Abstract
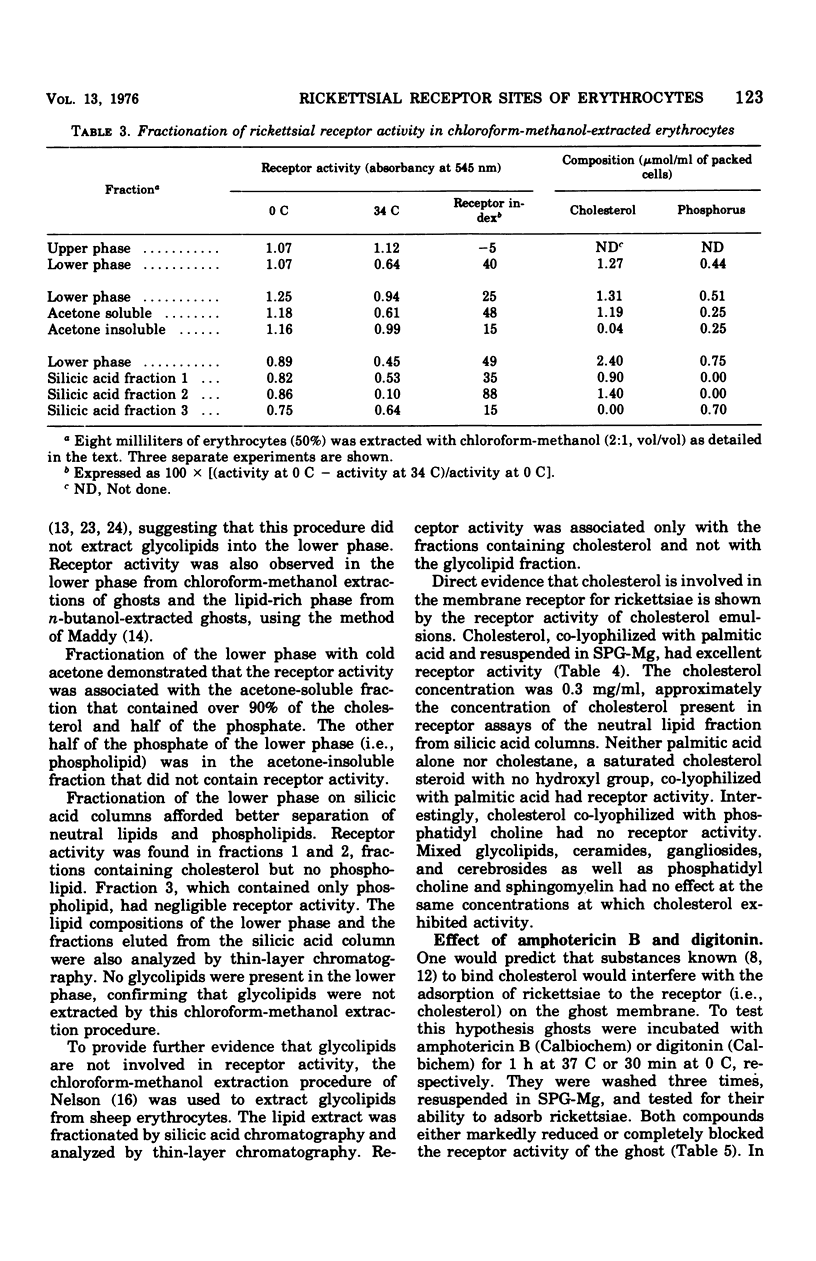
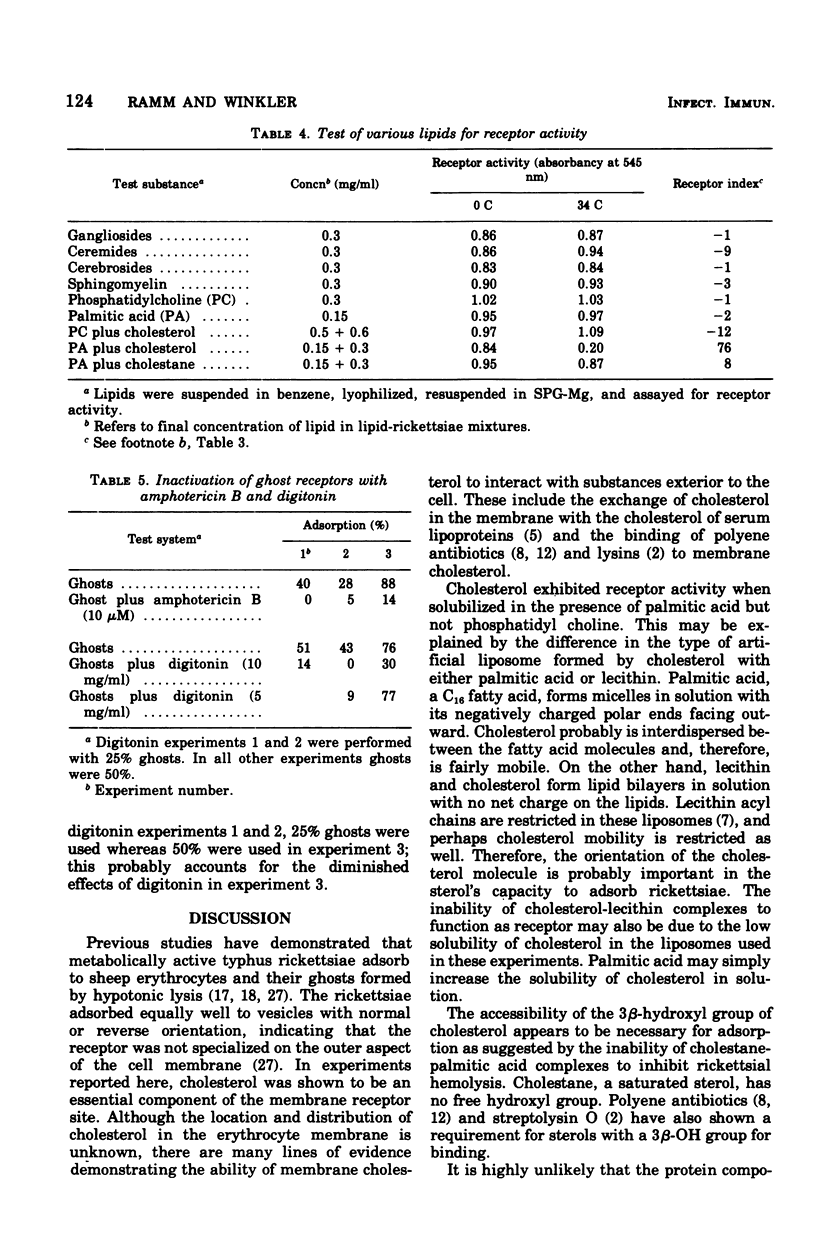
Cholesterol was identified as an essential component of the receptor on the sheep erythrocyte to which Rickettsia prowazeki adsorbs before lysing the cell. Erythrocytes or ghosts, derived by hypotonic lysis, were treated with proteolytic enzymes, sialidase, sulfhydryl reagents, and periodate without affecting their ability to adsorb rickettsiae. Lipid extracts of ghosts and erythrocytes, on the other hand, contained receptor activity. Fractionation of the lipid extracts by silicic acid column chromatogrphy resulted in the isolation of receptor activity in a neutral lipid fraction. The lipid fractions demonstrated receptor acitity at 34 C but not at 0 C. These properties are also characteristic of the receptor activity with erythrocytes and ghosts. Cholesterol, co-lyophilized with palmitic acid, was found to possess receptor activity. Palmitic acid alone, cholesterol-lecithin, cholestane-palmitic acid, and various phospholipids and glycolipids had no receptor activity. Ghosts treated with amphotericin B or digitonin, compounds that bind to cholesterol in the membrane, lost their ability to adsorb rickettsiae.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMES B. N., DUBIN D. T. The role of polyamines in the neutralization of bacteriophage deoxyribonucleic acid. J Biol Chem. 1960 Mar;235:769–775. [PubMed] [Google Scholar]
- BOVARNICK M. R., MILLER J. C., SNYDER J. C. The influence of certain salts, amino acids, sugars, and proteins on the stability of rickettsiae. J Bacteriol. 1950 Apr;59(4):509–522. doi: 10.1128/jb.59.4.509-522.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruckdorfer K. R., Green C. The exchange of unesterified cholesterol between human low-density lipoproteins and rat erythrocyte 'ghosts'. Biochem J. 1967 Jul;104(1):270–277. doi: 10.1042/bj1040270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuatrecasas P. Insulin receptor of liver and fat cell membranes. Fed Proc. 1973 Aug;32(8):1838–1846. [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Greenaway P. J., LeVine D. Binding of N-acetyl-neuraminic acid by wheat-germ agglutinin. Nat New Biol. 1973 Feb 7;241(110):191–192. doi: 10.1038/newbio241191a0. [DOI] [PubMed] [Google Scholar]
- Kinsky S. C., Luse S. A., Zopf D., van Deenen L. L., Haxby J. Interaction of filipin and derivatives with erythrocyte membranes and lipid dispersions: electron microscopic observations. Biochim Biophys Acta. 1967;135(5):844–861. doi: 10.1016/0005-2736(67)90055-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maddy A. H. The properties of the protein of the plasma membrane of ox erythrocytes. Biochim Biophys Acta. 1966 Mar 28;117(1):193–200. doi: 10.1016/0304-4165(66)90166-8. [DOI] [PubMed] [Google Scholar]
- Marchesi V. T., Andrews E. P. Glycoproteins: isolation from cellmembranes with lithium diiodosalicylate. Science. 1971 Dec 17;174(4015):1247–1248. doi: 10.1126/science.174.4015.1247. [DOI] [PubMed] [Google Scholar]
- Nelson G. J. Lipid composition of erythrocytes in various mammalian species. Biochim Biophys Acta. 1967 Oct 2;144(2):221–232. doi: 10.1016/0005-2760(67)90152-x. [DOI] [PubMed] [Google Scholar]
- Ramm L. E., Winkler H. H. Rickettsial hemolysis: adsorption of rickettsiae to erythrocytes. Infect Immun. 1973 Jan;7(1):93–99. doi: 10.1128/iai.7.1.93-99.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramm L. E., Winkler H. H. Rickettsial hemolysis: effect of metabolic inhibitors upon hemolysis and adsorption. Infect Immun. 1973 Apr;7(4):550–555. doi: 10.1128/iai.7.4.550-555.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouser G., Kritchevsky G., Simon G., Nelson G. J. Quantitative analysis of brain and spinach leaf lipids employing silicic acid column chromatography and acetone for elution of glycolipids. Lipids. 1967 Jan;2(1):37–40. doi: 10.1007/BF02531998. [DOI] [PubMed] [Google Scholar]
- SNYDER J. C., BOVARNICK M. R., MILLER J. C., CHANG R. S. M. Observations on the hemolytic properties of typhus rickettsiae. J Bacteriol. 1954 Jun;67(6):724–730. doi: 10.1128/jb.67.6.724-730.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabet S. F., Schnaitman C. A. Localization and solubilization of colicin receptors. J Bacteriol. 1971 Oct;108(1):422–430. doi: 10.1128/jb.108.1.422-430.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siakotos A. N. Analytical separation of nonlipid water soluble substances and gangliosides from other lipids by dextran gel column chromatography. J Am Oil Chem Soc. 1965 Nov;42(11):913–919. doi: 10.1007/BF02632444. [DOI] [PubMed] [Google Scholar]
- Strauss J. H., Jr, Burge B. W., Darnell J. E. Carbohydrate content of the membrane protein of Sindbis virus. J Mol Biol. 1970 Feb 14;47(3):437–448. doi: 10.1016/0022-2836(70)90313-x. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- WISSEMAN C. L., Jr, JACKSON E. B., HAHN F. E., LEY A. C., SMADEL J. E. Metabolic studies of rickettsiae. I. The effects of antimicrobial substances and enzyme inhibitors on the oxidation of glutamate by purified rickettsiae. J Immunol. 1951 Aug;67(2):123–136. [PubMed] [Google Scholar]
- Winkler H. H., Ramm L. E. Adsorption of typhus rickettsiae to ghosts of sheep erythrocytes. Infect Immun. 1975 Jun;11(6):1244–1251. doi: 10.1128/iai.11.6.1244-1251.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Gier J., Mandersloot J. G., van Deenen L. L. The role of cholesterol in lipid membranes. Biochim Biophys Acta. 1969 Jan 28;173(1):143–145. doi: 10.1016/0005-2736(69)90045-5. [DOI] [PubMed] [Google Scholar]
- de Kruijff B., Gerritsen W. J., Oerlemans A., Demel R. A., van Deenen L. L. Polyene antibiotic-sterol interactions in membranes of Acholeplasma laidlawii cells and lecithin liposomes. I. Specificity of the membrane permeability changes induced by the polyene antibiotics. Biochim Biophys Acta. 1974 Feb 26;339(1):30–43. doi: 10.1016/0005-2736(74)90330-7. [DOI] [PubMed] [Google Scholar]


