Abstract
Preventing mucosal transmission of HIV is critical to halting the HIV epidemic. Novel approaches to preventing mucosal transmission are needed. Hyaluronic acid (HA) is a major extracellular component of mucosa and the primary ligand for the cell surface receptor CD44. CD44 enhances HIV infection of CD4+ T cells, but the role of HA in this process is not clear. To study this, virions were generated with CD44 (HIVCD44) or without CD44 (HIVmock). Exogenous HA reduced HIV infection of unstimulated CD4+ T cells in a CD44-dependent manner. Conversely, hyaluronidase-mediated reduction of endogenous HA on the cell surface enhanced HIV binding to and infection of unstimulated CD4+ T cells. Exogenous HA treatment reduced activation of protein kinase C alpha via CD44 on CD4+ T cells during infection with HIVCD44. These results reveal new roles for HA during the interaction of HIV with CD4+ T cells that may be relevant to mucosal HIV transmission and could be exploitable as a future strategy to prevent HIV infection.
Prevention of HIV transmission is still the most direct way to stem the HIV/AIDS epidemic.1 However, to date, large-scale clinical trials of vaccines to produce an HIV-specific antibody or a T-cell response to prevent HIV infection have been disappointing.2, 3 As 80% of HIV infection occurs through sexual contact,4 there is intense interest in the prevention of HIV mucosal transmission. To design a better strategy to prevent mucosal transmission of HIV, we need to more fully understand the mechanism of HIV mucosal transmission.5
Mucosal tissues are the front-line defense against pathogen invasion and greatly impede HIV transmission. Studies using the simian immunodeficiency virus (SIV) rhesus macaque model demonstrate that the genital tract mucosal barrier limits exposure of CD4+ T cells, dendritic cells and macrophages to the majority of the viral inoculum, and only a small number of infectious virions pass through the mucosal barrier to establish the infected founder population.6, 7 These findings are confirmed by clinical studies showing that a small number of infectious virions breach the mucosal barrier to infect resting CD4+ T cells, generating a clonal or oligoclonal founder population.5, 8, 9
Mucosal integrity has an important role in HIV transmission, and mucosal inflammation can increase HIV transmission.10, 11, 12 The mucosal tissues are composed of epithelial cells, extracellular matrix, interstitial cells and surface mucus. In addition to providing a full complement of host immune cells that variably facilitate or impede HIV infection, the mucosal surface also serves as a physical barrier to mucosal HIV invasion. Mucosal mucus can trap HIV virions13 and reduce virion movement.14 An acidic vaginal mucosal environment can decrease the rate of HIV sexual transmission.15 How these effects on mucosal HIV transmission are mediated remains largely unknown.5, 9
The surface of the mucosal layer is a scaffold with extracellular matrix; a major component of the extracellular matrix is hyaluronic acid (HA, or hyaluronan). HA is a large glycosaminoglycan that can be remodeled and degraded by hyaluronidase. On the surface of the cells, HA polymers extend up to 25 μm in length, forming pericellular coats. HA interaction with its receptors can induce cellular signaling and is involved in mucosal tissue homeostasis and maintenance of tissue integrity.16, 17, 18 HA is also a regulator of immunity. HA interaction with its main receptor, CD44, regulates recruitment and extravasation of T cells into sites of inflammation19, 20 and participates in the inflammatory process.16, 21 HA interaction with CD44 can reduce cytokine production from macrophages in the setting of inflammation22 and lowers protein kinase C alpha (PKCa) activity to decrease histamine release from leukemic cell lines.23
There are reasons to believe that HA–CD44 receptor interactions may influence mucosal transmission of HIV. Clinical studies have found that mucosal integrity, activation of T cells and secretion of cytokines are each involved in mucosal HIV transmission,5, 9 and each is modulated by HA–CD44 receptor binding. Studies have also reported that the primary HA receptor, CD44, is incorporated into HIV-1 virions24, 25 and that CD44 on the HIV virion surface maintains its biological function, such as binding to HA.26 Moreover, CD44 on HIV virions enhances HIV-1 infectivity for primary CD4+ T cells.27 However, the effect of HA on HIV-1 infectivity remains poorly understood. The main aim of this study was to assess the role of HA in HIV infection. We observed that exogenous HA reduced HIV infectivity when both virions and CD4+ T cells expressed CD44. Effects were seen on both early infection events like viral binding and probably later events through reduction of PKCa activation, whereas treatment with hyaluronidase reduced endogenous HA thickness and enhanced susceptibility of CD4+ T cells to infection.
Results
Exogenous HA reduces HIV infectivity on unstimulated peripheral blood mononuclear cells, but only for virus bearing CD44
CD44 is found on HIV virions from either peripheral blood mononuclear cell (PBMC) cultures24 or directly in patient plasma.25 In contrast, 293T cells do not express appreciable levels of endogenous CD44.27 Transfection of an expression vector containing CD44 complementary DNA (cDNA) from donor CD4 T cells resulted in CD44 protein expression in 293T cells (Supplementary Figure 1), and this expressed CD44 protein was able to bind to HA (Supplementary Figure 2). Simultaneous transfection of the cloned CD44 expression vector with pNL4-3 into 293T cells was used to generate HIV virions containing CD44 (HIVCD44), whereas cotransfection of pNL4-3 along with an empty vector (pcDNA3.1) produced virions without CD44 (HIVmock). CD44 is a transmembrane protein whose extracellular domain can be cleaved by membrane proteases.28, 29 To test whether virions produced by CD44+ cells contain CD44, we utilized a centrifugation-based method30 for concentrating virions that should exclude free protein present in the supernatant. Supernatants were obtained from cells transfected with HIV plus empty vector (HIVmock), CD44 plus empty vector (CD44 alone) or CD44 plus HIV (HIVCD44), and aliquots of supernatant and virions (resuspended from centrifuged supernatant) were tested for CD44 by enzyme-linked immunosorbent assay (ELISA). No CD44 was detected in supernatant or virions from cells transfected with HIV plus empty vector. CD44 was present in similar concentrations in supernatants from cells transfected with CD44 plus empty vector and CD44 plus HIV, but was enriched only in the virions from cells transfected with CD44 plus HIV (Supplementary Figure 3). Both viral stocks (HIVCD44 and HIVmock) were tested on the reporter T cell line M7-Lue, which contains the luciferase gene under the control of the HIV long terminal repeat (LTR), providing a measure of infectivity.31 In the presence of 10% fetal calf serum (FCS), viral stock containing CD44 (HIVCD44) demonstrated higher infectivity than HIV without CD44 (HIVmock) in both M7-lue cell line (Supplementary Figure 4) and primary CD4 T cells (Supplementary Figure 5). HIV produced from 293T cells cotransfected with CD44 (HIVCD44) has a similar infectivity compared with HIV produced by PBMCs (HIVPMBC). This was not true for HIV generated from 293T cells without CD44 (HIVmock) (Supplementary Figure 6). Reports have indicated that FCS not only contains HA but it can also influence HA synthesis32 and hyaluronidase activity.33 To limit the confounding effects of FCS during the study of HA effects on HIV infection, we removed FCS when the cells inoculated with HIV. This resulted in reduced infectivity of viral stocks irrespective of virion CD44 expression (Supplementary Figure 7).
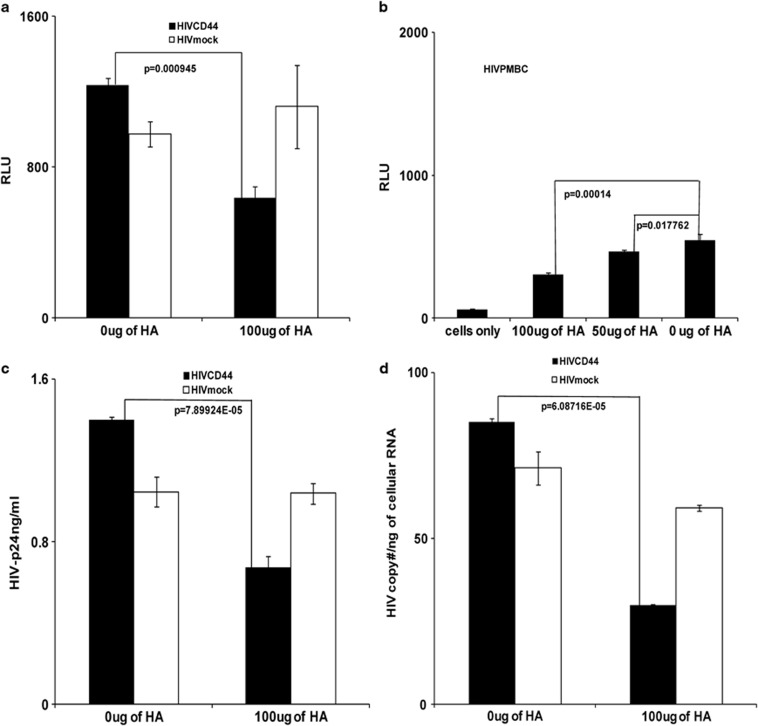
Earlier studies reported that exogenous HA could interrupt T-cell rolling and impede extravasation of T cells into an inflamed site through interactions with CD44.19 To study whether exogenous HA can impact HIV infectivity, M7-Lue cells were treated with exogenous HA for 1 h, then inoculated with HIVCD44 or HIVmock produced from 293T cells and normalized by p24. HIV infectivity was measured at day 3 post infection by luciferase activity as relative light units. Exogenous HA reduced infectivity of HIVCD44 significantly but had no effect on the infectivity of HIVmock (Figure 1a). This inhibitory effect of exogenous HA for HIV containing CD44 was confirmed in experiments with HIV produced from PBMCs (HIVPMBC), which contain CD44.25, 26 The reduction of infectivity of HIV by HA was dosage dependent (Figure 1b).
Figure 1.
Exogenous HA reduced infectivity of HIV virions containing CD44. (a and b) M7-Lue cell assays. Relative light unitsis a measurement of luciferase activity. (a) Exogenous HA (Sigma) reduced infectivity of HIVCD44 (filled bar), but not HIVmock (open bar). (b) Exogenous HA decreased HIVPBMC infectivity in a dose-dependent fashion. (a and b: data are mean±s.e.m. of triplicate samples and represent three independent experiments). (c and d) Primary healthy donor unstimulated PBMCs' assays. (c) Exogenous HA reduced HIV-1 p24 production from HIVCD44 (filled bar) infection, but failed to reduce HIVmock (open bar) infection. (d) Exogenous HA lowered HIV RNA in T-20-treated cells infected with HIVCD44 (filled bar), but not HIVmock (open bar). (c and d: data are mean±s.e.m. of duplicate samples and representative of three donors.)
To further confirm that exogenous HA treatment can reduce infectivity of HIV containing CD44, unstimulated, primary PBMCs from healthy donors were treated with exogenous HA, and then inoculated with viral stocks of HIVCD44 or HIVmock normalized by p24, then stimulated with phytomeagglutinin (PHA) and IL-2 for 24 h. HIV infectivity was assessed by measurement of supernatant HIV-1 p24 7 days post infection. Exogenous HA treatment significantly reduced HIV-1 p24 production during HIVCD44 infection, but had no impact on p24 production during HIVmock infection (Figure 1c).
Because exogenous HA was added before HIV infection, we attempted to distinguish effects of HA on binding vs entry by using the entry inhibitor enfuvirtide (T-20).34 Unstimulated, primary PBMCs were treated with exogenous HA for 1 h, then exposed to an equivalent inoculum by p24 of HIVCD44 or HIVmock for 5 h in the presence of 10 μg ml−1 of enfuvirtide (T-20). Afterwards, the cells were extensively washed, and the cellular HIV RNA was measured. Exogenous HA treatment significantly reduced cell surface HIV RNA in unstimulated PBMC infected with HIVCD44, but not cell surface HIV RNA in HIVmock infection (Figure 1d). These results indicate that exogenous HA may interfere with HIV binding, but only for HIV bearing CD44.
CD44 on recipient cells is involved in exogenous HA's reduction of HIV infection
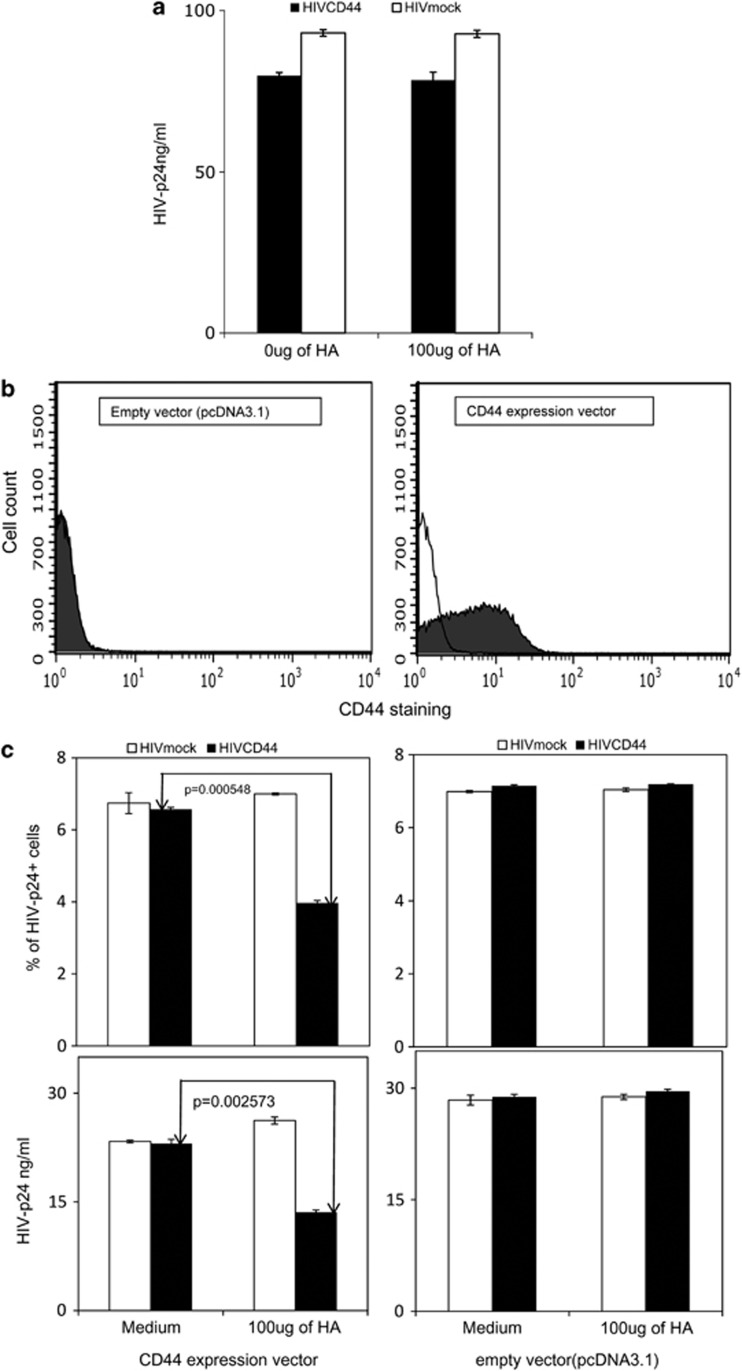
Although CD44 is the principal receptor for HA, HA also binds to other receptors on the cell surface, such as the receptor for hyaluronan-mediated motility (RHAMM), tumor necrosis factor (TNF)-stimulated gene-6/TNFα-induced protein six (TSG-6), lymphatic vessel endothelial HA receptor (LYVE-1), hyaluronan receptor for endocytosis (HARE), liver endothelial cell clearance receptor (LEC receptor) and TLR-4.16, 17 To assess whether CD44 on the target cell is required to reveal the inhibitory effect of exogenous HA on HIV infectivity, a cell line lacking expression of the CD44, the Jurkat-E6.1 cell line35 was studied. Jurkat-E6.1 cells were treated with exogenous HA before exposure to either HIVCD44 or HIVmock normalized by p24. HIV infectivity was assessed by measuring HIV-1 p24 production on day 7 post infection. Exogenous HA treatment had no impact on production of HIV-1 p24 for either HIVCD44 or HIVmock (Figure 2a). Next, Jurkat-E6.1 cells were transfected with either the CD44 expression vector or empty vector (pcDNA3.1). At 3 days post transfection, the cells were examined by flow cytometry (fluorescence-activated cell sorting) after staining with anti-human CD44 antibody. Fifty-three percent of the cells that were transfected with the CD44 expression vector showed CD44 protein expression in the cells (Figure 2b), but CD44 staining was absent in cells transfected with the empty vector. Next, both CD44-transfected and empty vector-transfected Jurkat-E6.1 cells were treated with exogenous HA before infection with either HIVCD44 or HIVmock normalized by p24; HIV infectivity was assessed by measuring HIV-1 p24 intracellular staining, and HIV-1 p24 on day 3 post infection. Exogenous HA reduced HIV-p24 intracellular staining and HIV-p24 production of HIVCD44 but not HIVmock in CD44-transfected E6.1 cells, whereas HA did not affect the ability of either virus to infect empty vector-transfected Jurkat-E6.1 cells (Figure 2c, Supplementary Figure 8). These data indicate that CD44 expression on target cells is also required for exogenous HA to reduce infection by HIVCD44.
Figure 2.
Exogenous HA-mediated reduction of HIV infection required CD44 on target cells. (a) Exogenous HA (Sigma) had no impact on either HIVCD44 (filled bar) or HIVmock (open bar) infection of Jurkat-E6.1 cells, which lack CD44. (b) Jurkat-E6.1 cells expressed CD44 after transfection with a CD44 expression vector, but not with an empty vector (pcDNA3.1). Intracellular staining was performed using isotype control (R&D systems; open histogram) or anti-human CD44-APC (R&D systems; closed histogram). (c) On top is percent of intracellular staining of HIV-p24+ cells (KC-57RD1-PE; Beckman Coulter) and bottom is the amount of HIV-p24 in the supernatant. Exogenous HA reduced HIV-p24 staining in CD44-transfected Jurkat cells infected with HIVCD44, but not HIVmock. Exogenous HA had no effect on HIV-p24 staining when either HIVCD44 or HIVmock were used to infect Jurkat-E6.1 cells transfected with empty vector. Viral production was assessed by measurement of p24 in the supernatant. The effects of HA were analogous to those seen with intracellular staining for p24 in c. (Data in a are mean±s.e.m. of triplicate samples and representative of three independent experiments, whereas data in b and c are from duplicate samples and representative of two independent experiments.)
Hyaluronidase treatment can enhance HIVCD44 infectivity on unstimulated CD4+ T cells
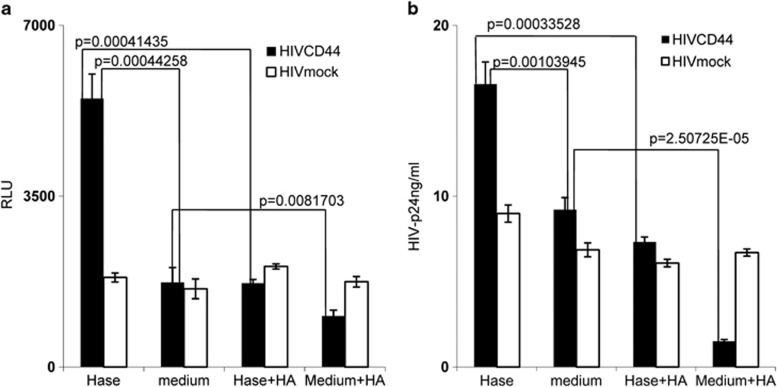
Endogenous HA has been observed to form a cushion on the cell surface, to have an anti-adhesive role18 and to impede ligand access to cell surface receptors.36 Hyaluronidase treatment can reduce endogenous HA from the cell surface, thus promoting cell–cell contact.17 To determine whether endogenous HA on cell surfaces has an effect on HIV infection, M7-Lue cells were treated with hyaluronidase and then exposed to p24-equivalent inocula of HIVCD44 or HIVmock. HIV infectivity was assessed on day 3 post infection by measuring luciferase activity. Hyaluronidase treatment significantly enhanced infectivity of HIVCD44; however, this enhancement was reduced when exogenous HA was added. In contrast, hyaluronidase treatment had no effect on infectivity of HIVmock (Figure 3a). This latter observation indicates that the inhibitory effect of HA for HIV infectivity is not simply through nonspecific steric hindrance.
Figure 3.
Hyaluronidase treatment enhanced HIV infection of unstimulated CD4+ T cells. Hase (hyaluronidase treatment; Sigma); medium (medium treatment); Hase+HA (hyaluronidase treatment with 100 μg of exogenous HA (Sigma)); and medium+HA (medium treatment with 100 μg of exogenous HA). (a) Hyaluronidase (Hase) treatment enhanced HIVCD44 (filled bar) infection of M7-lue cells, but exogenous HA reversed the enhancement (Hase+HA). However, hyaluronidase (Hase) treatment failed to impact HIVmock (open bar) infectivity. (All data are mean±s.e.m. of triplicate samples and representative of three independent experiments). (b) Treatment of healthy donor unstimulated CD4+ T cells by hyaluronidase (Hase) boosted HIVCD44 (filled bar) infection, but not HIVmock (open bar) infection, and exogenous HA reduced this enhancement (Hase+HA). (All data are mean±s.e.m. from duplicate samples and representative of at least three donors.)
In vivo studies of acute HIV and SIV infection demonstrate that virus initially targets resting CD4+ T cells and establishes an infected founder population during mucosal transmission.5, 8, 9 Hyaluronidase is highly activated during sexual intercourse as well as inflammation.16, 17, 18 Our initial experiments showed that exogenous HA reduced HIV infection of unstimulated PBMCs. Thus, we studied the impact of hyaluronidase treatment on HIV infection of primary unstimulated CD4+ T cells. Healthy donor, unstimulated, primary CD4+ T cells were treated with hyaluronidase before inoculation with an equivalent input (by p24) of either HIVCD44 or HIVmock, then stimulated with PHA and interleukin (IL)-2 for 24 h. At day 7 post infection, infectivity was assessed by measurement of HIV-1 p24 in culture supernatants. Consistent with the ability of endogenous HA to inhibit HIV infection, hyaluronidase treatment significantly increased HIVCD44 infection of unstimulated CD4+ T cells, but this enhancement could be completely reversed by addition of exogenous HA. However, hyaluronidase treatment had no effect on infectivity of HIVmock (Figure 3b). These results indicate that hyaluronidase treatment renders unstimulated CD4+ T cells more susceptible to HIVCD44 infection, possibly through reduction of cell surface HA.
Hyaluronidase treatment reduces the thickness of endogenous HA on unstimulated CD4+ T-cell surface and enhances HIV binding
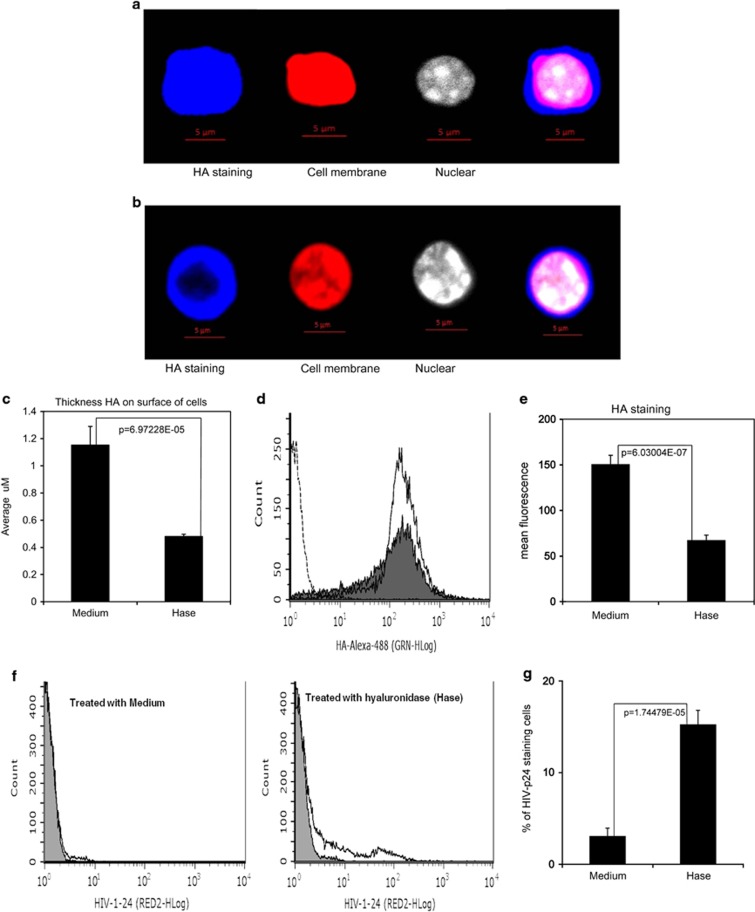
Next, we investigated how hyaluronidase treatment impacts endogenous HA on the surface of unstimulated CD4+ T cells. Unstimulated, primary CD4+ T cells were treated with hyaluronidase, stained with biotinylated hyaluronan-binding protein37 and studied using laser scanning microscopy Z-stack projections (Supplementary Figure 9). Hyaluronidase treatment significantly reduced endogenous HA thickness on surface of the cells (Figures 4a–c). This conclusion was corroborated by flow cytometry, which revealed that hyaluronidase treatment did not reduce the numbers of HA-positive cells (Figure 4d), but significantly reduced the mean fluorescence intensity of HA staining (Figure 4e). Because the thickness of endogenous HA has been postulated to prevent ligand binding to cognate receptors on the surface of T cells36 and hyaluronidase treatment enhanced infectivity of HIVCD44, we hypothesized that hyaluronidase-mediated reduction of HA on the cell surface may enhance HIV binding. To test this hypothesis, unstimulated, primary CD4+ T cells were treated with hyaluronidase and then incubated with HIVPBMC in the presence 10 μg ml−1 of the peptide fusion inhibitor T-2032 for 5 h. Cells were washed five times to remove unbound HIV, stained with anti-HIV-1 p24 antibody and assessed by flow cytometry. 15.4% of hyaluronidase-treated cells stained positive for HIV-p24 (Figure 4f), compared with only 3% of untreated cells (P<0.001) (Figure 4g). These data indicate that endogenous HA on the cell surface can modulate HIVPBMC binding to unstimulated CD4+ T cells.
Figure 4.
Hyaluronidase reduced the thickness of surface HA on unstimulated CD4+ T cells and enhanced HIV binding. (a–c) The images are Z-stack projections from laser scanning microscopy analysis of cell surface HA on CD4 T cells. Images are 40 × ; HA-staining withAlexa488 (blue); cell membrane staining with Texas Red (Life Technologies); and nuclear staining with DAPI (Life Technologies). (a) CD4 T cells without hyaluronidase treatment, and (b) CD4 T cells with hyaluronidase treatment. (c) The thickness of HA on the surface of the cell, from an average of 20 cells after hyaluronidase treatment (Hase) or no treatment (Medium). (d and e) A total of 50 000 cells of each staining were measured by flow cytometry (fluorescence-activated cell sorting (FACS)): healthy primary unstimulated CD4 T cells were treated with or without hyaluronidase (Hase; Sigma) for 1 h, then stained with Alexa488-labeled HA-binding protein. (d) FACS result showing cells without HA staining (dash line), cells treated with hyaluronidase (closed histogram) and cells treated with control medium (open histogram) followed by HA staining. (e) The mean fluorescence after treatment with hyaluronidase (Hase) or medium (Medium). (f and g) HIV-1 p24 staining on unstimulated CD4+ T cells. A total of 100 000 cells of each unstimulated CD4+ T cells were treated with or without hyaluronidase for 1 h, incubated with HIVPMBCs for 5 h, then stained mouse anti-HIV-1p24 (Santa Cruz Biotech) and Donkey anti-mouse IgG antibody with AlexaFluor@680 (Life Technologies). (f) FACS result showing cells treated with hyaluronidase (Hase) or medium (Medium) and stained with isotype control (closed histogram) or HIV-1p24-Alexa-680 (open histogram). (g) Percent of HIV-1-p24-positive cells treated with hyaluronidase (Hase) or with medium (Medium). (All data are mean±s.e.m. from duplicate experiments and representative of at least three donors.)
PKCa signaling is important for reduction of HIVCD44 infection of unstimulated CD4+ T cells by exogenous HA
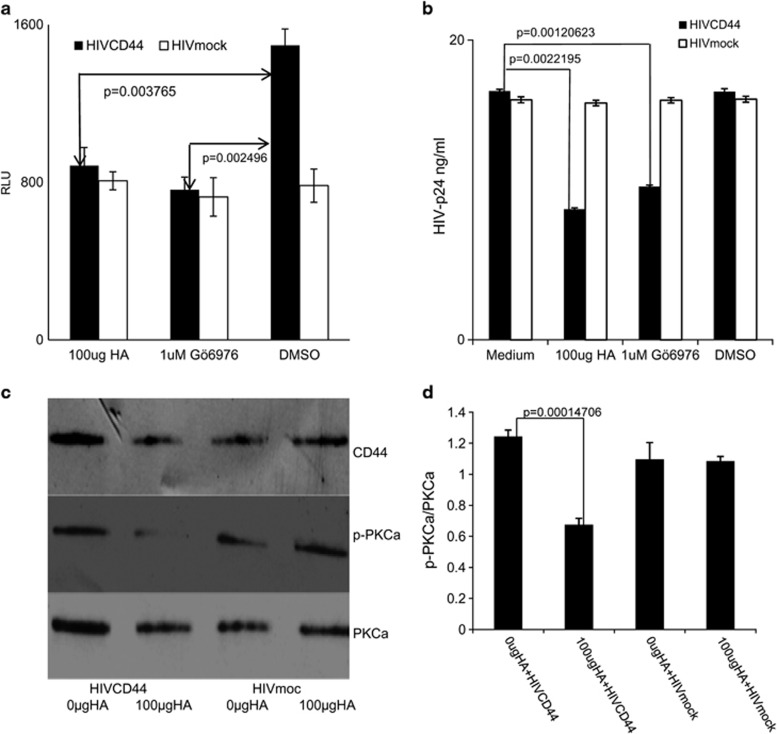
Others have reported that interaction of exogenous HA with CD44 can reduce PKCa activation,23 that PKCa has a critical role in HIV infectivity38 and that the selective PKCa inhibitor ‘Gö6976' can inhibit HIV infection.39 To further investigate the role of PKCa in HA–CD44 modulation of HIV infectivity, M7-Lue cells were treated with Gö6976, dimethylsulphoxide (DMSO) or exogenous HA, then incubated with either HIVCD44 or HIVmock normalized by p24. HIV infectivity was assessed on day 3 post infection by measurement of luciferase activity. Gö6976 significantly reduced infectivity of HIVCD44 compared with the DMSO control, and the magnitude of this reduction was comparable to exogenous HA treatment. However, Gö6976 had no impact on the infectivity of HIVmock (Figure 5a). Next, unstimulated, primary CD4+ T cells from healthy controls were first treated with either Gö6976 or exogenous HA, then exposed for 5 h to either HIVCD44 or HIVmock normalized by p24. The toxicity of these treatments on the primary unstimulated CD4+ T cells was evaluated using the ViaCount assay (Guava, Millipore, Billercia, MA, USA), and neither cell number nor viability was affected (Supplementary Figure 10). The infectivity of HIV was assessed 7 days post infection by measurement of HIV-1 p24 in the supernatant. As expected, both Gö6976 and exogenous HA significantly reduced infectivity of HIVCD44, but had no effect on infectivity of HIVmock (Figure 5b). These results suggest that CD44 is involved in reduction of HIV infection by both Gö6976 and exogenous HA and that both treatments have a similar effect on HIV infection.
Figure 5.
PKCa is involved in exogenous HA-mediated reduction of HIV infectivity. Gö6976 treatment reduced HIVCD44 infection (filled bar) to a degree similar to exogenous HA in M7-Lue cells (a) and primary healthy donor unstimulated CD4+ T cells (b). However, Gö6976 treatment had no impact on infection of HIVmock (open bar). DMSO (Sigma) is a solvent for Gö6976 (Sigma). (All data are mean±s.d. of triplicate samples and representative of three independent experiments). (c) CD44 co-immunoprecipitation western blot. Unstimulated CD4+ T cells were treated with 0 μg or 100 μg of exogenous HA (Sigma), then infected with either HIVCD44 or HIVmock for 5 h. Cellular protein was extracted and immunoprecipitated with anti-CD44 antibody. The western blots were done using three antibodies: (1) anti-human CD44 (CD44); (2) anti-phosphorylated PKCa (p-PKCa); and (3) anti-PKCa (PKCa). (d) The ratio of p-PKCa and PKCa from the mean intensity of each band shown in c. (All data are mean±s.e.m. from duplicate experiments representing at least three donors.)
From these results, we hypothesize that reduction of infectivity of HIVCD44 by exogenous HA may involve the PKCa signaling pathways distal to CD44. To test this hypothesis, we evaluated PKCa phosphorylation, which corresponds to activation of PKCa,23, 40 in unstimulated CD4+ T cells treated with exogenous HA and exposed to HIV. Following treatment with exogenous HA and exposure to HIV, CD4+ T cells were lysed and cellular proteins were extracted, co-immunoprecipitated with anti-CD44 antibody and analyzed using western blot. Treatment with exogenous HA had no effect on the amount of total PKCa protein in any condition (Figure 5c), but phosphorylated PKCa was reduced following exogenous HA treatment, and the ratio of phosphorylated PKCa/PKCa was significantly reduced in exogenous HA-treated unstimulated CD4+ T cells infected with HIVCD44 (Figure 5d). These results suggest that reduction of HIVCD44 infectivity of unstimulated CD4 T cells by exogenous HA is mediated by inhibition of PKCa activation.
Discussion
Devising more effective interventions to prevent mucosal HIV transmission will require a deeper understanding of the interplay between virus and target cells in the mucosal environment. Studies of both human and rhesus macaque models indicate that mucosal tissues provide multiple mechanisms to prevent HIV and SIV infection, including physical barriers and innate and adaptive immune responses.5, 9 However, a small amount of HIV-1 and SIV can penetrate the mucosal barrier, gaining access to the submucosa and the initial recipient cell targets, which appear to be predominantly resting CD4+ T cells.8 Details of the interaction of HIV and SIV with resting CD4+ T cells in the submucosa are not fully understood.5, 9 In this study, we show for the first time that endogenous HA on unstimulated CD4+ T cells is an obstacle for HIV infection and that hyaluronidase treatment can reduce endogenous HA, increase binding of HIV particles and enhance HIV infectivity. Furthermore, addition of exogenous HA at 100 μg ml−1, the concentration of HA in human epidermis,41 reduces HIV infectivity on unstimulated CD4+ T cells. These data collectively argue that HA interferes with HIV infection during early stage, perhaps by directly impacting the interactions between CD44 and other molecules, or altering the distribution and/or activation status of local CD4+ T cells, thus reducing targets that are needed for efficient transmission.
HA is the main component of the extra cellular matrix in the mucosal tissue. The main receptor for HA, CD44, is highly expressed in all cells, including CD4+ T cells, and in mucosal tissues, especially those along the reproductive tract.18, 42 HA interactions with CD44 are involved in mucosal immunity and participate in mucosal inflammation.16, 21 It has been reported that exogenous HA can interfere with interaction of endogenous HA with CD44, limiting recruitment of T cells by reducing T-cell adhesion,43 preventing T-cell rolling and inhibiting T-cell extravasation into an inflamed site.19, 20 Other studies identify the recruitment of T cells into submucosa as important and necessary for mucosal HIV transmission.5, 9
Our study demonstrates that exogenous HA reduces HIV infection of unstimulated PBMCs and unstimulated CD4+ T cells and that this reduction of HIV infectivity is CD44 dependent. This inhibitory effect of HA was only observed when both HIV virions (HIVCD44 and HIVPBMC) and recipient cells (Figure 2c) were CD44+. These results indicate that exogenous HA may reduce HIV infectivity by interfering with interactions between CD44 on HIV virions, endogenous HA (or other CD44 ligands) and CD44 on unstimulated CD4+ T cells. Others have reported that the endogenous HA coat on the surface of cells can prevent extraneous or indiscriminant ligand binding18 and can prevent infection with Newcastle disease virus, vesicular stomatitis virus and rubella virus. Reducing the thickness of HA on the cell surface by hyaluronidase treatment can make target cells more susceptible to Newcastle disease virus, vesicular stomatitis virus and rubella virus infection.44 We also observed that treatment of unstimulated CD4+ T cells with hyaluronidase reduced the thickness of endogenous HA on the cell surface, allowing more HIV particles to bind (Figures 4c and d), an effect that could be reversed by the addition of exogenous HA (Figure 3b). Although these experimental results suggest that the inhibitory effects of HA on HIV infection occur at least in part at an early stage of the viral life cycle, including effects localizable to viral binding, further work is needed.
Interactions of HA with CD44 induce cellular signaling and regulate immune responses.21 Others have observed that exogenous HA can reduce PKCa activity through CD4423 to decrease expression of inflammatory regulators such as TNFa, IL-6, IL-18 and MMP-7.45 PKCa is a classic isoform of PKC that is highly expressed in CD4 T cells.46 PKCa and CD44 are localized in lipid microdomains (lipid rafts),47 and HIV engagement with CD4 in lipid rafts is required for HIV infection.48, 49 In turn, PKCa has been implicated as important for HIV infection,38, 39 and Gö6976, a PKCa selective inhibitor, has been observed to reduce HIV infection.39 This study is the first to show that virion CD44 is necessary for the reduction of HIV infectivity by Gö6976 and that Gö6976 treatment has an effect analogous to treatment with exogenous HA (Figures 5a and b). This finding is compatible with a model where PKCa signaling is triggered by bridging of virion and cell surface CD44 by multivalent ligands. Our findings are reminiscent of the report by Chang et al.50 that alpha-defensin-1 reduces HIV infectivity through decreased cellular phosphorylation of PKC, but not the amount of PKC protein. These investigators further determined that blocking phosphorylation of PKC resulted in inhibitory effects at later stages of the viral life cycle, including nuclear import and viral transcription. Such effects therefore represent at least a second, later stage in the viral life cycle that can be modulated by manipulating HA–CD44 interactions.
These findings are also similar to other recent data showing that anti-CD44 antibody beads enhance HIV infection only for HIV produced by PBMC, but not for HIV produced by 293T cells.27 Under some conditions, different HA fragments are known to mediate CD44 interactions that augment T-cell activation.21 In the current work, a commercial source of HA was used, as has been reported by many other investigators.19, 34, 43 Although several experiments were performed with different sizes of HA (both high and low molecular weight), we did not observe consistent differences, but rather found that all sizes of exogenous HA inhibited HIV infection under the conditions tested (Supplementary Figure 11). One possible effect of exogenous HA regardless of size may be its ability to compete with ligands (perhaps including some natural species of HA) that bind to CD44. We also observed that exogenous HA reduced HIV infectivity in unstimulated CD4+ T cells to a degree greater than that observed in stimulated CD4+ T cells (Supplementary Figures 12 and 13). In such a model, excess exogenous HA may disrupt the optimal stoichiometry necessary for endogenous ligands to bind and bridge CD44, an effect that may be more important for the infection of resting CD4 T cells.
It is notable that HA, hyaluronidase and CD44 are all highly expressed and have important roles in maintaining the integrity and function of the mucosal tissue along the reproductive tract. Hyaluronidase is also a major constituent of semen.42 Both HA and hyaluronidase are highly upregulated during sexual intercourse as well as inflammation.18 The processes of reproductive fertilization and HIV transmission intersect at many levels anatomically and functionally. Both involve the reproductive tract and permissiveness of mucosal tissue and surface barriers. Clinical studies have demonstrated that inflammation in the mucosal tissue can facilitate HIV transmission,51 and that transmission rates are higher for HIV in semen compared with blood.52 The identification of topical agents that can be applied to mucosal surfaces to prevent mucosal HIV transmission has been identified as a priority area for research and development in the effort to control the HIV/AIDS epidemic.53
In summary, HA present on the surface of unstimulated CD4+ T cells can influence HIV infection. Both HA and hyaluronidase have well-described roles in reproductive and inflammatory processes. Our data now show that the status of the HA coat may have an important role in the initial interaction of HIV virions with resting CD4+ T cells, a feature that may be particularly relevant for mucosal HIV transmission. Intriguingly, exogenous HA appears to block HIV engagement and infection of target cells. Furthermore, HA interactions with virion and cell surface CD44 modulate PKCa activation and may have additional inhibitory effects on later stages of the viral life cycle. HA is a non-immunogenic natural biopolymer that has been used in a variety of clinical applications. These findings should be further explored in vivo and, if confirmed, could lead to the development of novel interventions to reduce HIV mucosal transmission.
Methods
CD44 gene construction
Cloning of CD44 gene
A cDNA of the standard isoform of human CD44 was generated from RNA extracted from healthy donor CD4 T cells using SuperScript III Reverse Transcriptase (Life Technologies, Carlsbad, CA, USA) according the manufacturer's instructions and amplified using Platinum TaqDNA High Fidelity Polymerase (Life Technologies). Oligonucleotides used in reverse-transcription PCR were 5-CD44 (5′-CAGCCTCTGCCAGGTTCGGTCCGCCATCCTCG-3′) and 3-CD44 (5′-TGAAGATCGAAGAAGTACAGATATTTATTATG-3′). The CD44 gene was cloned into pcDNA3.1/V5-His TOPO TA Expression vector (Life Technologies). The CD44 expression plasmid was purified and sequenced, and the CD44 clone was verified to be a perfect match to the standard isoform of human CD44 (GenBank AY101192.1).
Isolation of PBMCs and CD4 T cells from healthy donors
PBMCs were isolated from healthy donors as previously published,31 and CD4+ T cells were isolated from PBMCs using the EasySep Human CD4+ T cell Enrichment kit (STEMCell, Vancouver, BC, Canada) according to the manufacturer's instructions.
Generation of HIV virus stock with or without CD44 from 293T cells and from PBMCs
Viral stock generated from 293T cells
293T cells (ATCC, Manassas, VA, USA) were cultured in complete Dulbecco's modified Eagle's medium (Life Technologies) supplemented with penicillin (50 U ml−1), streptomycin (50 μg ml−1), 2 mM of L-glutamine (Life Technologies) and 10% heat-inactivated FCS (Sigma, St Louis, MO, USA), then cotransfected with 10 μg of pNL4-3 (NIH AIDS Research and Reference Reagent Program) and 5 μg CD44 plasmid (to generate HIVCD44), or with empty pcDNA3.1/V5-His TOPO TA Expression vector (to make HIVMOCK) using FuGENE HD Transfection Reagent (Roche, Indianapolis, IN, USA) according to the manufacturer's instructions. Three days later, the supernatant was collected and HIV production was assessed using an HIV-1 p24 ELISA assay (PerkinElmer, Waltham, MA, USA).
HIV viral stock generated from PBMCs
Isolated PBMCs were stimulated with 3 μg ml−1 of PHA-M (Roche) and 20 U ml−1 of human-IL 2 (hIL-2; Roche) for 3 days; viral supernatant from transfection of 293T cells was used to infect pooled mixed stimulated PBMCs prepared from two healthy donors. The supernatant from HIV-infected PBMCs was collected at day 7 and day 11. Viral titers were determined by HIV-1 p24 ELISA (PerkinElmer).
Reduction of HA from cell surface
A total of 1 × 106 ml−1 of unstimulated CD4+ T cells or M7-lue cells were washed three times in RPMI without FCS and incubated in RPMI with 12 U ml−1 of hyaluronidase (Sigma)19 for 60 min, followed by removal of supernatant and three washes with RPMI without FCS.
Co-immunoprecipitation with CD44 western blotting
A total of 2 × 106 unstimulated healthy donor CD4+ T cells were washed twice with cold phosphate-buffered saline and lysed using the Mammalian Cell Lysis kit (Sigma). The lysates were centrifuged at 4 °C for 10 min at 12 000 g to pellet cellular debris, and the clarified lysate was transferred to a fresh pre-chilled tube. Lysates were precleared by incubation with protein A/Sepharose (Sigma) for 1 h at 4 °C, subjected to immunoprecipitation with anti-CD44 (R&D systems, Minneapolis, MN, USA) overnight at 4 °C, then treated with 50 μl of protein G/Sepharose (Sigma) for 2 h. The immunoprecipitates were washed 10 times with 1 ml of cold wash buffer (lysis buffer plus 0.5 M NaCl), then eluted from the protein G/Sepharose using 50 μl of elution buffer (1 M glycine, 0.25 M NaCl, pH 2.8). The Eluate was neutralized by 2 μl of 1 M Tris-Cl (pH 9.5), then subjected to10% SDS-polyacrylamide gel electrophoresis and transferred to nitrocellulose membrane. The membrane was treated with blocking buffer (5% fat-free milk, 50 mM Tris–HCl (pH 7.5), 0.2 M NaCl and 0.2% volume Tween-20), then incubated overnight at 4 °C in blocking buffer containing either 1:250 dilution of antibody to PKCa (Santa Cruz Biotech, Santa Cruz, CA, USA) or antibody to phosphoryated-PKCa (Santa Cruz Biotech). Detection was performed using goat anti-mouse immunoglobulin G (IgG)-conjugated horseradish peroxidase (Sigma) and FATDAB tablets (Sigma). The bands were analyzed using Quantity One 1-D analysis Software (Bio-Rad, Hercules, CA, USA).
Staining HA and HIV (p24) on unstimulated CD4+ T cells
HA staining
We adapted a method from Lin37 to stain HA on the unstimulated CD4+ T cell surface. Unstimulated CD4+ T cells were fixed by acid–formalin–ethanol and stained with biotinylated hyaluronan-binding protein (Calbiochem, Gibbstown, NJ, USA) overnight, then stained with Alexa488-streptavidin (Life Technologies) for 2 h. HA staining was measured by two methods: laser scanning microscopy and flow cytometry (fluorescence-activated cell sorting). For laser scanning microscopy analysis, the cells were further stained, cell membranes were stained with Texas Red (Life Technologies) and nuclei stained with DAPI (4,6-diamidino-2-phenylindole; Life Technologies). LSM510 META laser scanning microscope (Carl Zeiss Microimaging Inc., Thornwood, NY, USA) was used to scan the cells with Z-stack projections. For fluorescence-activated cell sorting analysis, 50 000 HA-stained cells were analyzed by EasyCyte6HT-2L (Millipore, Billercia, MA, USA).
HIV staining
Unstimulated CD4+ T cells were incubated with HIV for 5 h, fixed with 2% formaldehyde and incubated with mouse anti-HIV-1 p24 antibody at 4 °C overnight, then stained with donkey anti-mouse IgG antibody conjugated with Alexa Fluor647 (Life Technologies) for 2 h. The HIV-1-p24-positive cells were measured by flow cytometry (fluorescence-activated cell sorting), 50 000 cells from each condition were analyzed by EasyCyte6HT-2L (Millipore).
Assays for HIV infection
M7-lue cell (5.25.EGFP.Lue.M7 cell, a gift from Dr David Montefiori (Duke University) and Dr Ned Landau (NYU))
A total of 1 × 106 cells were washed with complete RPMI without FCS, then treated with study reagents, inoculated with 1000 pg HIV-p24 of HIV for 5 h, washed three times with complete RPMI and resuspended in complete RPMI. HIV infectivity was measured by using Bright-Glo Luciferase assay system (Promega, Madison, WI, USA) and measured with a plate reader (VICTOR3; PerkinElmer) at 3 days post infection.31 The luciferase activity is presented as relative light units.
Jurkat (E6.1) cell (NIH AIDS Research and Reference Reagent Program)
For untransfected Jurkat cells, HIV infection conditions were the same as for the M7-Lue cell line, but HIV infectivity was measured by HIV-1 p24 production at 7 days post infection using the HIV-1p24 ELISA kit (PerkinElmer). For CD44− or empty vector-transfected Jurkat cells, after 3 days of transfection, the cells were infected with 5 ng of HIV-p24 for 5 h, then washed three times with complete RPMI, resuspended in complete RPMI and cultured for 3 days. These cells were analyzed for HIV infection by two methods: first, by intracellular staining for HIV-p24 (KC-57RD1-PE; Beckman Coulter, Fullerton, CA, USA) and CD44 (anti-huCD44-APC; R&D system); and second, measuring HIV-1 p24 in the supernatant by p24 ELISA (PerkinElmer).
Unstimulated CD4+ T cells and unstimulated PBMCs
A total of 1 × 106 healthy donor unstimulated cells were washed with RPMI without FCS, treated with different reagents, inoculated with 1000 pg HIV-p24 of HIV for 5 h, washed three times with complete RPMI, resuspended in complete RPMI with 5 μg of PHA-M and 20 U of hIL-2 for 24 h, washed three times with completed RPMI and finally resuspended in completed RPMI with 20 U of hIL-2. HIV infectivity was measured by HIV-1 p24 production in supernatant at day 7 post infection by using the HIV-1p24 ELISA kit (PerkinElmer).
Measurement of cell-associated HIV RNA
Cellular RNA was extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA) and treated with DNase (Qiagen). RNA concentrations were measured using a ND-1000 Spectrophotometer (NanoDrop, Thermo Scientific, Wilmington, DE, USA). HIV RNA copy numbers were measured using a locked nucleic acid Taqman assay. Briefly, we performed a single-tube, one-step assay using 12.5 μl of TaqMmanRNA-to-CT-1STEP KIT (Life Technologies), 7.5 μl of primers (400 nM both forward and reverse) and probe (200 nM), and 5 μl of RNA. Cycling conditions were 50 °C for 30 min, 95 °C for 5 min, then 50 cycles of 95 °C for 15 s, 59 °C for 1 min. Primers were: G19-2-F-7Y (5′-AGCAGCYATGCAAATGTTA-3′ 1374–1392); and G-20-R (5′-AGAGAACCAAGGGGAAGTGA-3′ 1474–1493). For detection, a dual-labeled fluorescent locked nucleic acid probe was used with 6-FAM (Sigma-Aldrich, The Woodlands, TX, USA) at the 5′ end and Black hole quencher (BHQ-1, Sigma-Aldrich) at the 3′ end: G-probe (5′-CCATCAATGAGGA-3′ 1400–1412; underlined bases are locked nucleic acid).
Statistical analyses
All M7-lue cell and Jurkat cell assays were run in triplicate and repeated at least three times. Experiments with primary PBMCs and CD4+ T cells were run in duplicate and repeated with at least three different donors. Data averages and s.d. were calculated in Microsoft Excel 2008 and presented as means±s.d. Significance was assessed by a t-test, with P<0.05 considered significant.
Acknowledgments
This work was supported by the National Institutes of Health (grants 1R21AI104445-01A1 (PL) and R56AI091573-01 (JKW)), and the Department of Veterans Affairs (1 IK2 CX000520-01 (SY) and Merit Review Award 5101 BX001048 (JKW)).
The authors declare no conflict of interest.
Footnotes
The Supplementary Information that accompanies this paper is available on the Immunology and Cell Biology website (http://www.nature.com/icb)
Supplementary Material
References
- Padian NS, McCoy SI, Karim SS, Hasen N, Kim J, Bartos M, et al. HIV prevention transformed: the new prevention research agenda. Lancet. 2011;378:269–278. doi: 10.1016/S0140-6736(11)60877-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitisuttithum P, Gilbert P, Gurwith M, Heyward W, Martin M, van Griensven F, et al. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis. 2006;194:1661–1671. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- Watkins DI, Burton DR, Kallas EG, Moore JP, Koff WC. Nonhuman primate models and the failure of the Merck HIV-1 vaccine in humans. Nat Med. 2008;14:617–621. doi: 10.1038/nm.f.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS Global report 2010. Geneva http://www.unaids.org/globalreport/global_report.htm .
- Shaw GM, Hunter E. HIV transmission. Cold Spring Harb Perspect Med. 2012;2:1–23. doi: 10.1101/cshperspect.a006965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CJ, Li Q, Abel K, Kim EY, Ma ZM, Wietgrefe S, et al. Propagation and dissemination of infection after vaginal transmission of simian immunodeficiency virus. J Virol. 2005;79:9217–9227. doi: 10.1128/JVI.79.14.9217-9227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Schuler T, Zupancic M, Wietgrefe S, Staskus KA, Reimann KA, et al. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- Haase AT. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu Rev Med. 2011;62:127–139. doi: 10.1146/annurev-med-080709-124959. [DOI] [PubMed] [Google Scholar]
- Giavedoni LD, Chen HL, Hodara VL, Chu L, Parodi LM, Smith LM, et al. Impact of mucosal inflammation on oral simian immunodeficiency virus transmission. J Virol. 2013;87:1750–1758. doi: 10.1128/JVI.02079-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajoie J, Juno J, Burgener A, Rahman S, Mogk K, Wachihi C, et al. A distinct cytokine and chemokine profile at the genital mucosa is associated with HIV-1 protection among HIV-exposed seronegative commercial sex workers. Mucosal Immunol. 2012;5:277–287. doi: 10.1038/mi.2012.7. [DOI] [PubMed] [Google Scholar]
- Haaland RE, Hawkins PA, Salazar-Gonzalez J, Johnson A, Tichacek A, Karita E, et al. Inflammatory genital infections mitigate a severe genetic bottleneck in heterosexual transmission of subtype A and C HIV-1. PLoS Pathog. 2009;5:e1000274. doi: 10.1371/journal.ppat.1000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher D, Wu X, Schacker T, Horbul J, Southern P. HIV binding, penetration, and primary infection in human cervicovaginal tissue. Proc Natl Acad Sci USA. 2005;102:11504–11509. doi: 10.1073/pnas.0500848102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shukair SA, Allen SA, Cianci GC, Stieh DJ, Anderson MR, Baig SM, et al. Human cervicovaginal mucus contains an activity that hinders HIV-1 movement. Mucosal Immunol. 2013;6:427–434. doi: 10.1038/mi.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SK, Hida K, Shukair S, Wang YY, Figueiredo A, Cone R, et al. Human immunodeficiency virus type 1 is trapped by acidic but not by neutralized human cervicovaginal mucus. J Virol. 2009;83:11196–11200. doi: 10.1128/JVI.01899-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4:528–539. doi: 10.1038/nrc1391. [DOI] [PubMed] [Google Scholar]
- Girish KS, Kemparaju K. The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life Sci. 2007;80:1921–1943. doi: 10.1016/j.lfs.2007.02.037. [DOI] [PubMed] [Google Scholar]
- Evanko SP, Tammi MI, Tammi RH, Wight TN. Hyaluronan-dependent pericellular matrix. Adv Drug Deliv Rev. 2007;59:1351–1365. doi: 10.1016/j.addr.2007.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGrendele HC, Estess P, Siegelman MH. Requirement for CD44 in activated T cell extravasation into an inflammatory site. Science. 1997;278:672–675. doi: 10.1126/science.278.5338.672. [DOI] [PubMed] [Google Scholar]
- Siegelman MH, DeGrendele HC, Estess P. Activation and interaction of CD44 and hyaluronan in immunological systems. J Leukoc Biol. 1999;66:315–321. doi: 10.1002/jlb.66.2.315. [DOI] [PubMed] [Google Scholar]
- Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases. Physiol Rev. 2011;91:221–264. doi: 10.1152/physrev.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muto J, Yamasaki K, Taylor KR, Gallo RL. Engagement of CD44 by hyaluronan suppresses TLR4 signaling and the septic response to LPS. Mol Immunol. 2009;47:449–456. doi: 10.1016/j.molimm.2009.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Lee YS, Hahn JH, Choe J, Kwon HJ, Ro JY, et al. Hyaluronic acid targets CD44 and inhibits FcepsilonRI signaling involving PKCdelta, Rac1, ROS, and MAPK to exert anti-allergic effect. Mol Immunol. 2008;45:2537–2547. doi: 10.1016/j.molimm.2008.01.008. [DOI] [PubMed] [Google Scholar]
- Bastiani L, Laal S, Kim M, Zolla-Pazner S. Host cell-dependent alterations in envelope components of human immunodeficiency virus type 1 virions. J Virol. 1997;71:3444–3450. doi: 10.1128/jvi.71.5.3444-3450.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn SD, Roberts BD, Griffin GE, Folks TM, Butera ST. Cellular compartments of human immunodeficiency virus type 1 replication in vivo: determination by presence of virion-associated host proteins and impact of opportunistic infection. J Virol. 2000;74:139–145. [PMC free article] [PubMed] [Google Scholar]
- Guo MM, Hildreth JE. HIV acquires functional adhesion receptors from host cells. AIDS Res Hum Retroviruses. 1995;11:1007–1013. doi: 10.1089/aid.1995.11.1007. [DOI] [PubMed] [Google Scholar]
- Terry VH, Johnston IC, Spina CA. CD44 microbeads accelerate HIV-1 infection in T cells. Virology. 2009;388:294–304. doi: 10.1016/j.virol.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano O, Saya H. Mechanism and biological significance of CD44 cleavage. Cancer Sci. 2004;95:930–935. doi: 10.1111/j.1349-7006.2004.tb03179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cichy J, Puré E. The liberation of CD44. J Cell Biol. 2003;161:839–843. doi: 10.1083/jcb.200302098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yukl SA, Li PL, Fujimoto K, Liegler T, Pandori M, Havlir DV, et al. Modification of the Abbott m2000 assay to detect HIV-1 plasma RNA viral loads less than one copy per milliliter. J Virol Methods. 2011;175:261–265. doi: 10.1016/j.jviromet.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen RA, Hofmann-Lehmann R, Li PL, Vlasak J, Schmitz JE, Reimann KA, et al. Neutralizing antibodies as a potential secondary protective mechanism during chronic SHIV infection in CD8+ T-cell-depleted macaques. AIDS. 2002;16:829–838. doi: 10.1097/00002030-200204120-00002. [DOI] [PubMed] [Google Scholar]
- Kastner S, Thomas GJ, Jenkins RH, Davies M, Steadman R. Hyaluronan induces the selective accumulation of matrix- and cell-associated proteoglycans by mesangial cells. Am J Pathol. 2007;171:1811–1821. doi: 10.2353/ajpath.2007.070085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JM, Stokes JM, MacDonald MH, Benton HP. Evaluation of hyaluronidase activity in equine and bovine sera and equine synovial fluid samples by use of enzyme zymography. Am J Vet Res. 2005;66:984–990. doi: 10.2460/ajvr.2005.66.984. [DOI] [PubMed] [Google Scholar]
- Derdeyn CA, Decker JM, Sfakianos JN, Wu X, O'Brien WA, Ratner L, et al. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120. J Virol. 2000;74:8358–8367. doi: 10.1128/jvi.74.18.8358-8367.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang LS, Ma HW, Greyner HJ, Zuo W, Mummert ME. Inhibition of cell proliferation by CD44: Akt is inactivated and EGR-1 is down-regulated. Cell Prolif. 2010;43:385–395. doi: 10.1111/j.1365-2184.2010.00689.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WH, Bard JB. Hyaluronidase-sensitive halos around adherent cells. Their role in blocking lymphocyte-mediated cytolysis. J Exp Med. 1979;149:507–515. doi: 10.1084/jem.149.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Shuster S, Maibach HI, Stern R. Patterns of hyaluronan staining are modified by fixation techniques. J Histochem Cytochem. 1997;45:1157–1163. doi: 10.1177/002215549704500813. [DOI] [PubMed] [Google Scholar]
- Trushin SA, Bren GD, Asin S, Pennington KN, Paya CV, Badley AD. Human immunodeficiency virus reactivation by phorbol esters or T-cell receptor ligation requires both PKCalpha and PKCtheta. J Virol. 2005;79:9821–9830. doi: 10.1128/JVI.79.15.9821-9830.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qatsha KA, Rudolph C, Marmé D, Schächtele C, May WS. Gö 6976, a selective inhibitor of protein kinase C, is a potent antagonist of human immunodeficiency virus 1 induction from latent/low-level-producing reservoir cells in vitro. Proc Natl Acad Sci USA. 1993;90:4674–4678. doi: 10.1073/pnas.90.10.4674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aaltonen V, Peltonen J. PKCalpha/beta I inhibitor Go6976 induces dephosphorylation of constitutively hyperphosphorylated Rb and G1 arrest in T24 cells. Anticancer Res. 2010;30:3995–3999. [PubMed] [Google Scholar]
- Volpi N, Schiller J, Stern R, Soltés L. Role, metabolism, chemical modifications and applications of hyaluronan. Curr Med Chem. 2009;16:1718–1745. doi: 10.2174/092986709788186138. [DOI] [PubMed] [Google Scholar]
- Rodriguez HI, Stewart AJ, Wolfe DF, Caldwell FJ, Harrie M, Whitley EM. Immunolocalization of the hyaluronan receptor CD44 in the reproductive tract of the mare. Theriogenology. 2010;75:276–286. doi: 10.1016/j.theriogenology.2010.08.014. [DOI] [PubMed] [Google Scholar]
- Mohamadzadeh M, DeGrendele H, Arizpe H, Estess P, Siegelman M. Proinflammatory stimuli regulate endothelial hyaluronan expression and CD44/HA-dependent primary adhesion. J Clin Invest. 1998;101:97–108. doi: 10.1172/JCI1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson RL, Peterson DA, Deinhardt F, Howard F. Rubella and rheumatoid arthritis: hyaluronic acid and susceptibility of cultured rheumatoid synovial cells to viruses. Proc Soc Exp Biol Med. 1975;149:594–598. doi: 10.3181/00379727-149-38859. [DOI] [PubMed] [Google Scholar]
- Campo GM, Avenoso A, Micali A, Nastasi G, Squadrito F, Altavilla D, et al. High-molecular weight hyaluronan reduced renal PKC activation in genetically diabetic mice. Biochim Biophys Acta. 2010;1802:1118–1130. doi: 10.1016/j.bbadis.2010.08.004. [DOI] [PubMed] [Google Scholar]
- Iwamoto T, Hagiwara M, Hidaka H, Isomura T, Kioussis D, Nakashima I. Accelerated proliferation and interleukin-2 production of thymocytes by stimulation of soluble anti-CD3 monoclonal antibody in transgenic mice carrying a rabbit protein kinase C alpha. J Biol Chem. 1992;267:18644–18648. [PubMed] [Google Scholar]
- Thankamony SP, Knudson W. Acylation of CD44 and its association with lipid rafts are required for receptor and hyaluronan endocytosis. J Biol Chem. 2006;281:34601–34609. doi: 10.1074/jbc.M601530200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popik W, Alce TM, Au WC. Human immunodeficiency virus type 1 uses lipid raft-colocalized CD4 and chemokine receptors for productive entry into CD4(+) T cells. J Virol. 2002;76:4709–4722. doi: 10.1128/JVI.76.10.4709-4722.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiyama H, Yoshii H, Tanaka Y, Sato H, Yamamoto N, Kubo Y. Raft localization of CXCR4 is primarily required for X4-tropic human immunodeficiency virus type 1 infection. Virology. 2009;386:23–31. doi: 10.1016/j.virol.2008.12.033. [DOI] [PubMed] [Google Scholar]
- Chang TL, Vargas J, Jr, DelPortillo A, Klotman ME. Dual role of alpha-defensin-1 in anti-HIV-1 innate immunity. J Clin Invest. 2005;115:765–773. doi: 10.1172/JCI200521948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvin SR, Cohen MS. The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol. 2004;2:33–42. doi: 10.1038/nrmicro794. [DOI] [PubMed] [Google Scholar]
- Pilcher CD, Tien HC, Eron JJ, Jr, Vernazza PL, Leu SY, Stewart PW, et al. Brief but efficient: acute HIV infection and the sexual transmission of HIV. J Infect Dis. 2004;189:1785–1792. doi: 10.1086/386333. [DOI] [PubMed] [Google Scholar]
- Hladik F, Doncel GF. Preventing mucosal HIV transmission with topical microbicides: challenges and opportunities. Antiviral Res. 2010;88S:S3–S9. doi: 10.1016/j.antiviral.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.