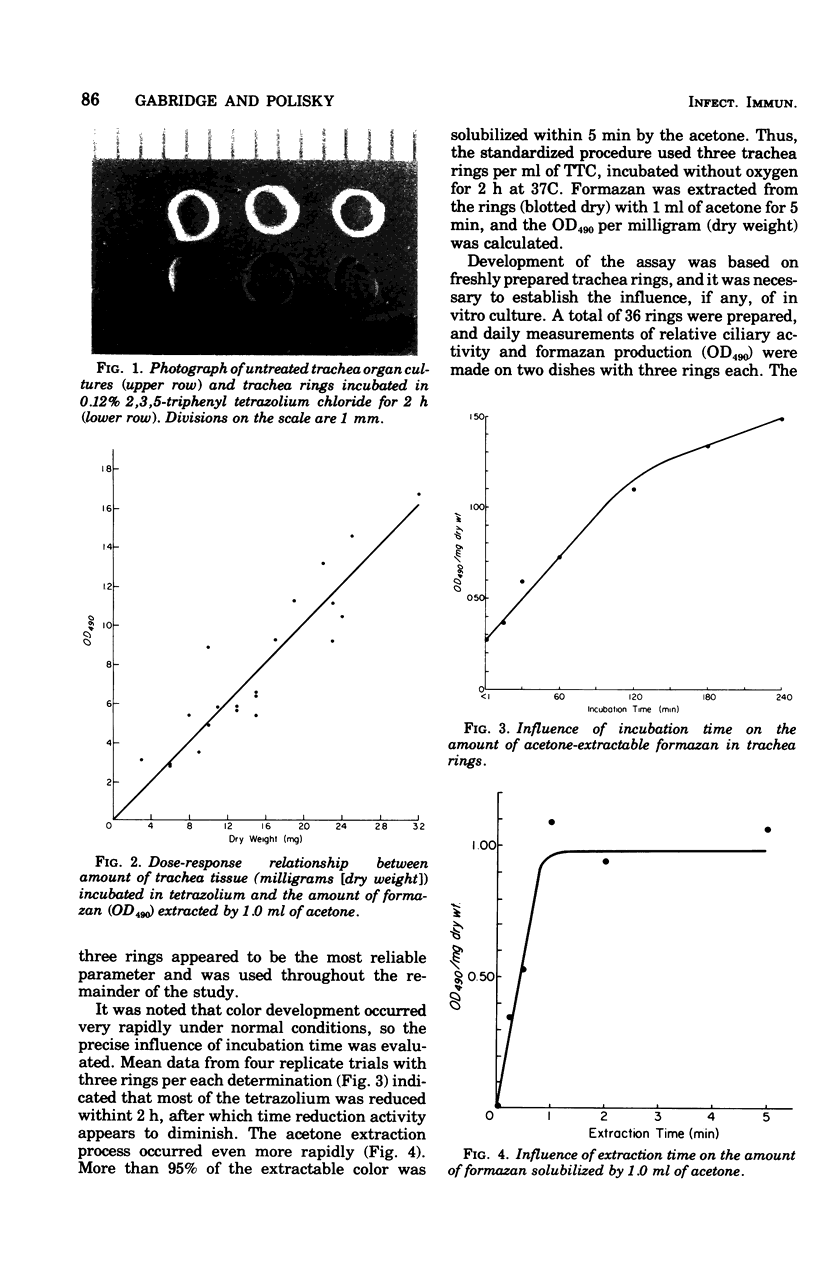
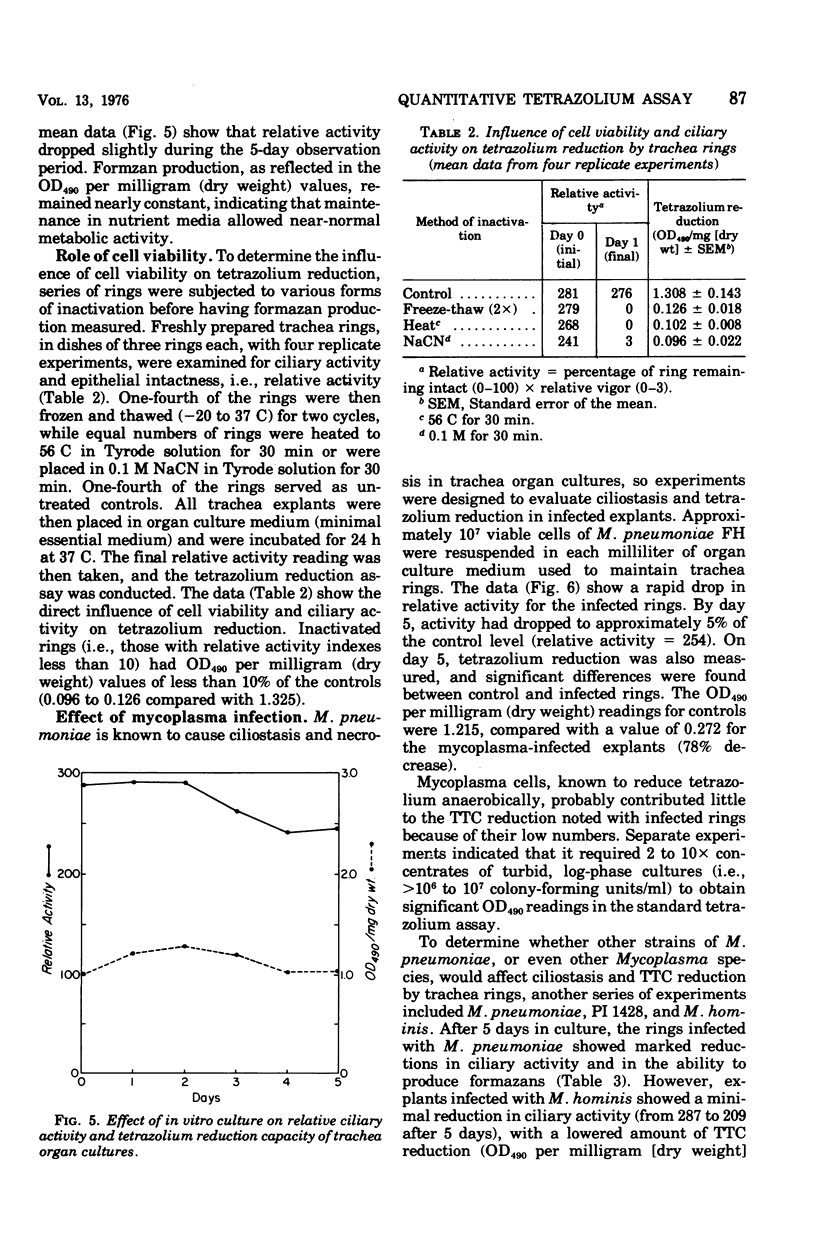
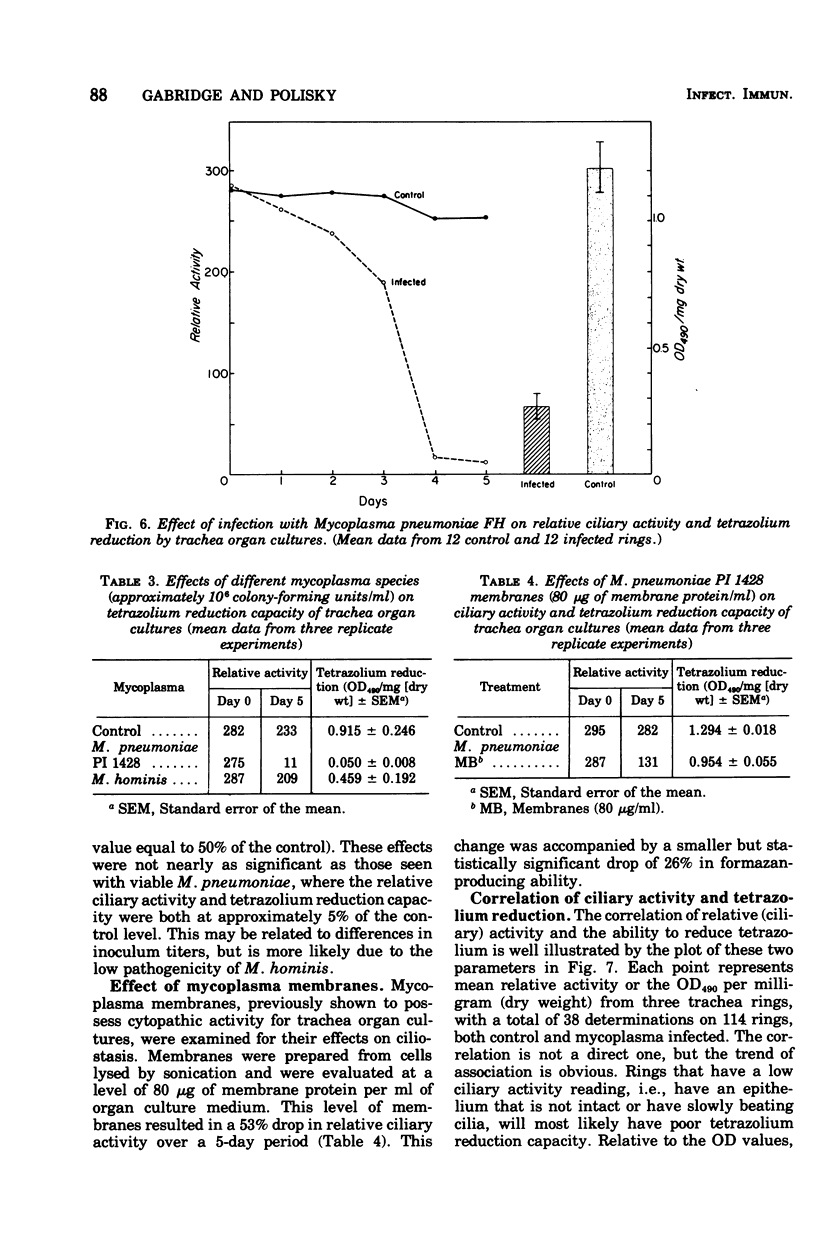
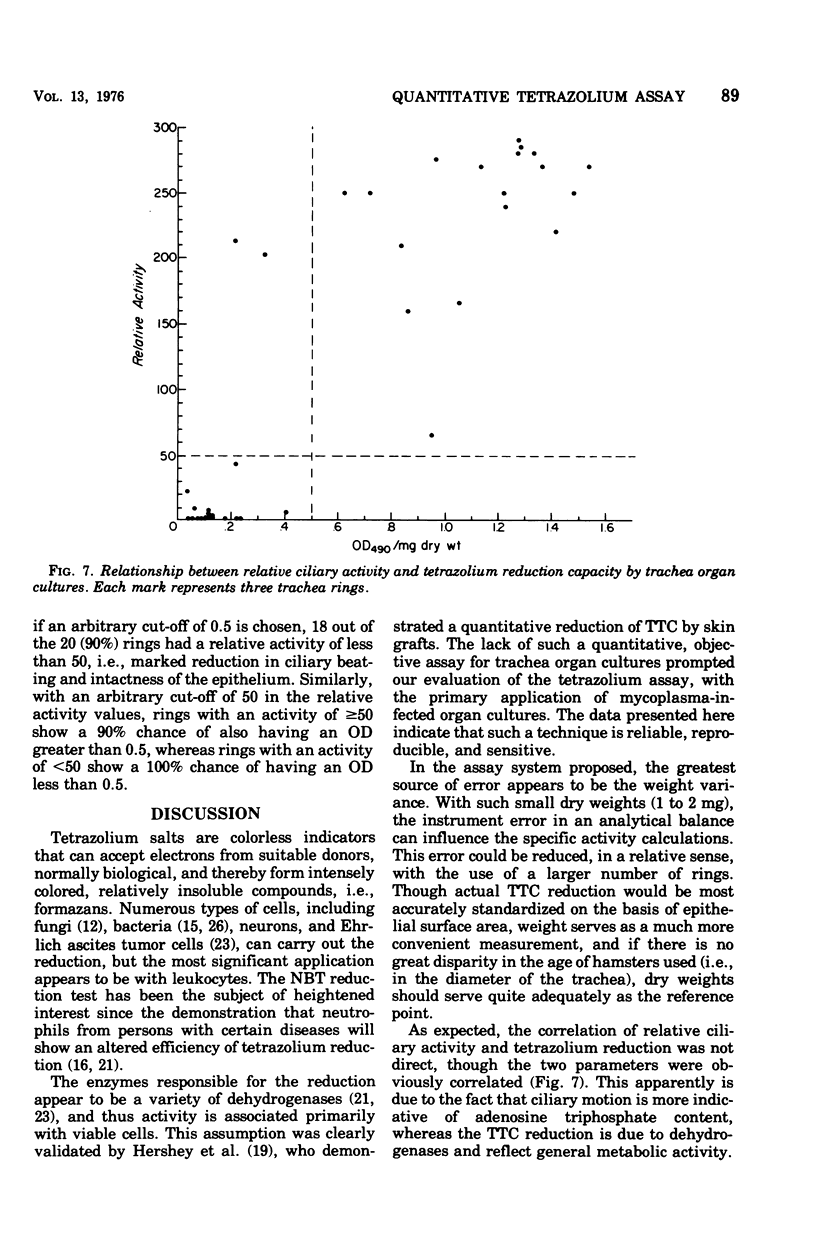
Abstract
The ability of Mycoplasma pneumoniae cells and membranes to affect tetrazolium reduction by hamster trachea organ cultures was evaluated. Uninfected trachea explants reduced 2,3,5-triphenyl tetrazolium chloride (TTC) and nitro-blue tetrazolium when incubated at 37 C in the absence of air. Reduced tetrazolium salts (formazans) were extractable with acetone or ethylene glycol and could be quantitated spectrophotometrically. The optimal assay system involved the use of three or more tracheal rings incubated for 2 h in 0.12% TTC in Tyrode balanced salts supplemented with 1.2% sodium succinate. Formazan was extracted for 5 min with acetone, and the optical density (490 nm) was determined. Trachea explants with metabolic activity reduced or obliterated by freeze-thaw lysis, heat (56 C X 30 min), or cyanide (0.1 M NaCN X 30 min) had negligible ciliary activity and tetrazolium reduction activity (optical density at 490 nm [dry weight]). Tracheas exposed to mycoplasma cells or membranes also showed significantly decreased ciliary activity and tetrazolium reduction; e.g., only 5pc of the ciliary activity and reduction capacity remained after 5 days in culture when infected with M. pneumonia PI 1428 cells. The data indicate that the exposure of ciliated respiratory epithelium to mycoplasma cells or membranes results in diminished oxidative metabolism, and that the ability to reduce TTC to its formazan is correlated with relative ciliary activity.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehner R. L., Nathan D. G. Quantitative nitroblue tetrazolium test in chronic granulomatous disease. N Engl J Med. 1968 May 2;278(18):971–976. doi: 10.1056/NEJM196805022781801. [DOI] [PubMed] [Google Scholar]
- Blacklow N. R., Rose F. B., Whalen R. A. Organ culture of human aorta: prolonged survival with support of viral replication. J Infect Dis. 1975 May;131(5):575–578. doi: 10.1093/infdis/131.5.575. [DOI] [PubMed] [Google Scholar]
- Clyde W. A., Jr An experimental model for human mycoplasma disease. Yale J Biol Med. 1968 Apr-Jun;40(5-6):436–443. [PMC free article] [PubMed] [Google Scholar]
- Collier A. M., Clyde W. A., Jr, Denny F. W. Biologic effects of Mycoplasma pneumoniae and other mycoplasmas from man on hamster tracheal organ culture. Proc Soc Exp Biol Med. 1969 Dec;132(3):1153–1158. doi: 10.3181/00379727-132-34385. [DOI] [PubMed] [Google Scholar]
- Collier A. M., Clyde W. A. Relationships Between Mycoplasma pneumoniae and Human Respiratory Epithelium. Infect Immun. 1971 May;3(5):694–701. doi: 10.1128/iai.3.5.694-701.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conover J. H., Bonforte R. J., Hathaway P., Paciuc S., Conod E. J., Hirschhorn K., Kopel F. B. Studies on ciliary dyskinesia factor in cystic fibrosis. I. Bioassay and heterozygote detection in serum. Pediatr Res. 1973 Apr;7(4):220–223. doi: 10.1203/00006450-197304000-00027. [DOI] [PubMed] [Google Scholar]
- Curreri P. W., Heck E. L., Browne L., Baxter C. R. Stimulated nitroblue tetrazolium test to assess neutrophil antibacterial function: prediction of wound sepsis in burned patients. Surgery. 1973 Jul;74(1):6–13. [PubMed] [Google Scholar]
- Donnelly G. M., McKean H. E., Heird C. S., Green J. Ciliostasis as a bioassay: sources of variation and their control. Arch Environ Health. 1974 Jun;28(6):350–355. doi: 10.1080/00039896.1974.10666507. [DOI] [PubMed] [Google Scholar]
- Fred R. B., Knight S. G. The Reduction of 2,3,5-Triphenyltetrazolium Chloride by Penicillium chrysogenum. Science. 1949 Feb 18;109(2825):169–170. doi: 10.1126/science.109.2825.169. [DOI] [PubMed] [Google Scholar]
- GERSHENFELD L., WEBER L. S., Jr Bacterial variants produced in culture media containing 2,3,5-triphenyltetrazolium chloride. Am J Pharm Sci Support Public Health. 1951 Jun;123(6):203–207. [PubMed] [Google Scholar]
- Gabridge M. G., Johnson C. K., Cameron A. M. Cytotoxicity of Mycoplasma pneumoniae Membranes. Infect Immun. 1974 Nov;10(5):1127–1134. doi: 10.1128/iai.10.5.1127-1134.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabridge M. G. Oxygen consumption by trachea organ cultures infected with Mycoplasma pneumoniae. Infect Immun. 1975 Sep;12(3):544–549. doi: 10.1128/iai.12.3.544-549.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. M., Rowan R. M., Brown T., Carson H. G. Routine application of the nitroblue tetrazolium test in the clinical laboratory. J Clin Pathol. 1973 Jan;26(1):52–56. doi: 10.1136/jcp.26.1.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERSHEY F. B., CRUICKSHANK C. N., MULLINS L. I. The quantitative reduction of 2,3,5-triphenyl tetrazolium chloride by skin in vitro. J Histochem Cytochem. 1958 May;6(3):191–196. doi: 10.1177/6.3.191. [DOI] [PubMed] [Google Scholar]
- Hara K., Izumikawa K., Kinoshita I., Ota M., Ikebe A. Experimental infection with Mycoplasma pneumoniae in the young hamster: location of ferritin-labeled antibody binding to infective tissue. Tohoku J Exp Med. 1974 Dec;114(4):315–337. doi: 10.1620/tjem.114.315. [DOI] [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- Hu P. C., Collier A. M., Baseman J. B. Alterations in the metabolism of hamster tracheas in organ culture after infection by virulent Mycoplasma pneumoniae. Infect Immun. 1975 Apr;11(4):704–710. doi: 10.1128/iai.11.4.704-710.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU C. Studies on primary atypical pneumonia. I. Localization, isolation, and cultivation of a virus in chick embryos. J Exp Med. 1957 Oct 1;106(4):455–466. doi: 10.1084/jem.106.4.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lace J. K., Tan J. S., Watanakunakorn C. An appraisal of the nitroblue tetrazolium reduction test. Am J Med. 1975 May;58(5):685–694. doi: 10.1016/0002-9343(75)90505-7. [DOI] [PubMed] [Google Scholar]
- Martinez-Rodriguez R., Toledano A., Gonzalez M. Reduction of the tetrazolium salts in cells and tissue sections incubated without substrate. Ann Histochim. 1972 Jul-Sep;17(3):233–242. [PubMed] [Google Scholar]
- Pollack J. D., Razin S., Cleverdon R. C. Localization of Enzymes in Mycoplasma. J Bacteriol. 1965 Sep;90(3):617–622. doi: 10.1128/jb.90.3.617-622.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S. E., Boyde A. Organ cultures of respiratory epithelium infected with rhinovirus or parainfluenza virus studied in a scanning electron microscope. Infect Immun. 1972 Jul;6(1):68–76. doi: 10.1128/iai.6.1.68-76.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOMERSON N. L., MORTON H. E. Reduction of tetrazolium salts by pleuropneumonialike organisms. J Bacteriol. 1953 Mar;65(3):245–251. doi: 10.1128/jb.65.3.245-251.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson D., Cherry J. D. A non-pathogenic mycoplasma inhibiting the effect of a pathogenic mycoplasma in organ culture. J Med Microbiol. 1972 Aug;5(3):291–298. doi: 10.1099/00222615-5-3-291. [DOI] [PubMed] [Google Scholar]
- Toms G. L., Rosztoczy I., Smith H. The localization of influenza virus: minimal infectious dose determinations and single cycle kinetic studies on organ cultures of respiratory and other ferret tissues. Br J Exp Pathol. 1974 Apr;55(2):116–129. [PMC free article] [PubMed] [Google Scholar]
- Ward M. E., Watt P. J., Robertson J. N. The human fallopian tube: a laboratory model for gonococcal infection. J Infect Dis. 1974 Jun;129(6):650–659. doi: 10.1093/infdis/129.6.650. [DOI] [PubMed] [Google Scholar]
- Watanabe T. Proteolytic activity of Mycoplasma salivarium and Mycoplasma orale 1. Med Microbiol Immunol. 1975;161(2):127–132. doi: 10.1007/BF02121754. [DOI] [PubMed] [Google Scholar]
- Westerberg S. C., Smith C. B., Wiley B. B., Jensen C. Mycoplasma-virus interrelationships in mouse tracheal organ cultures. Infect Immun. 1972 Jun;5(6):840–846. doi: 10.1128/iai.5.6.840-846.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]




