Abstract
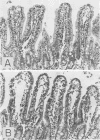
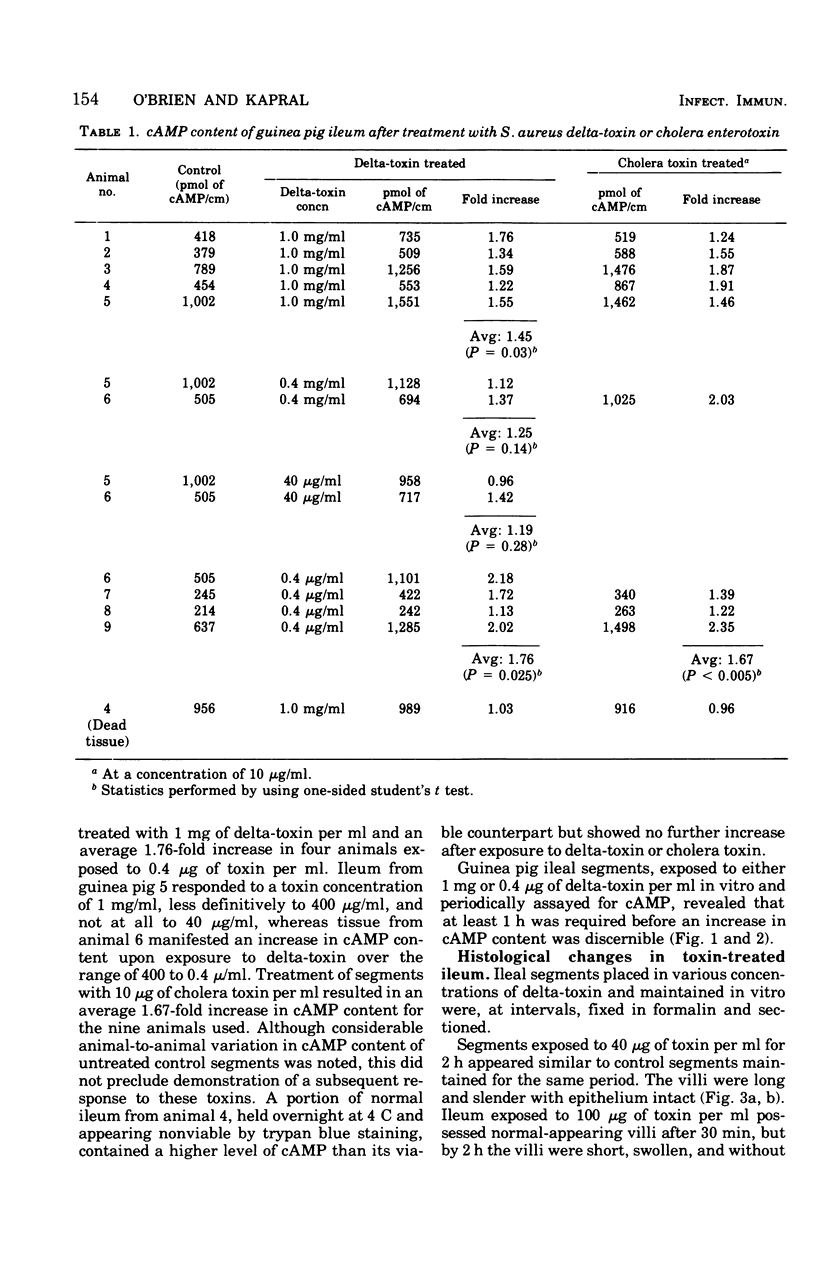
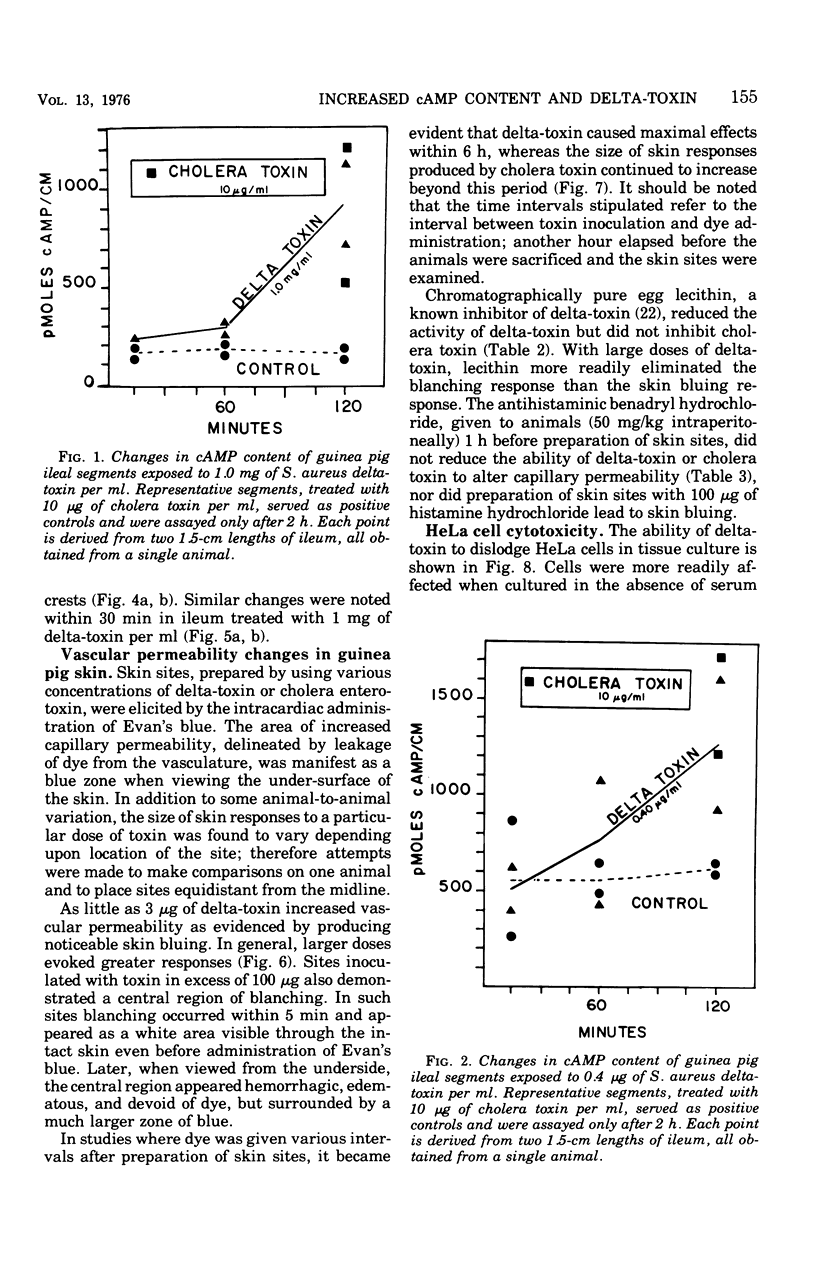
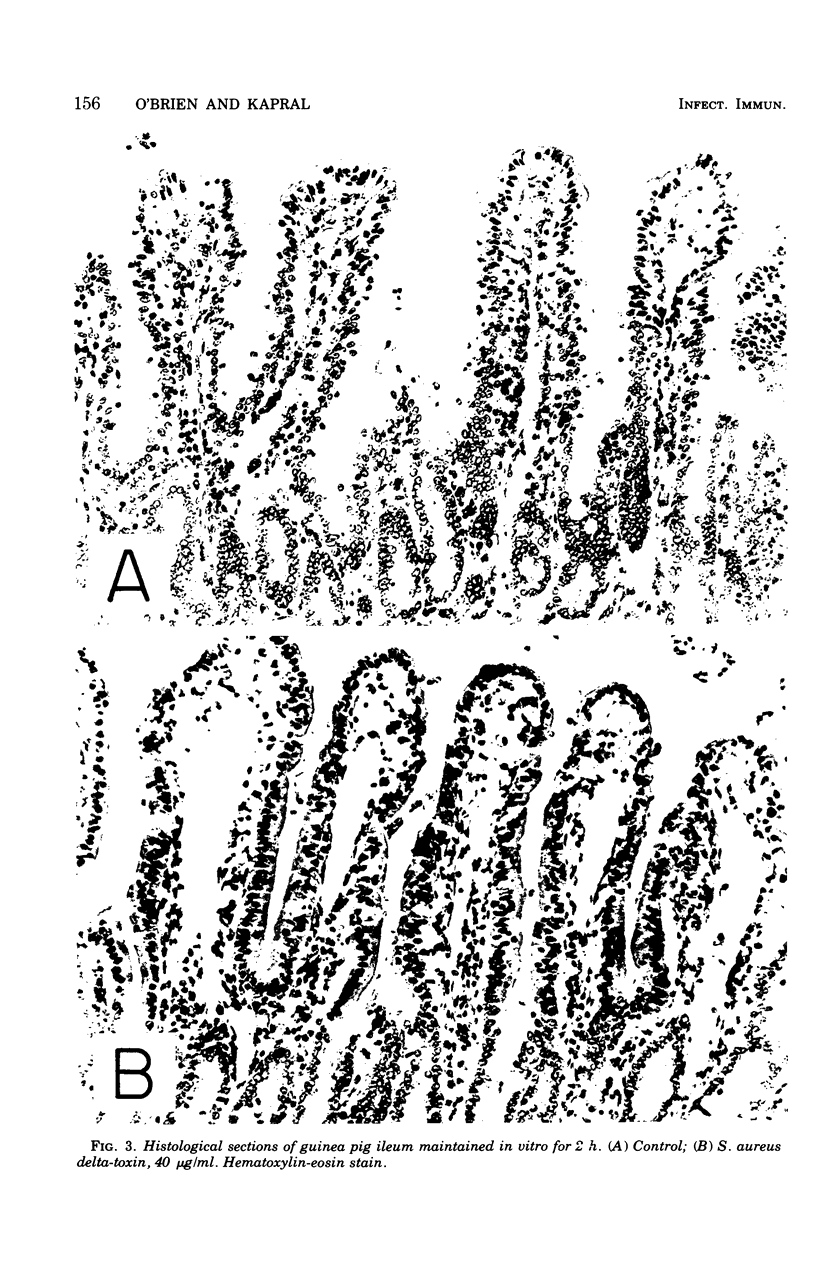
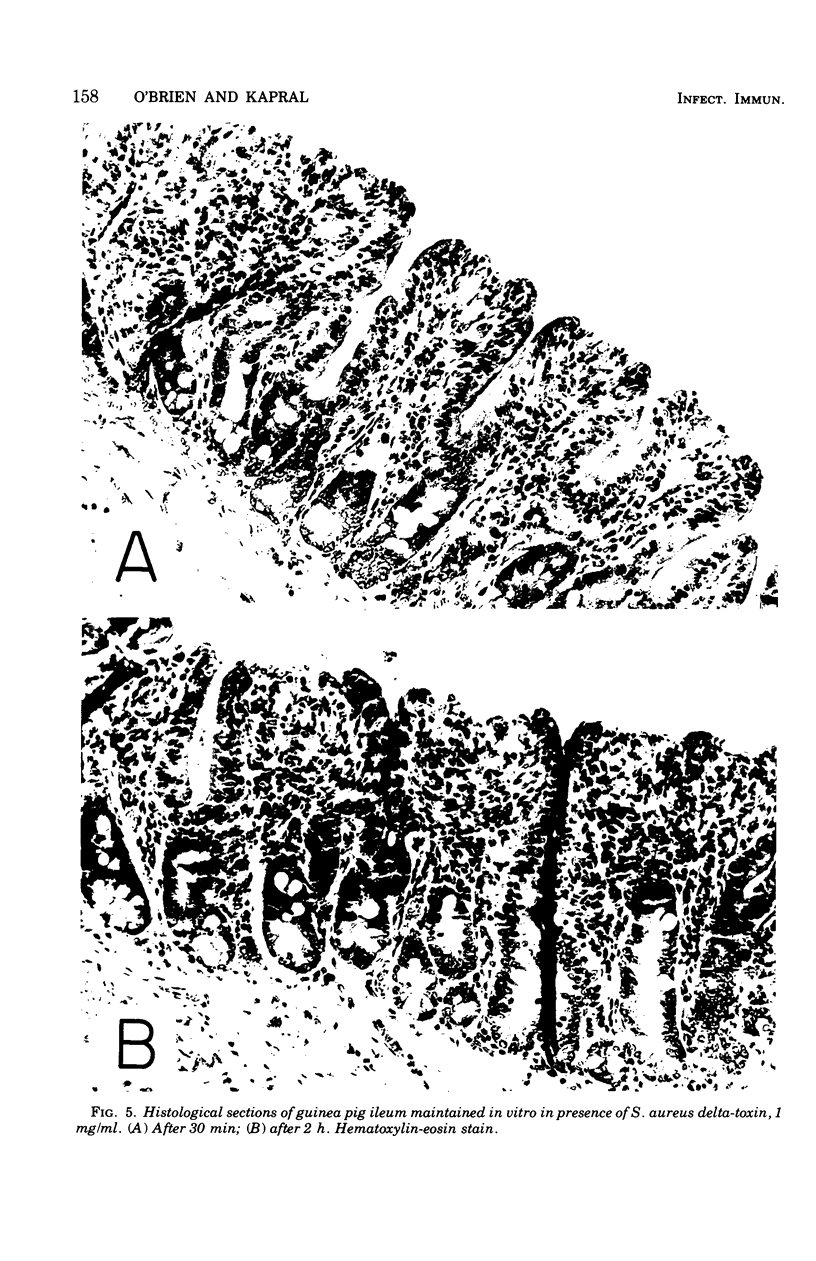
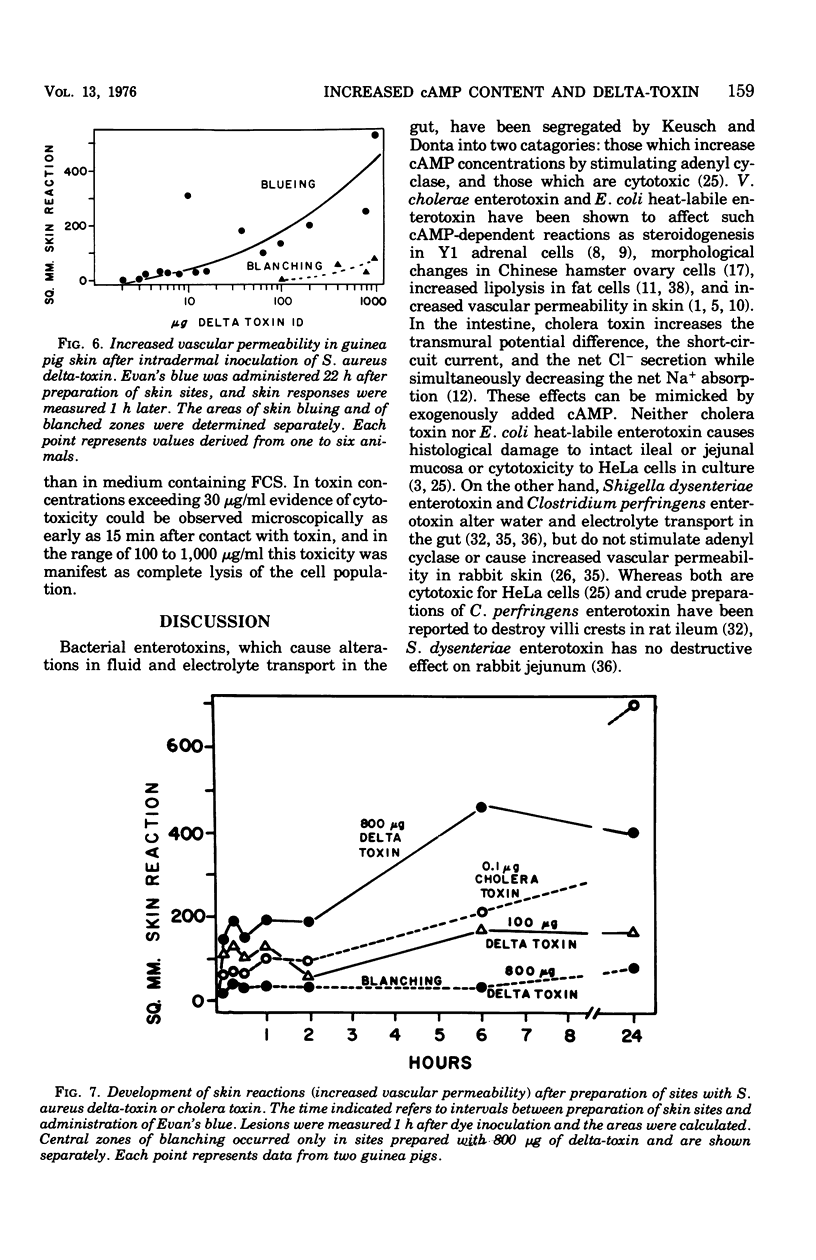
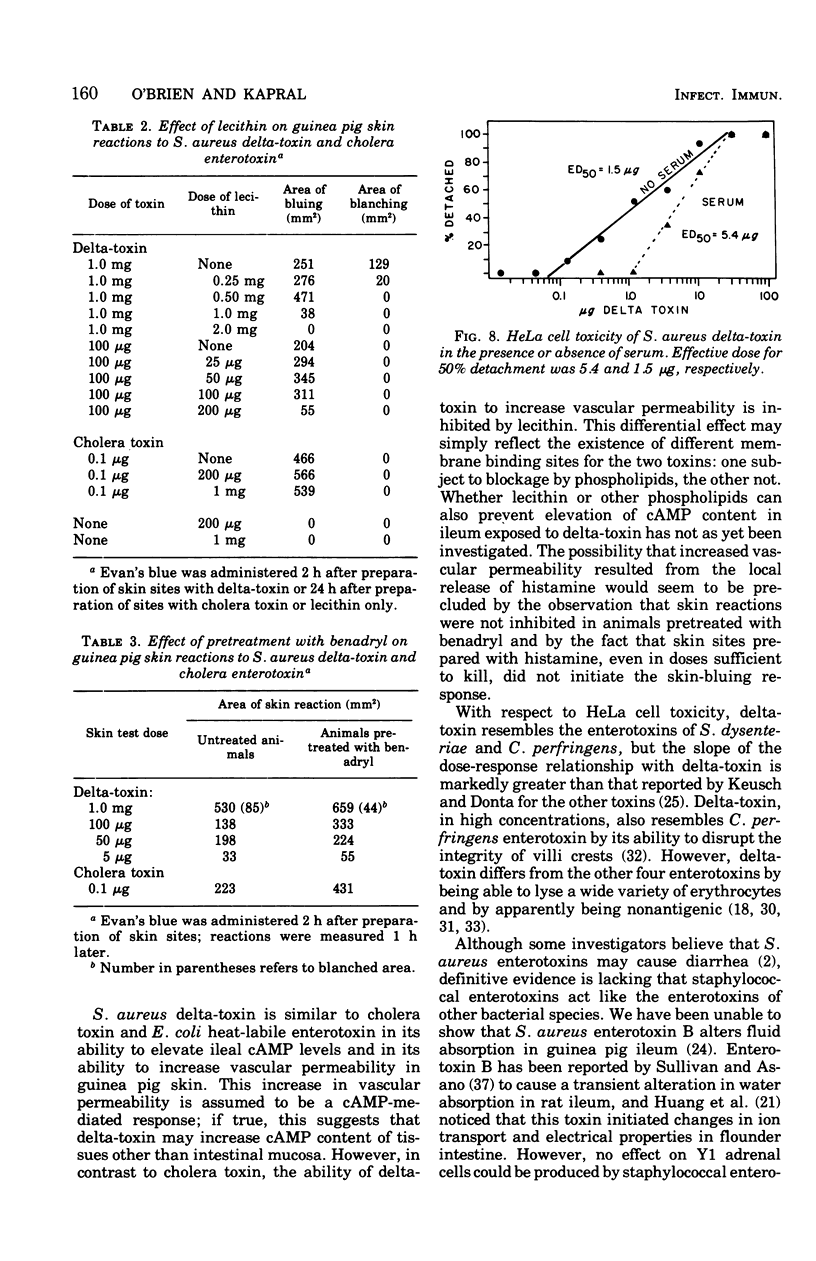
To compare Staphylococcus aureus delta-toxin with cholera toxin, which is known to increase cellular cyclic adenosine 3',5'-monophosphate (cAMP), studies were undertaken to determine the effect of delta-toxin on the cAMP content of guinea pig ileum maintained in vitro. Concentrations of delta-toxin as low as 0.40 mug/ml increased cAMP levels in guinea pig ileum after 2 h of incubation. Histological damage was seen in ileum exposed for 2 h to delta-toxin concentrations of 100 mug/ml. As little as 3 mug of delta-toxin increased vascular permeability in guinea pig skin. Permeability changes became evident within 5 min and were maximal within 6 h, whereas those produced by cholera toxin required 24 h to become maximal. Benadryl did not interfere with the ability of these toxins to alter vascular permeability. Purified egg lecithin reduced the effectiveness of delta-toxin in the skin but did not inhibit cholera toxin. Delta-toxin in concentrations as low as 0.1 mug/ml caused dislodgement of HeLa cells in tissue cultures. Therefore, delta-toxin appears unique in being the only bacterial toxin, currently known to alter water absorption in the ileum, that is capable of both increasing cAMP levels and being cytotoxic. These findings suggest a possible role for delta-toxin in the pathogenesis of staphylococcal enteritis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BASUMALLIK K. C., GANGULI N. C. SOME OBSERVATIONS ON THE PATHOGENESIS OF CHOLERA. Indian J Med Res. 1964 Aug;52:894–901. [PubMed] [Google Scholar]
- Carpenter C. C. Cholera and other enterotoxin-related diarrheal diseases. J Infect Dis. 1972 Nov;126(5):551–564. doi: 10.1093/infdis/126.5.551. [DOI] [PubMed] [Google Scholar]
- Chen L. C., Rohde J. E., Sharp G. W. Properties of adenyl cyclase from human jejunal mucosa during naturally acquired cholera and convalescence. J Clin Invest. 1972 Apr;51(4):731–740. doi: 10.1172/JCI106867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig J. P. A permeability factor (toxin) found in cholera stools and culture filtrates and its neutralization by convalescent cholera sera. Nature. 1965 Aug 7;207(997):614–616. doi: 10.1038/207614a0. [DOI] [PubMed] [Google Scholar]
- Donahue J. A. Antistaphylococcal hemolysins and delta hemolysin inhibitor in adult human serum. Can J Microbiol. 1969 Aug;15(8):957–959. doi: 10.1139/m69-167. [DOI] [PubMed] [Google Scholar]
- Donta S. T., King M., Sloper K. Induction of steroidogenesis in tissue culture by cholera enterotoxin. Nat New Biol. 1973 Jun 20;243(129):246–247. doi: 10.1038/newbio243246a0. [DOI] [PubMed] [Google Scholar]
- Donta S. T., Smith D. M. Stimulation of steroidogenesis in tissue culture by enterotoxigenic Escherichia coli and its neutralization by specific antiserum. Infect Immun. 1974 Mar;9(3):500–505. doi: 10.1128/iai.9.3.500-505.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Chen L. C., Curlin G. T., Evans D. G. Stimulation of adenyl cyclase by Escherichia coli enterotoxin. Nat New Biol. 1972 Apr 5;236(66):137–138. doi: 10.1038/newbio236137a0. [DOI] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., Gorbach S. L. Production of vascular permeability factor by enterotoxigenic Escherichia coli isolated from man. Infect Immun. 1973 Nov;8(5):725–730. doi: 10.1128/iai.8.5.725-730.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M., Fromm D., al-Awqati Q., Greenough W. B., 3rd Effect of cholera enterotoxin on ion transport across isolated ileal mucosa. J Clin Invest. 1972 Apr;51(4):796–804. doi: 10.1172/JCI106874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores J., Grady G. F., McIver J., Witkum P., Beckman B., Sharp G. W. Comparison of the effects of enterotoxins of Shigella dysenteriae and Vibrio cholerae on the adenylate cyclase system of the rabbit intestine. J Infect Dis. 1974 Oct;130(4):374–379. doi: 10.1093/infdis/130.4.374. [DOI] [PubMed] [Google Scholar]
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady G. F., Keusch G. T. Pathogenesis of bacterial diarrheas. I. N Engl J Med. 1971 Oct 7;285(15):831–841. doi: 10.1056/NEJM197110072851505. [DOI] [PubMed] [Google Scholar]
- Guerrant R. L., Brunton L. L., Schnaitman T. C., Rebhun L. I., Gilman A. G. Cyclic adenosine monophosphate and alteration of Chinese hamster ovary cell morphology: a rapid, sensitive in vitro assay for the enterotoxins of Vibrio cholerae and Escherichia coli. Infect Immun. 1974 Aug;10(2):320–327. doi: 10.1128/iai.10.2.320-327.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallander H. O. Characterization and partial purification of staphylococcal delta-lysin. Acta Pathol Microbiol Scand. 1968;72(4):586–600. doi: 10.1111/j.1699-0463.1968.tb00471.x. [DOI] [PubMed] [Google Scholar]
- Hallanger H. O., Bengtsson S. Studies on the cell toxicity and species specificity of purified staphylococcal toxins. Acta Pathol Microbiol Scand. 1967;70(1):107–119. doi: 10.1111/j.1699-0463.1967.tb01274.x. [DOI] [PubMed] [Google Scholar]
- Hendrix T. R. The pathophysiology of cholera. Bull N Y Acad Med. 1971 Oct;47(10):1169–1180. [PMC free article] [PubMed] [Google Scholar]
- Huang K. C., Chen T. S. Ion transport across intestinal mucosa of winter flounder, Pseudopleuronectes americanus. Am J Physiol. 1971 Jun;220(6):1734–1738. doi: 10.1152/ajplegacy.1971.220.6.1734. [DOI] [PubMed] [Google Scholar]
- Kapral F. A. Inhibition of Staphylococcus aureus delta hemolysin by phospholipids. Proc Soc Exp Biol Med. 1972 Nov;141(2):519–521. doi: 10.3181/00379727-141-36812. [DOI] [PubMed] [Google Scholar]
- Kapral F. A., Miller M. M. Product of Staphylococcus aureus responsible for the scalded-skin syndrome. Infect Immun. 1971 Nov;4(5):541–545. doi: 10.1128/iai.4.5.541-545.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapral F. A., O'Brien A. D., Ruff P. D., Drugan W. J., Jr Inhibition of water absorption in the intestine by Staphylococcus aureus delta-toxin. Infect Immun. 1976 Jan;13(1):140–145. doi: 10.1128/iai.13.1.140-145.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keusch G. T., Donta S. T. Classification of enterotoxins on the basis of activity in cell culture. J Infect Dis. 1975 Jan;131(1):58–63. doi: 10.1093/infdis/131.1.58. [DOI] [PubMed] [Google Scholar]
- Keusch G. T., Grady G. F., Mata L. J., McIver J. The pathogenesis of Shigella diarrhea. I. Enterotoxin production by Shigella dysenteriae I. J Clin Invest. 1972 May;51(5):1212–1218. doi: 10.1172/JCI106915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keusch G. T., Jacewicz M., Hirschman S. Z. Quantitative microassay in cell culture for enterotoxin of Shigella dysenteriae. J Infect Dis. 1972 May;125(5):539–541. doi: 10.1093/infdis/125.5.539. [DOI] [PubMed] [Google Scholar]
- Kimberg D. V., Field M., Gershon E., Henderson A. Effects of prostaglandins and cholera enterotoxin on intestinal mucosal cyclic AMP accumulation. Evidence against an essential role for prostaglandins in the action of toxin. J Clin Invest. 1974 Mar;53(3):941–949. doi: 10.1172/JCI107635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimberg D. V., Field M., Johnson J., Henderson A., Gershon E. Stimulation of intestinal mucosal adenyl cyclase by cholera enterotoxin and prostaglandins. J Clin Invest. 1971 Jun;50(6):1218–1230. doi: 10.1172/JCI106599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleck J. L., Donahue J. A. Production of thermostable hemolysin by cultures of Staphylococcus epidermidis. J Infect Dis. 1968 Jun;118(3):317–323. doi: 10.1093/infdis/118.3.317. [DOI] [PubMed] [Google Scholar]
- Kreger A. S., Kim K. S., Zaboretzky F., Bernheimer A. W. Purification and properties of staphylococcal delta hemolysin. Infect Immun. 1971 Mar;3(3):449–465. doi: 10.1128/iai.3.3.449-465.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonel J. L. In Vivo Effects of Clostridium perfringens Enteropathogenic Factors on the Rat Ileum. Infect Immun. 1974 Nov;10(5):1156–1162. doi: 10.1128/iai.10.5.1156-1162.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp G. W., Hynie S. Stimulation of intestinal adenyl cyclase by cholera toxin. Nature. 1971 Jan 22;229(5282):266–269. doi: 10.1038/229266a0. [DOI] [PubMed] [Google Scholar]
- Stark R. L., Duncan C. L. Purification and biochemical properties of Clostridium perfringens type A enterotoxin. Infect Immun. 1972 Nov;6(5):662–673. doi: 10.1128/iai.6.5.662-673.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg S. E., Banwell J. G., Yardley J. H., Keusch G. T., Hendrix T. R. Comparison of secretory and histological effects of shigella and cholera enterotoxins in rabbit jejunum. Gastroenterology. 1975 Feb;68(2):309–317. [PubMed] [Google Scholar]
- Sullivan R., Asano T. Effects of staphylococcal enterotoxin B on intestinal transport in the rat. Am J Physiol. 1971 Jun;220(6):1793–1797. doi: 10.1152/ajplegacy.1971.220.6.1793. [DOI] [PubMed] [Google Scholar]
- Vaughan M., Pierce N. F., Greenough W. B., 3rd Stimulation of glycerol production in fat cells by cholera toxin. Nature. 1970 May 16;226(5246):658–659. doi: 10.1038/226658a0. [DOI] [PubMed] [Google Scholar]