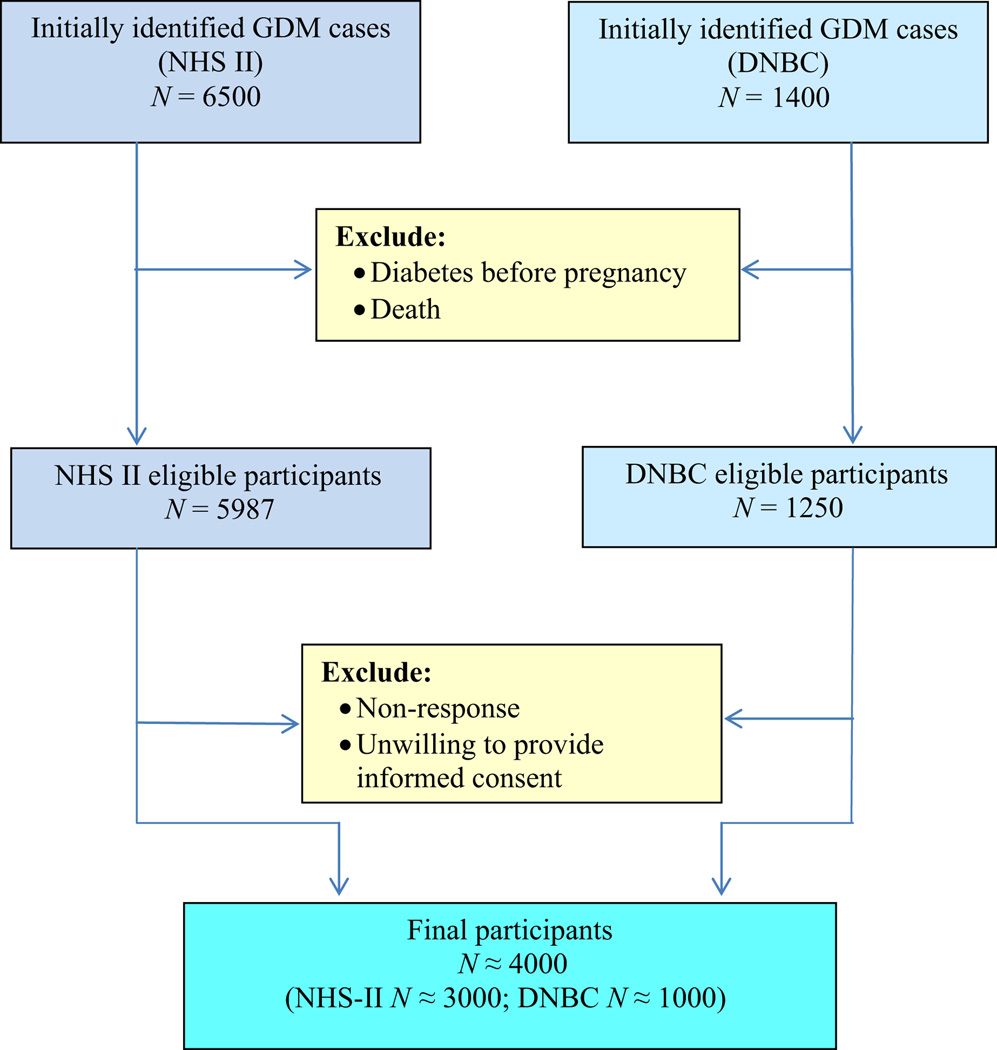
The DWH study applies a hybrid design, combining prospective data collection with resources from two existing cohorts, the DNBC and the Nurses’ Health Study II (NHS-II), to address the study aims. The NHS-II study is an ongoing prospective cohort that was established in 1989 among 116 430 female registered nurses from across the USA without a history of cancer who were aged from 25 to 42 years and who completed a four-page baseline questionnaire (16). Study participants have been completing follow-up questionnaires every 2 years since the inception of the cohort. The DNBC started in 1996 and enrolled 91 827 women between January 1996 and October 2002 (17–19). These two cohorts collectively enrolled 208 257 women, who were eligible for the present study. We anticipate enrolling approximately 4000 women from among the 7500 women who reported a medical history of GDM (53%). The target is to obtain approximately 1000 T2DM cases at the end of the study (Figure 2) The inclusion criteria are women with a medical history of GDM, and women who were alive in 1 March 2012 (death records are updated every year in NHS-II and more often in the DNBC). Women who had diabetes before pregnancy were excluded.
Figure 2.
Flow-chart of anticipated number of study participants. GDM, gestational diabetes mellitus; NHS-II, Nurses’ Health Study II; DNBC, Danish National Birth Cohort.
At the DWH study baseline, eligible study participants are enrolled and followed to collect medical data and updated information on major environmental factors including diet, physical activity, anthropometric and other information that may predict T2DM risk. Biospecimens including blood, urine and toenails are collected to measure genetic and biochemical markers implicated in the etiology of hyperglycemia and T2DM related disorders. Newly collected data will be appended to the relevant existing data for the creation of a new database inclusive of genetic and environmental data needed. The progression from GDM to T2DM and co-morbidities can be a long-term process. The hybrid design enables us to capture this long latency period as well as to accumulate a sufficient number of T2DM events efficiently.
Identification of gestational diabetes
In general, to maximize the possibility of capturing all women who may have had glucose intolerance during pregnancy, in both cohorts, all eligible and potentially suspected GDM cases were identified, enrolled and included as part of the study source population. In NHSII, identification of GDM was based on self-reported physician diagnosis, which was validated by medical records in a previous study showing high validity (20). Confirmed GDM cases were most likely to have been diagnosed by the National Diabetes Data Group criteria (21). From a supplemental questionnaire sent to a random sample of parous women who did not report GDM, 83% reported a glucose screening test during pregnancy and 100% reported frequent prenatal urine screening (20), suggesting a high level of GDM surveillance among both cases and non-cases in NHS-II.
Gestational diabetes mellitus cases in DNBC were identified by a reported GDM event through self-reporting during study interviews at 30 weeks of gestation or 6 months postpartum, or a GDM-related diagnosis recorded in the Danish National Patient Registry in relation to the index pregnancy [International Classification of Diseases (ICD)-10 codes O24.4 and O24.9], or both. Copies from the hospital records of pages relating to the index pregnancy, and copies of all pages with information about oral glucose tolerance test (OGTT) and measurements of HbA1c are available and will be included as part of the DNBC historical database.
Data collection
DWH new data collection of key medical and environmental factors and covariates
New data collection of key medical and environmental factors and covariates began in March 2012 and will be collected again commencing in fall 2014, to complement existing exposure data. Participants have the option of completing either a paper or an online questionnaire. Briefly, exposure data will include data on the women, such as current medical conditions, medical history and medications, pregnancy history, anthropometry, body weight retention after the index pregnancy, diet and dietary supplements, physical activity, sleep duration and quality, socio-demographic status, socio-behavior factors, family disease history and reproductive characteristics.
All exposure data are collected using standardized questionnaires according to a study manual of procedures. For instance, dietary data are collected using a food frequency questionnaire on the different types of food the participants eat, as well as the frequency with which they consume the food. The food frequency questionnaire includes questions about dairy, fruits, vegetables, meats, fats, nuts/seeds, starches, beverages, and desserts and snacks. A dietary supplements form will be administered to collect detailed information on what dietary supplements the participants take, as well as on manufacturer, dose, form, and length of use.
Data collected on the offspring will be reported by the women and will include medical history, body adiposity characteristics over the life course (such as birthweight and body shapes at different ages) and infant feeding information of the offspring.
Women in the NHS-II may complete the standardized questionnaire online via a web link provided by mail or email. For those preferring to respond on paper, a self-addressed postage-paid envelope is provided to return the questionnaire. Up to seven mailings are sent to persistent non-respondents. For women in the DNBC, a standardized baseline questionnaire will be administered during the clinical examination visit. The majority of the study participants enrolled from the DNBC have the clinical examination performed at one of the two main hospitals in Denmark: Rigshospitalet University Hospital, Copenhagen (where the main physical examination team is located) or Aarhus University Hospital, Aarhus. The majority of study participants live within a 2-h drive from one of the two clinical sites. For women who live at a greater distance from the primary clinical sites, the clinical examination will be done at a smaller hospital or clinic located closer to their home. The clinical examination aims to evaluate glycemic control and diabetes status by an OGTT. Height, weight, waist-to-hip ratio, blood pressure and resting heart rate are measured during the clinical examination according to a standardized protocol. At Rigshospitalet University Hospital, women will be scanned with a dual-energy X-ray absorptiometry scanner to measure body composition, including fat and muscle distribution and bone mineral density.
Bio-specimen collection
Study participants in both NHS-II and DNBC will be asked to provide first morning urine specimen, a fasting venous blood sample, and toenail clipping at baseline. Biospecimen collection in the follow-up visit includes first morning urine, fasting blood sample or dried blood spot, and saliva. In addition, blood samples are also collected from the blood drawn at the 2h OGTT among DNBC participants. All blood samples will be processed within 30–60 min of collection according to standardized protocol. Cryovials of serum, plasma, white blood cells, red blood cells, PAXgeneTM RNA tubes, urine and toenail samples will be stored temporarily at the sites at −80°C, then shipped to the Eunice Kennedy Shriver National Institute of Child Health and Human Development’s repository in Rockville, MD, USA and stored at −80°C.
Ascertainment of outcomes
Identical diagnostic criteria for defining T2DM will be applied in NHS-II and DNBC, although procedures for the identification of disease outcomes differ between the two sites (see below). Confirmation and validation mechanisms will be in place in both cohorts to ensure rigorous ascertainment of data.
Nurses’ Health Study II
A medical history of T2DM is initially identified by self-report in the biennial questionnaires. NHS-II participants are instructed to report only physician-diagnosed conditions. Confirmation of T2DM cases is a two-stage process. Women who report T2DM in the biennial follow-up questionnaire are sent a supplementary questionnaire for confirmation of the report, including the date of diagnosis and details of the diagnostic tests performed, presenting symptoms, and medications prescribed. Nonrespondents to the supplementary questionnaire receive a second and third computer-generated mailing. Those who remain non-respondents are called by a study coordinator, who administers the supplementary questionnaire by telephone. Consistent with the criteria of the National Diabetes Data Group (21), the diagnosis of T2DM for cases newly identified between baseline and 1996 is established if one or more of the following criteria are met:
an elevated glucose concentration (fasting plasma glucose level ≥7.8 mmol/L (140 mg/dL), random plasma glucose level ≥11.1 mmol/L (200 mg/dL), or plasma glucose level ≥11.1 mmol/L (200 mg/dL) after an oral glucose load) and at least one symptom related to diabetes (excessive thirst, polyuria, weight loss, or hunger);
no symptoms but elevated glucose concentrations on two occasions;
treatment with insulin or oral hypoglycemic medication.
For cases identified after 1997, the cut-off for fasting plasma glucose concentration was lowered to 7.0 mmol/L (126 mg/dL) in accordance with the American Diabetes Association criteria (8).
The validity of this diagnostic procedure has been verified in a subsample of this study population (22). In a random sample of 62 participants reporting T2DM, 61 cases (98%) were confirmed after medical record review by an endocrinologist blinded to the supplementary questionnaire information. A subsequent sub-study assessing the frequency of undiagnosed diabetes in this cohort found a prevalence of 0.5% in a randomly selected sample of NHS-II participants who had never reported a diagnosis of diabetes. The results of these validation studies give us confidence that T2DM is correctly classified by the process of self-reporting and subsequent confirmation by the supplementary questionnaire.
Danish National Birth Cohort
Confirmation of T2DM cases will follow a two-stage process. First, potential cases will be identified through linkage with the National Diabetes Registry (23). Second, the diagnosis will be re-evaluated in combination with results of OGTT conducted during physical examinations during the study.
Denmark has a healthcare system that covers all citizens, with visits to general practitioners and specialists being free of charge. All Danish residents have a unique personal identification number kept in the Danish Civil Registration System. The National Patient Register, established in 1977, contains electronic records of all patient-discharges from hospitals, and since 1994 it also includes all treatments in outpatient clinics. Each contact is recorded with one or more diagnostic codes in ICD-10 (ICD-8 before 1999). Coverage is 100%, as reporting is compulsory. The National Health Insurance Service Registry, established in 1973, contains information on all services provided by general and specialist practitioners to patients in Denmark, including provision for institutionalized persons. The Register of Medicinal Product Statistics (RMPS prescription register), established in 1993 and complete since 1995, contains information on all prescriptions dispensed at Danish pharmacies. Each prescription is identified by date, type and amount (ATC-DDD codes), as well as the personal identification number. Inclusion criteria for National Diabetes Registry have been established by linking information from these three registers (National Patient Register, National Health Insurance Service Registry and Register of Medicinal Product Statistics). The sensitivity of the National Diabetes Registry algorithm to diagnose diabetes has been reported to be 86%, with a positive predictive value close to 90%.
Data management and integrity
A secure web-based data management structure has been designed to support the DWH Study (http://www.DWH-study.org). All data collection forms have been developed into online data entry systems with automatic quality control procedures including range checking, illogical entries, verification of blanks and missing values. All data are de-identified and encrypted prior to transmission and uploaded via a secure Internet data entry website. A Data Coordinating Center oversees data collection in English and Danish, data editing, and maintain an audit report of such activities, and it oversees data management, including site-monitoring visits. All data are backed up daily with the second copy of the data stored offsite in a secure and fire-resistant location. All research staff are trained and certified in the proper procedures for form completion and are assigned a unique staff identifier, which is recorded in the audit history of each form they complete or change.
Data analysis
We will perform time-to-event analyses to examine the association of environmental factors with the risk of developing T2DM. Only data on environmental factors from before the T2DM development will be used in the analysis. Follow-up will be calculated from date of delivery of the index GDM pregnancy until the date of diagnosis of T2DM, death due to any cause or the end of follow up. First, we will evaluate the univariate associations using Kaplan–Meier curves and calculate log-rank statistics. To account for potential confounding, we will consider the following Cox proportional hazards model: Log λ(t|x) = log λ(t) + βx + γz, where x is the vector of variables indicating the environmental factor of interest that may be updated during follow up, and z is the vector of covariates. Multivarible analyses will take into account both the plausibility of biological effects and statistical evidence of confounding of each covariate. Age, smoking, and race/ethnicity will be included in all models; other covariates with less certain biological effects or confounding potentials will be examined individually in stratified and regression analyses. We will report estimates of hazard ratio parameters and absolute risk of T2DM for women with different levels of environmental exposure following the index pregnancy.
For genetic factors and gene–environment interactions, we will conduct nested case-control studies to investigate the associations of genetic variants of candidate genes with risk of progression from GDM to T2DM and further examine their interactions with diet/physical activity in determining risk of progression. Following the nested case-control design described by Prentice et al. (24), for each incident T2DM case with available biospecimens, we will randomly (i.e. a computer program will be used to generate a random selection of eligible study participant’s ID) select two control women with available specimens from the overall analytical source population. Controls will be matched for year and age of woman at the index pregnancy. We will attempt to use the concept of risk set sampling, whereby controls are selected at random from the sub-group at risk to become a case in a given time period. One advantage of data from the NHS-II and DNBC for these investigations, particularly the genetic studies, is that case and control status in the prospective cohort setting is determined only by the subsequent development of disease, thus minimizing bias due to controls that do not adequately represent the population from which the cases arise (i.e. selection bias). To account for the matched design in the analyses, data will be analyzed using conditional logistic regression, which permits simultaneous adjustment for multiple confounding variables while accounting for the proposed matched design. Given the proposed study design, the estimates from these models are unbiased estimates of incidence rate ratios, thus allowing direct comparisons with the analyses involving the entire cohort, where applicable. To estimate the effect of genetic variants, we will conduct single locus analyses and multiple locus analyses. To account for the joint effects of genetic variants in which we are interested, a genetic risk score will be calculated based on the single nucleotides (SNPs) that have been reproducibly associated loci reaching genome-wide levels of significance.
Two methods will be used to create the genetic risk score: a simple count method and a weighted method as described in detail previously (25). To test for interactions between environmental risk factors and genotypes, we will create covariates corresponding to genotype (such as AA = 1, Aa = 0, aa = 0) or the genetic risk score, the environmental risk factor (such as low physical activity = 1 or high physical activity = 0), and the genotype–environmental risk factor interaction term (the product of the recoded genotype and environmental factor variables), and will include them in multiple logistic regression models. Likelihood ratio tests for interaction can be performed by comparing the model with haplotype and T2DM risk factor main effects, with the model with main effects and interaction terms.
Discussion
Findings from the DWH study could identify genomic and environmental determinants underlying the progression from GDM to T2DM and co-morbidities and will possibly delineate underlying molecular mechanisms. Such information is critical for the development of targeted and thus more effective prevention strategies of these chronic conditions for this high-risk population. Both cohorts remain predominantly Caucasian, which could limit the generalizability of the results to other populations. However, this minimizes bias related to population stratification with regard to genetic analyses.
Data from this study should be very useful for developing the early and personalized prevention strategies of T2DM in a high-risk population, such as women with a history of GDM, and would assist clinicians and public health practitioners to evaluate the personalized risk of T2DM for women with a history of GDM given their genes, lifestyle, and medical conditions. The study will also provide an opportunity to investigate pathogenesis of early stages of T2DM or pre-diabetes. In addition, there is added value in the research framework since it offers future potentials for investigating long-term health of children born from a hyperglycemia pregnancy.
Acknowledgments
Funding
The study is funded by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (contract # HHSN275201000020C). The NHS II cohort is funded by NIH grants R01 CA50385, R01 CA67262, and 1UM1CA176726-01. The DNBC is supported by grants from The Danish Research Council (09-067124 (Center for Fetal Programming) and 09-075611.
Abbreviations
- DNBC
Danish National Birth Cohort
- DWH study
Diabetes & Women’s Health study
- GDM
gestational diabetes mellitus
- ICD
International Classification of Diseases
- NHS-II
Nurses’ Health Study II
- OGTT
oral glucose tolerance test
- T2DM
type 2 diabetes mellitus
Footnotes
Conflict of interest
The authors have stated explicitly that there are no conflicts of interest in connection with this article.
References
- 1.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–321. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Nathan DM. Long-term complications of diabetes mellitus. N Engl J Med. 1993;328:1676–1685. doi: 10.1056/NEJM199306103282306. [DOI] [PubMed] [Google Scholar]
- 3.Cheung NW, Byth K. Population health significance of gestational diabetes. Diabetes Care. 2003;26:2005–2009. doi: 10.2337/diacare.26.7.2005. [DOI] [PubMed] [Google Scholar]
- 4.American Diabetes Association. Gestational diabetes mellitus. Diabetes Care. 2004;27(Suppl 1):S88–S90. doi: 10.2337/diacare.27.2007.s88. [DOI] [PubMed] [Google Scholar]
- 5.Dabelea D, Snell-Bergeon JK, Hartsfield CL, Bischoff KJ, Hamman RF, McDuffie RS. Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort: Kaiser Permanente of Colorado GDM Screening Program. Diabetes Care. 2005;28:579–584. doi: 10.2337/diacare.28.3.579. [DOI] [PubMed] [Google Scholar]
- 6.Buckley BS, Harreiter J, Damm P, Corcoy R, Chico A, Simmons D, et al. Gestational diabetes mellitus in Europe: prevalence, current screening practice and barriers to screening. A review. Diabet Med. 2012;29:844–854. doi: 10.1111/j.1464-5491.2011.03541.x. [DOI] [PubMed] [Google Scholar]
- 7.Jensen DM, Molsted-Pedersen L, Beck-Nielsen H, Westergaard JG, Ovesen P, Damm P. Screening for gestational diabetes mellitus by a model based on risk indicators: a prospective study. Am J Obstet Gynecol. 2003;189:1383–1388. doi: 10.1067/s0002-9378(03)00601-x. [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33(Suppl 1):S62–S69. doi: 10.2337/dc10-S062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Metzger BE. Long-term outcomes in mothers diagnosed with gestational diabetes mellitus and their offspring. Clin Obstet Gynecol. 2007;50:972–979. doi: 10.1097/GRF.0b013e31815a61d6. [DOI] [PubMed] [Google Scholar]
- 10.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25:1862–1868. doi: 10.2337/diacare.25.10.1862. [DOI] [PubMed] [Google Scholar]
- 11.Feig DS, Zinman B, Wang X, Hux JE. Risk of development of diabetes mellitus after diagnosis of gestational diabetes. CMAJ. 2008;179:229–234. doi: 10.1503/cmaj.080012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee AJ, Hiscock RJ, Wein P, Walker SP, Permezel M. Gestational diabetes mellitus: clinical predictors and long-term risk of developing type 2 diabetes: a retrospective cohort study using survival analysis. Diabetes Care. 2007;30:878–883. doi: 10.2337/dc06-1816. [DOI] [PubMed] [Google Scholar]
- 13.O’Rahilly S, Barroso I, Wareham NJ. Genetic factors in type 2 diabetes: the end of the beginning? Science. 2005;307:370–373. doi: 10.1126/science.1104346. [DOI] [PubMed] [Google Scholar]
- 14.Visscher PM, Brown MA, McCarthy MI, Yang J. Five years of GWAS discovery. Am J Hum Genet. 2012;90:7–24. doi: 10.1016/j.ajhg.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Andersson EA1, Allin KH, Sandholt CH, Borglykke A, Lau CJ, Ribel-Madsen R, et al. Genetic risk score of 46 type 2 diabetes risk variants associates with changes in plasma glucose and estimates of pancreatic beta-cell function over 5 years of follow-up. Diabetes. 2013;62:3610–3617. doi: 10.2337/db13-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Solomon CG, Willett WC, Carey VJ, Rich-Edwards J, Hunter DJ, Colditz GA, et al. A prospective study of pregravid determinants of gestational diabetes mellitus. Jama. 1997;278:1078–1083. [PubMed] [Google Scholar]
- 17.Olsen J, Melbye M, Olsen SF, Sørensen TI, Aaby P, Andersen AM, et al. The Danish National Birth Cohort – its background, structure and aim. Scand J Public Health. 2001;29:300–307. doi: 10.1177/14034948010290040201. [DOI] [PubMed] [Google Scholar]
- 18.Andersen AM, Olsen J. The Danish National Birth Cohort: selected scientific contributions within perinatal epidemiology and future perspectives. Scand J Public Health. 2011;39:115–120. doi: 10.1177/1403494811407674. [DOI] [PubMed] [Google Scholar]
- 19.Olsen SF, Mikkelsen TB, Knudsen VK, Orozova-Bekkevold I, Halldórsson TI, Strøm M, et al. Data collected on maternal dietary exposures in the Danish National Birth Cohort. Paediatr Perinat Epidemiol. 2007;21:76–86. doi: 10.1111/j.1365-3016.2007.00777.x. [DOI] [PubMed] [Google Scholar]
- 20.Solomon CG, Willett WC, Carey VJ, Rich-Edwards J, Hunter DJ, Colditz GA, et al. A prospective study of pregravid determinants of gestational diabetes mellitus. JAMA. 1997;278:1078–1083. [PubMed] [Google Scholar]
- 21.National Diabetes Data Group. Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28:1039–1057. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 22.Manson JE, Rimm EB, Stampfer MJ, Colditz GA, Willett WC, Krolewski AS, et al. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet. 1991;338:774–778. doi: 10.1016/0140-6736(91)90664-b. [DOI] [PubMed] [Google Scholar]
- 23.Carstensen B, Kristensen JK, Marcussen MM, Borch-Johnsen K. The National Diabetes Register. Scand J Public Health. 2011;39:58–61. doi: 10.1177/1403494811404278. [DOI] [PubMed] [Google Scholar]
- 24.Prentice RL, Kalbfleisch JD, Peterson AV, Jr, Flournoy N, Farewell VT, Breslow NE. The analysis of failure times in the presence of competing risks. Biometrics. 1978;34:541–554. [PubMed] [Google Scholar]
- 25.Cornelis MC, Qi L, Zhang C, Kraft P, Manson J, Cai T, et al. Joint effects of common genetic variants on the risk for type 2 diabetes in U.S. men and women of European ancestry. Ann Intern Med. 2009;150:541–550. doi: 10.7326/0003-4819-150-8-200904210-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]