Abstract
Results of the present study support ocular epithelia-specific LOXL1 functions in exfoliation glaucoma that may include both dysregulated extracellular matrix cross-linking activity and cellular mechanisms involving a role for LOXL1, in direct interaction with Snail1, in promoting epithelial to mesenchymal transition (EMT) and a potential shift towards fibrogenic epithelial cell phenotypes.
Introduction
Exfoliation syndrome (XFS) is a late onset systemic disorder characterized by the pathological deposition of fibrillar material in multiple tissues. XFS is the most common recognizable cause of open-angle glaucoma (XFG) worldwide, comprising the majority of XFG cases in some countries. XFG has an aggressive course, with a worse prognosis than primary open-angle glaucoma, and either predisposes to or is associated with a range of intraocular complications. In the XFG eye, fibrillo-granular aggregates accumulate on the anterior lens capsule and in other tissues of the anterior segment. These aggregates are considered to result from aberrant production, conformation, and function of both structural (including microfibrils1), and enzymatic (such as LOXL1) components of the extracellular matrix (ECM). Exfoliation material (XFM) released from both the lens surface by iridolenticular friction and synthesized endogenously in the trabecular meshwork, combined with pigment released from ruptured iris pigmented epithelial cells, block the trabecular outflow pathway.
Genome-wide associations and genetic linkage studies have consistently demonstrated in populations world-wide, single nucleotide polymorphisms at the chromosome 15q24 locus, including alleles of the LOXL1 gene, that predispose to a high risk for both XF syndrome and XF glaucoma2-5, yet the mechanisms underlying the involvement of LOXL1 remains to be elucidated.
LOXL1 is one of five members of the lysyl oxidase (LOX) family of copper-dependent ECM amine oxidases characterized by a conserved carboxyl terminal domain required for the catalysis of lysine-derived cross-linking of collagen and elastic fibers. While the exact pathomechanisms of XFS and XFG remain unknown, characterization of the aggregated intra-and extra ocular material, and aberrant production of microfibrillar proteins, fibrillin-1, micro fibril-associated glycoprotein-1 (MAGP-1), latent TGF-β binding proteins (LTBP-1 and LTBP-2,) fibulin-2, elastin, and LOXL1 have led to the concept that XFG may result from primary disturbances in LOXL1 regulation and elastic fiber6,7 or microfibrillar1 network homeostasis. More in-depth analyses, however, indicate disordered function of a wider range of ECM components, including those of the basement membrane, various glycoproteins, ECM remodeling enzymes, and cell-ECM signaling involving TGFβ. The TGFβ-regulated LOXL1 also has significant intra-cellular functions within regulatory networks that have not been analyzed in the context of XFS or XFG. XFM is known to be produced by mesenchymal, epithelial, and endothelial cell types, yet there is an overall lack of understanding of the contribution of cell-type specific mechanisms to disorderly ECM production and aberrant LOXL1 functions in XFS and XFG.
Methods
In order to analyze LOXL1-associated epithelial functions, panels of mesenchymal and epithelial cell lines, including kidney, and retinal epithelial cells (ARPE-19) suitable for LOXL1 analysis8, were established and genotyped for the major risk-associated alleles R141G and G153D. In cultured cells, LOXL1 synthesis and processing were evaluated by Western blot analysis using LOXL1 polyclonal rabbit primary antibody (Zymed Laboratories, San Francisco), and polyclonal anti-BMP-1 antibody (Affinity Bioreagents). LOXL1 catalytic activity assays were performed in media fractions of cells cultured for 48 hours in phenol red and serum free media using the Amplex Red assay (Life Technologies) as previously described9. LOXL1 sub-cellular and ECM localizations were determined in fluorescent immuno-cytochemistryexperiments. Cell membrane staining was performed with WGA, and nuclear staining using DAPI or propidium iodide. Stably transfected epithelial cell lines over-expressing the human full-length hLOXL1 (allele 153G/G), were generated and in these cells, LOXL1 over-expression, sub cellular localization, processing, and activity were analyzed. LOXL1-induced cell phenotype changes were monitored under different culture conditions using cell migration assays, and tests for filopodia for cell mobility/spindling. LOXL1 activity-dependent and independent functions were evaluated using an irreversible inhibitor of LOXL1, BAPN, and catalase. Cultured cells were pretreated with BAPN or catalasefor 48 hrs prior to protein extraction or immuno fluorescent microscopy, or for migration assays, 24 hrs prior to assays and an additional 4 hrs during the assays. Earlier established non-cytotoxic concentrations of 100 μM for BAPN (Sigma), and 200 U/mL for catalase (Sigma) were used in these experiments10. To monitor relative expression changes in the levels of E-cadherin, whole cell lysates were probed in Western blot protein assays using polyclonal rabbit anti–E-cadherin (Santa Cruz Biotechnology). Optical density on autoradiographs was normalized to GAPDH control. For LOXL1-Snail1 interaction tests, LOXL1 over expressing adherent cells were washed with PBS and harvested in high salt RIPA lysis buffer, protein lysates were analyzed using SDS gel electrophoresis. LOXL1-Snail1 interactions were detected using co-immune precipitation of whole cell lysates and Snail1 (Cell Signaling Technology) and LOXL1-specific antibodies.
Results
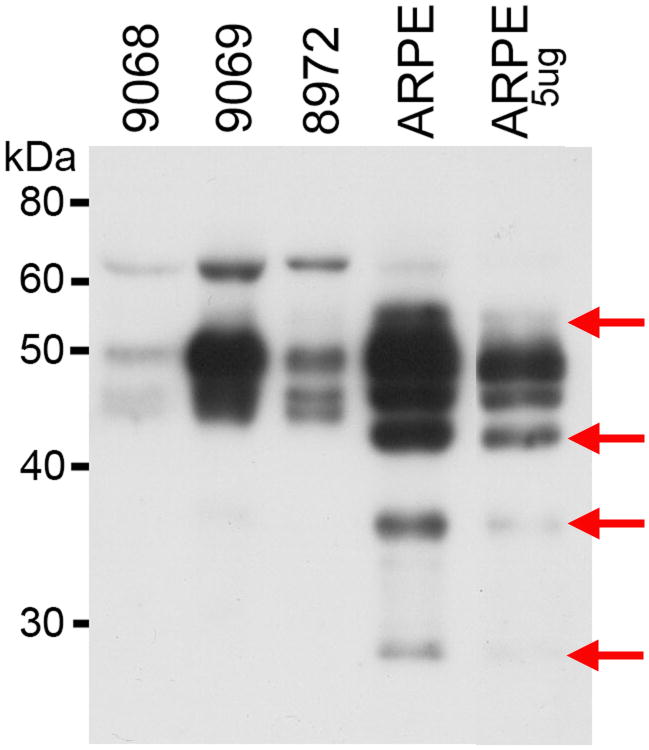
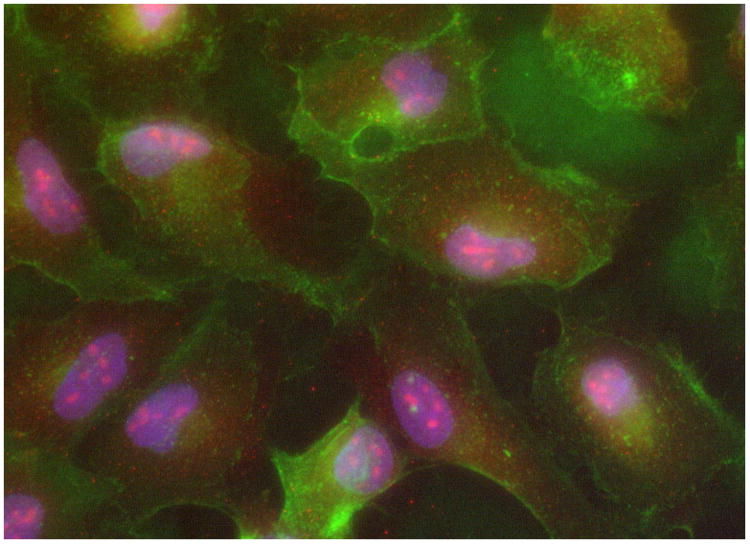
While traditionally, mesenchymal cells are believed to be the primary source of LOXL1, in our experiments, the levels of LOXL1 in E-cadherin expressing cultured ARPE-19 retinal epithelial cells exceeded LOXL1 levels detected in mesenchymal fibroblasts (Figure 1). In retinal epithelial cells, LOXL1 appeared in both cellular and secreted compartments in various molecular masses including the full length 65 kD and a 55 kD form likely resulting from Furine-like processing11. The secreted retinal epithelial cell type-specific forms (Figure 1) (44, 40 and 30 kD), by analogy to known post-translational intra- and extra cellular modifications of the LOXL1 protein in various cell types, were presumed to be the product of activating BMP-1 processing and suggesteda high level of retinal epithelial LOXL1 activity. A further evaluation of BMP-1 processing of LOXL1 in retinal epithelial cells using Western blot analysis of the culture media, confirmed significant levels of BMP-1 (data not shown). In addition to cytoplasmic and secreted LOXL1, abundant amounts of the LOXL1 protein were detected within the nuclei of retinal epithelial cells using both Westen and immuno-cytochemisty analyses (Figure 2).
Figure 1.
Western analysis of LOXL1 in cultured fibroblasts and retinal epithelial cells (ARPE-19). Arrows: novel processed LOXL1 forms in retinal epithelial cells.
Figure 2.
immunofluorescent microscopy of LOXL1 (red) nuclear localization in retinal epithelial cells (ARPE-19). Cell membranes (WGA-green), and nuclei (DAPI-blue) immuno-staining. 40×.
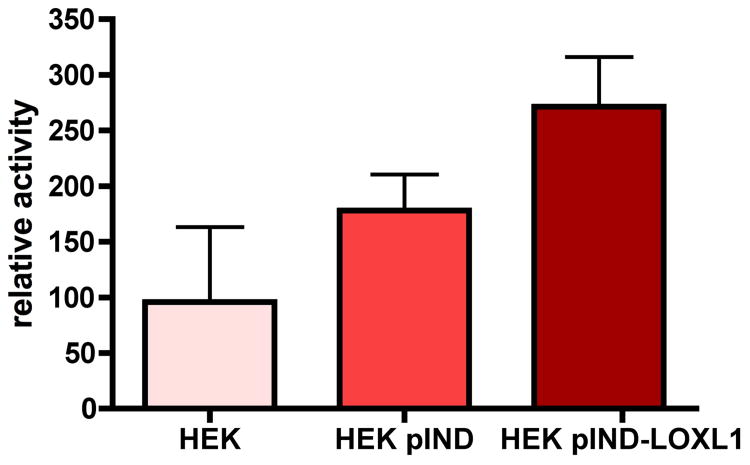
Over-expression of the full-length human LOXL1 (allele 153G/G) in stably transfected epithelial cells (HEK293), resulted in high levels of the LOXL1 protein. The hLOXL1 transfected (pIND-LOXL1) cells showed robust expression of both the 65 kD full-length protein and processed forms immuno-localized in the cytoplasm and nuclei and less prominently in the ECM. In activity assays of 3-day post-confluent pIND-LOXL1 stable over-expressing HEK293 cells, LOXL1 proved to have increased catalytic activity compared to native and empty vector (pIND) transfected cells (Figure 3).
Figure 3.
Catalytic activity assay data for LOXL1 in the media of native, empty vector (pIND), or pIND-LOXL1 over-expressing epithelial cells (HEK293).
Cellular over-expression of LOXL1, or exogenous LOXL1 in HEK293 or ARPE-19 cells treated for 48 hours with LOXL1 in conditioned culture media derived from either high expressing ARPE-19 or over-expressing HEK293 cells cultures, did not result in visible cell phenotype changes. In cell migration assays, the number of migrating cells (the average number of migrated cells in 6 randomly chosen field in 3 parallel experiments per conditions) in pIND-LOXL1 stably transfected HEK293 cells, did not change significantly (p=0.42) compared to native (HEK) or empty vector transfected (pIND HEK) cells (data not shown). In order to test for LOXL1 activity-dependent or activity-independent functions, cultured epithelial cells were treated with BAPN, an irreversible inhibitor of LOXL1 activity, or catalase that promotes the degradation of hydrogen peroxide, a by-product of the LOXL1 cross-linking reaction and itself an important regulator of cellular functions. There were no significant differences in the number of filopodia formed under different culture conditions, suggesting that LOXL1 activity-related function did not have a significant effect on cell mobility/spindling, a phenotypic marker of EMT.
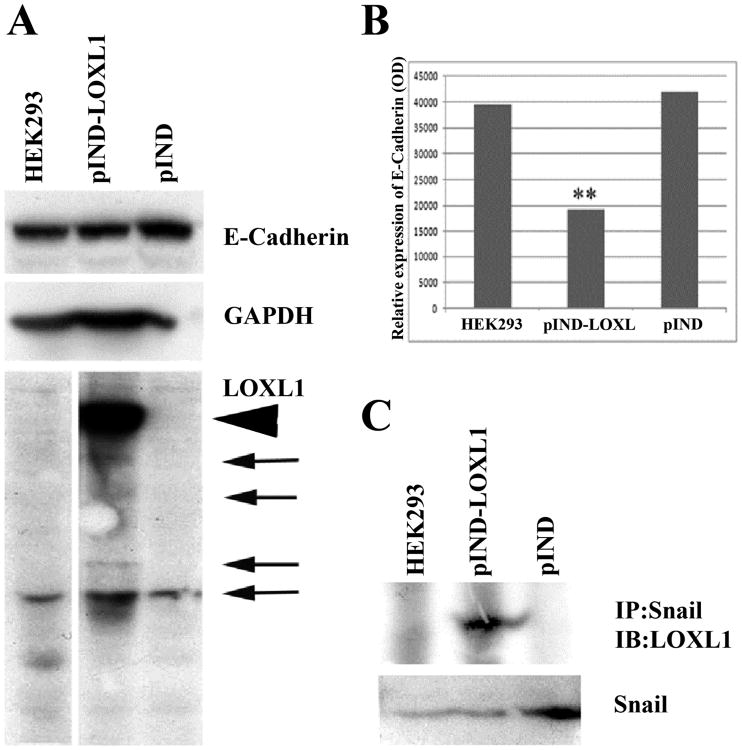
Among epithelial features affected by LOXL1, the epithelial marker and cell adhesion protein E-cadherin, highly expressed in ARPE-19 cells, was found significantly reduced by LOXL1 over-expression in stable transfected HEK293 cells (Fig.4A, B). E-cadherin expression is controlled by Snail, a transcriptional regulator of EMT. Therefore, we considered the possibility that reduced E-cadherin was an indicator for the initiation of LOXL1-mediated EMT in these cells. The basal levels of Snail expression did not increase significantly in response to LOXL1 over-expression. However, in immuno-precipitation experiments, we detected direct interaction between Snail and the full length LOXL1 in over-expressing cells, an interaction that was not detectable in native HEK293 cells or in vector-transfected cells (Figure 4C). These results confirmed our earlier data reported for various epithelial cell types 12-14 that LOXL1 in interactions with Snail may be directly involved in the induction of EMT and the generation of fibrogenic cell types, a mechanism that in ocular epithelial cells, may contribute to disorderly ECM and the pathomechanism of XFG.
Figure 4.
Western analysis in native, vector-only, and LOXL1 over-expressing, stable transfected HEK293 cells. A: E-cadherin and LOXL1 immunoblot. LOXL1 full length (arrowhead) and processed (arrows) forms. B: LOXL1 over-expression reduced E-cadherin levels. Relative expression of E-cadherin was normalized to GAPDH loading control. C.Immuno-precipitated Snail1 and LOXL1 were probed for LOXL1 and Snail1 respectively. Snail1 expression was detected in all three cell lines.
Discussion
To date, pathomechanistic explanations for XFG have largely focused on downstream scenarios, deduced from the components of the irreversible aberrant fibrillo-granular aggregates in eye compartments. Attempts to identify the cell type-specific mechanisms in the initiating pathological steps, meanwhile, have remained extremely difficult due to limited access to tissue and cell specimens suitable to experimentally test and challenge these hypotheses and lack of animal models that would recapture the XFG process. In this study, therefore, we have established a cell model to address firstly, the contribution of epithelial cells to LOXL1-associated pathological mechanisms most relevant to its aberrant regulation and cross-linking function of ECM aggregates in XFG and, secondly, to explore cellular roles for LOXL1 within regulatory functions that may additionally contribute to the wide-spread ECM disturbance and aberrant production of fibrotic material that occurs in XFG.
Results from the current study demonstrated that, compared to mesenchymal cells considered as major sources of LOXL1, E-cadherin-expressing polarized epithelial cells produce higher levels of LOXL1, express abundant amounts of BMP-1 critical for LOXL1 activation, and process and activate LOXL1, supporting high levels of epithelia-derived LOXL1 cross-linking activity in the ECM. The broader epithelial cell type-specificity and functional significance of some of the processed forms unique to retinal epithelial cells needs further study. While there are variations in LOXL1 expression levels among the cell lines analyzed, this is unlikely due to allelic differences at R141L or G153D (Arg/Leu, Gly/Asp) associated with risk for XFS and/or XFG, similar to an earlier report on LOXL1 haplotypes (GG, GA, TG, TA) at these loci that did not affect LOXL1 catalytic activity in vitro15.
Over-expression or inhibition of cellular or exogenous LOXL1 did not induce cell phenotype changes in terms of migratory ability, filopodia, or cell mobility/spindling, visible signs of mesenchymal features. LOXL1, however, significantly down-regulatedthe epithelial marker E-cadherin, suggesting the initiation of LOXL1-mediated EMT in these cells. Consistent with this novel cellular/nuclear LOXL1 function, LOXL1 localized not only to the ECM, but was also prominently present in nuclear compartments, in agreement with our earlier in vivo16 and in vitro17 observations.
EMT has been reported to occur, as part of various eye pathologies, in eye epithelial compartments18, in the trabecular meshwork19, under conditions such as ocular tissue fibrosis and injury20, and with the involvement of Snail121. EMT-like phenomenon has been also proposed to directly result in aberrant conditions in the aqueous outflow pathway in glaucomatous eyes19.
We have previously reported the involvement of LOX family members, LOXL2 and LOXL3, in EMT including interactions with Snail1 to down-regulate E-cadherin expression. Furthermore, we have also noted that Snail1 lysine residues 98 and 137 are required for stability, functional cooperation with LOXs, and induction of EMT17,22. We have also described an in vivo role for LOXL1 in cooperation with Snail1 during the transition of liver epithelial cells towards a fibrogenic phenotype23. Results of the current study further support interactions between LOXL1 and Snail1 in epithelial cells, and LOXL1-induced repression of E-cadherin, a hallmark of EMT, and a mechanism that in eye epithelial cells, may contribute to disorderly ECM production during the pathogenesis of XFG.
Trans differentiation reported in retinal epithelial cells induced by TGF-β1-and EGF, included gain of α-smooth muscle act in (α-SMA), an indicator of a transition towards a myofibroblast phenotype, and enhanced migration24. While the progression of LOXL1-induced EMT including expression patterns of early (vimentin) and late (α-SMA) markers need to be defined, enhanced epithelial cell migration does not appear to be a LOXL1-associated feature.
Not only epitihelial, but mesenchymal cells also become activated in XFG and under reactive conditions including injury, repair, and inflammation, and differentiate towards pro-fibrotic myofibroblast phenotypes resulting in persistent and excessive production and disorderly deposition of fibrotic material. Under these conditions, there is an additional, TGF-β-driven, expansion of connective tissue cell population25. In XFG, the pro-fibrotic TGF-β, has been noted to induce LOXL1 in Tenon's capsule cells26. In cells of the trabecular meshwork, TGF-β I induced LOXL1 expression involving both Smad and non-Smad signaling pathways, and the resulting increase in LOXL1 cross-linking activity was proposed to be partly responsible for TGF-β-mediated IOP elevation27. Of interest is the fact that in the characteristic XFG fibrotic profile, while numerous fibrotic elements are present, unlike in tissue fibrosis, there is no increase in fibrillar and type IV collagens. In epithelial cells, however, TGF-β did not appear to affect LOXL1 expression (A546 cells, NCBI GEO array data). Thus alterations in multiple epithelial and mesenchymal cell-specific regulatory pathways may converge and contribute to the XFG phenotype.
Data collectively support that in XFG, LOXL1-associated pathology involves dysregulated ECM activity of the secreted LOXL1 produced by both mesenchymal and epithelial cells that may directly contribute to irreversible cross-linking of fibrillar ECM aggregates, and additionally, in eye epithelia, a cellular mechanism with a role for LOXL1 in interactions with Snail1, in the regulation of EMT, a process that activates multiple regulatory networks to promote fibrogenic cell phenotypes.
Acknowledgments
This study was supported The Glaucoma Foundation and NIH grants U54RR022762 and U54RR026136 to KC.
References
- 1.Kuchtey J, Kuchtey RW. The microfibril hypothesis of glaucoma: implications for treatment of elevated intraocular pressure. J Ocul Pharmacol Ther. 2014 Mar-Apr;30:170–180. doi: 10.1089/jop.2013.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Challa P. Glaucoma genetics. Int Ophthalmol Clin. 2008 Fall;48:73–94. doi: 10.1097/IIO.0b013e318187e71a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y, Allingham RR. Molecular genetics in glaucoma. Exp Eye Res. 2011 Oct;93:331–339. doi: 10.1016/j.exer.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlötzer-Schrehardt U. Genetics and genomics of pseudoexfoliation syndrome/glaucoma. Middle East Afr J Ophthalmol. 2011 Jan;18:30–36. doi: 10.4103/0974-9233.75882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakano M, Ikeda Y, Tokuda Y, et al. Novel common variants and susceptible haplotype for exfoliation glaucoma specific to Asian population. Sci Rep. 2014;4:5340. doi: 10.1038/srep05340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schlötzer-Schrehardt U. Molecular pathology of pseudoexfoliation syndrome/glaucoma--new insights from LOXL1 gene associations. Exp Eye Res. 2009 Apr;88:776–785. doi: 10.1016/j.exer.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Schlötzer-Schrehardt U, Hammer CM, Krysta AW, et al. LOXL1 deficiency in the lamina cribrosa as candidate susceptibility factor for a pseudoexfoliation-specific risk of glaucoma. Ophthalmology. 2012 Sep;119:1832–1843. doi: 10.1016/j.ophtha.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Coral K, Madhavan J, Pukhraj R, et al. High glucose induced differential expression of lysyl oxidase and its isoform in ARPE-19 cells. Curr Eye Res. 2013 Jan;38:194–203. doi: 10.3109/02713683.2012.720341. [DOI] [PubMed] [Google Scholar]
- 9.Szauter KM, Ordas A, Laxer RM, et al. A novel fibrotic disorder associated with increased dermal fibroblast proliferation and downregulation of genes of the microfibrillar network. Br J Dermatol. 2010 Nov;163:1102–1115. doi: 10.1111/j.1365-2133.2010.09911.x. [DOI] [PubMed] [Google Scholar]
- 10.Kirschmann DA, Seftor EA, Fong SF, et al. A molecular role for lysyl oxidase in breast cancer invasion. Cancer Res. 2002 Aug 1;62:4478–4483. [PubMed] [Google Scholar]
- 11.Borel A, Eichenberger D, Farjanel J, et al. Lysyl oxidase-like protein from bovine aorta. Isolation and maturation to an active form by bone morphogenetic protein-1. J Biol Chem. 2001 Dec 28;276:48944–48949. doi: 10.1074/jbc.M109499200. [DOI] [PubMed] [Google Scholar]
- 12.Peinado H, Del Carmen Iglesias-de la Cruz M, Olmeda D, et al. A molecular role for lysyl oxidase-like 2 enzyme in snail regulation and tumor progression. Embo J. 2005 Oct 5;24:3446–3458. doi: 10.1038/sj.emboj.7600781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moreno-Bueno G, Salvador F, Martin A, et al. Lysyl oxidase-like 2 (LOXL2), a new regulator of cell polarity required for metastatic dissemination of basal-like breast carcinomas. EMBO Mol Med. 2011 Sep;3:528–544. doi: 10.1002/emmm.201100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diaz R, Kim JW, Hui JJ, et al. Evidence for the epithelial to mesenchymal transition in biliary atresia fibrosis. Hum Pathol. 2008 Jan;39:102–115. doi: 10.1016/j.humpath.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 15.Kim S, Kim Y. Variations in LOXL1 associated with exfoliation glaucoma do not affect amine oxidase activity. Mol Vis. 2012;18:265–270. [PMC free article] [PubMed] [Google Scholar]
- 16.Hayashi K, Fong KS, Mercier F, et al. Comparative immunocytochemical localization of lysyl oxidase (LOX) and the lysyl oxidase-like (LOXL) proteins: changes in the expression of LOXL during development and growth of mouse tissues. J Mol Histol. 2004 Nov;35:845–855. doi: 10.1007/s10735-004-2340-1. [DOI] [PubMed] [Google Scholar]
- 17.Peinado H, Del Carmen Iglesias-de la Cruz M, Olmeda D, et al. A molecular role for lysyl oxidase-like 2 enzyme in snail regulation and tumor progression. Embo J. 2005 Oct 5;24:3446–3458. doi: 10.1038/sj.emboj.7600781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li P, Jing J, Hu J, et al. RNA Interference Targeting Snail Inhibits the Transforming Growth Factor beta 2-Induced Epithelial-Mesenchymal Transition in Human Lens Epithelial Cells. J Ophthalmol. 2013;2013:869101. doi: 10.1155/2013/869101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi E, Inoue T, Fujimoto T, et al. Epithelial mesenchymal transition-like phenomenon in trabecular meshwork cells. Exp Eye Res. 2014 Jan;118:72–79. doi: 10.1016/j.exer.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 20.Saika S, Yamanaka O, Flanders KC, et al. Epithelial-mesenchymal transition as a therapeutic target for prevention of ocular tissue fibrosis. Endocr Metab Immune Disord Drug Targets. 2008 Mar;8:69–76. doi: 10.2174/187153008783928343. [DOI] [PubMed] [Google Scholar]
- 21.Li H, Li M, Xu D, et al. Overexpression of Snail in retinal pigment epithelial triggered epithelial-mesenchymal transition. Biochem Biophys Res Commun. 2014 Mar 28;446:347–351. doi: 10.1016/j.bbrc.2014.02.119. [DOI] [PubMed] [Google Scholar]
- 22.Moreno-Bueno G, Salvador F, Martin A, et al. Lysyl oxidase-like 2 (LOXL2), a new regulator of cell polarity required for metastatic dissemination of basal-like breast carcinomas. EMBO Mol Med. 2011 Sep;3:528–544. doi: 10.1002/emmm.201100156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diaz R, Kim JW, Hui JJ, et al. Evidence for the epithelial to mesenchymal transition in biliary atresia fibrosis. Hum Pathol. 2008 Jan;39:102–115. doi: 10.1016/j.humpath.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 24.Lee H, O'Meara SJ, O'Brien C, et al. The role of gremlin, a BMP antagonist, and epithelial-to-mesenchymal transition in proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 2007 Sep;48:4291–4299. doi: 10.1167/iovs.07-0086. [DOI] [PubMed] [Google Scholar]
- 25.Hinz B, Gabbiani G. Fibrosis: recent advances in myofibroblast biology and new therapeutic perspectives. F1000 Biol Rep. 2010;2:78. doi: 10.3410/B2-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zenkel M, Krysta A, Pasutto F, et al. Regulation of lysyl oxidase-like 1 (LOXL1) and elastin-related genes by pathogenic factors associated with pseudoexfoliation syndrome. Invest Ophthalmol Vis Sci. 2011 Oct;52:8488–8495. doi: 10.1167/iovs.11-8361. [DOI] [PubMed] [Google Scholar]
- 27.Sethi A, Mao W, Wordinger RJ, et al. Transforming growth factor-beta induces extracellular matrix protein cross-linking lysyl oxidase (LOX) genes in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2011 Jul;52:5240–5250. doi: 10.1167/iovs.11-7287. [DOI] [PMC free article] [PubMed] [Google Scholar]