Abstract
Neural larva migrans (NLM) with eosinophilic meningoencephalitis secondary to raccoon roundworm (Baylisascaris procyonis) infection has been reported in rural and suburban areas of North America and Europe with extant raccoon populations. Most cases have occurred in infants less than two years of age exposed to areas of raccoon fecal contamination. Here, we present a case of Baylisascaris-induced NLM from the densely populated borough of Brooklyn in New York City and alert urban pediatricians to consider this cause of clinical neurologic disease even in areas not typically thought to be associated with endemic risk factors. Infected raccoons also occur in urban settings, and urban children may be exposed to environmental areas or materials contaminated with their feces and the parasite’s eggs.
Keywords: Baylisascaris procyonis, Raccoon roundworm, Eosinophilic meningoencephalitis, Neural larva migrans
1 Introduction
Neural larva migrans (NLM) is invasion of the brain and/or spinal cord by migrating larvae of helminth parasites, most commonly ascarids of carnivores [11,13]. The most common cause of clinical NLM in animals is the raccoon roundworm, Baylisascaris procyonis, which has produced fatal or severe neurologic disease in over 130 species of mammals and birds in North America [13, K. Kazacos, unpublished]. Because of its high prevalence and widespread distribution, B. procyonis is being increasingly recognized as a cause of eosinophilic meningoencephalitis in humans, primarily children with significant environmental contact [8, 20]. Cases of B. procyonis NLM are usually observed where infected raccoons occur, such as in rural and suburban communities in the northern and midwestern United States and on the west coast [13]. NLM has been less often reported within densely urban communities, even though raccoons are also known to occur in this setting [8]. Unfortunately, this geographical bias may obscure consideration of this etiology and lead to delayed diagnosis and poor neurological outcomes. As raccoons have migrated from rural to urban settings, more city dwellers will be exposed to B. procyonis infection and may suffer grave consequences [8,20,22].
Herein we present a case of an infant in New York City in order to alert pediatricians in urban settings to consider NLM as well as B. procyonis in patients with eosinophilic meningoencephalitis. Recently, a New York City Department of Health public health advisory was released, in response to this case as well as another of our urban patients, a teenager with B. procyonis-induced ocular larva migrans (OLM), who suffered permanent severe vision impairment [21].
2 Case report
A 12-month-old male presented to the Pediatric Emergency Center with a three-week history of unexplained irritability and a three-day history of progressive weakness. The patient developed head lag, loss of truncal tone, vacant stare, inability to crawl or stand, and unresponsiveness to visual or auditory stimuli. However, the patient was afebrile, had a normal appetite, and did not manifest gastrointestinal, respiratory, or cutaneous symptoms.
The infant had an unremarkable medical history and was born by spontaneous vaginal delivery in Brooklyn, New York, to a healthy 25-year-old woman, without complications. He lived with his healthy parents, three-year-old sister, and five-year-old brother, in a single family dwelling in an urban area of Brooklyn known to have dogs, cats, and raccoons. The infant was never previously hospitalized, had up-to-date vaccinations, and was progressing normally through developmental milestones.
At presentation, the patient was alert but irritable. His vital signs were temperature 100.1°F, heart rate 124 beats per minute (bpm), respiratory rate 20 breaths per minute, oxygen saturation 98%, weight 8.9 kg (10th percentile), and height 78 cm (75th percentile). Physical exam was remarkable for markedly decreased tone in the head and trunk, decreased deep tendon reflexes in all extremities, and a searching-type nystagmus, with associated unresponsiveness to track any visual stimuli. Fundoscopic examination was within normal limits.
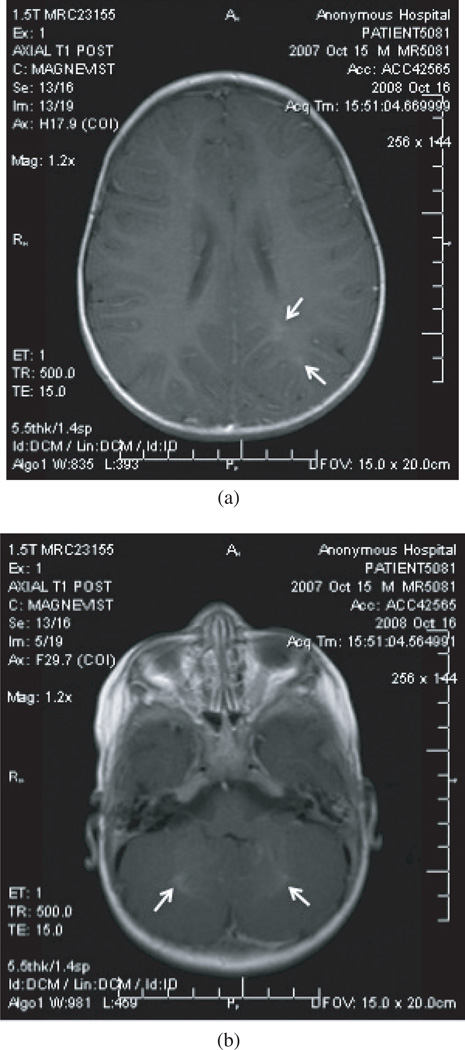
A peripheral complete blood count revealed white blood cells (WBCs) 31,200/µL, hemoglobin 10.7 mg/dL, and platelets 428,000/µL, with a manual differential of neutrophils 24%, bands 9%, lymphocytes 33%, and eosinophils 30% (Table 1). A repeat CBC performed the next day confirmed the marked eosinophilia (24%). The erythrocyte sedimentation rate was 35 mm/hr and serum chemistries were within normal limits. A lumbar puncture (LP) at the time of admission revealed red blood cells (RBCs) 21 cells/µL, WBC 7 cells/µL (lymphocytes 14%, monocytes 43%, eosinophils 29%), glucose 76 mg/dL, protein 21 mg/dL, herpes simplex virus PCR negative, and West Nile virus antibody negative. Radiologic imaging included a normal chest X-ray; however, the brain MRI (Figure 1) was consistent with an atypical pattern of acute demyelinating encephalomyelitis (ADEM).
Table 1.
Cerebrospinal fluid results and regimen
| WBC (cells/µL) | Lymph. | Mono. | Eosino. | Glucose (mg/dl) | Protein (mg/dl) | Regimen | |
|---|---|---|---|---|---|---|---|
| Day 1 | 7 | 14% | 43% | 29% | 76 | 21 | Started intravenous immunoglobulin |
| Day 7 | 110 | 56% | 48% | 46% | 81 | 38 | Started pulse methylprednisolone |
| Day 18 | 16 | 56% | 75% | 19% | 61 | 58 | Started albendazole and prednisone |
Figure 1.
(a) and (b): T1 MRI of patient consistent with atypical pattern of acute disseminated encephalomyelitis (arrows).
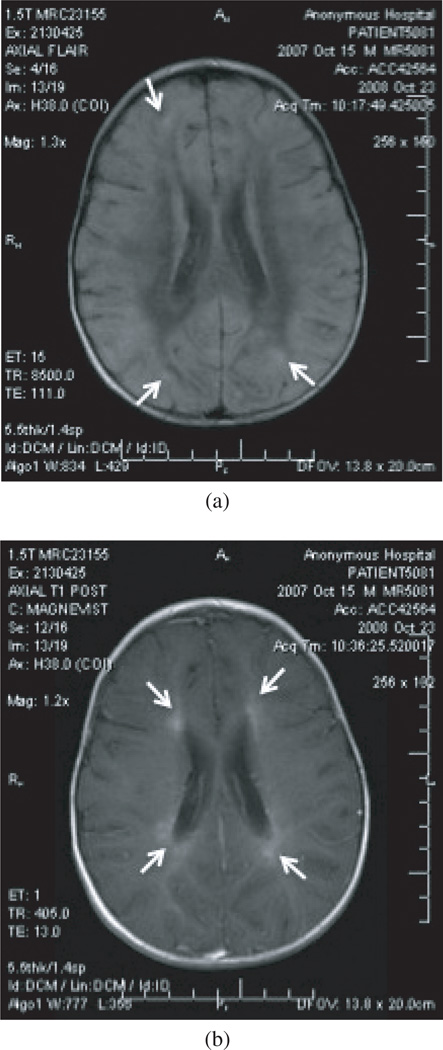
Given the marked eosinophilia at presentation, infectious disease consultation was requested at the time of admission. Pending the serological analysis from the LP, the leading differential diagnosis was considered ADEM over NLM, and hence, the patient was administered 2 g/kg intravenous immunoglobulin (IVIG). However, for the next several days, the patient developed lip smacking, worsening horizontal nystagmus, and decerebrate posturing. On hospital day 7, a repeat LP revealed RBC 4 cells/µL, WBC 110 cells/µL (lymphocytes 56%, monocytes 48%, eosinophils 46%), glucose 81 mg/dL, protein 38 mg/dL. On hospital day 8, a repeat brain MRI (Figure 2) demonstrated increased demyelination. As the admission LP serologies were not yet available, the patient was then started on high-dose pulse methylprednisolone (15 mg/kg/day) for refractory ADEM, without a clinical benefit.
Figure 2.
A second (a) TI and (b) FLAIR MRI demonstrating worsened demyelination (arrows).
A third LP performed during hospital week three revealed RBC 250 cells/µL, WBC 16 cells/µL (lymphocytes 56%, monocytes 75%, eosinophils 19%), glucose 61 mg/dL, and protein 58 mg/dL. Given the lack of response to the current treatment for ADEM, the leading differential diagnosis was now more consistent with NLM, and the patient was immediately treated with albendazole (20 mg/kg/day orally, divided every 12 hours) and prednisone (1 mg/kg/day). The admission stool analysis for ova and parasites was negative as were serum and cerebrospinal fluid (CSF) Toxocara canis and T. cati IgM and IgG serologies.
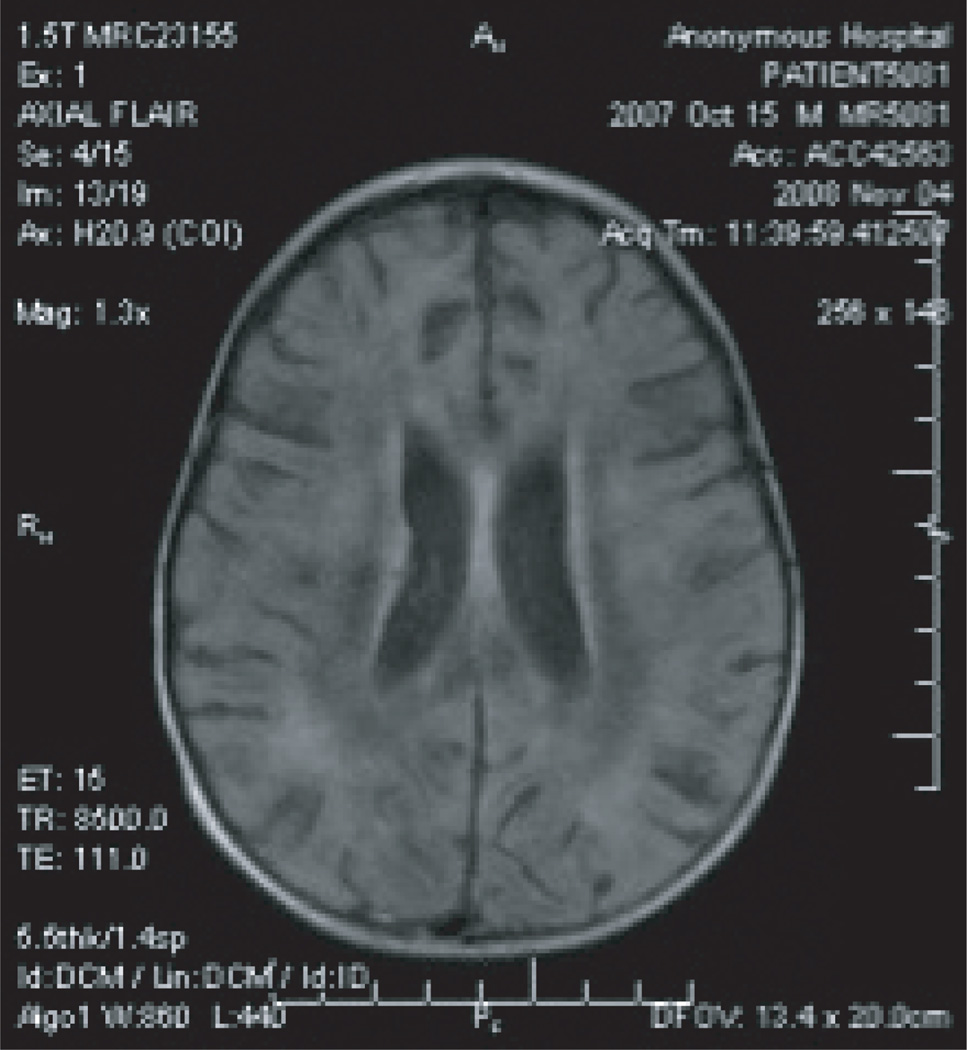
The admission serum and CSF samples, obtained prior to the administration of IVIG, returned as strongly positive via ELISA for anti-Baylisascaris procyonis antibodies, with optical density readings of 1.385 for serum and 1.453 for CSF (putative cutoff 0.250). Upon confirmation of the diagnosis of B. procyonis NLM, the dose of albendazole was doubled starting hospital day 33, and a second high-dose pulse of methylprednisolone (4 mg/kg/dose, every 6 hours) was given for 4 days due to the lack of clinical improvement and worsening atrophy noted on the third MRI (Figure 3).
Figure 3.
A third T1 MRI showing worsened atrophy, as demonstrated by sulcal prominence.
Throughout his hospital course, the patient remained on a normal oral diet and did not require respiratory support. The patient did not demonstrate recognition of his parents or any awareness of his environment, despite being awake most of the day. Unfortunately, the patient further developed hand contractures which worsened progressively despite daily physical therapy and splinting. At the time of discharge, the patient’s clinical status was assessed as stable with significant neurological impairment, including apparent cortical blindness, total lack of cognitive function, and spastic diplegia. Dilated retinal examination failed to demonstrate any presence of chorioretinitis, neuroretinitis, or parasite larvae.
After a period of approximately one month under our care, the patient was discharged to a specialized pediatric rehabilitation center, continuing on albendazole (200 mg twice daily) and hydrocortisone (5 mg crushed tablets in water every six hours).
3 Discussion
The patient’s diffuse neurological deficits, peripheral serum and LP eosinophilia, and MRI findings, initially suggested a broad differential diagnosis. The eosinophilia, in particular, could have been secondary to leukemias, carcinomas, idiopathic hypereosinophilic syndrome, collagen vascular diseases, allergic angitis, hyperimmunoglobulin E syndromes, and eosinophilia myopathy syndromes. Alternatively, eosinophilia may be secondary to infectious organisms such as Ascaris lumbricoides, Toxocara canis, Trichinella spiralis, Angiostrongylus cantonensis, Baylisascaris procyonis, Strongyloides stercoralis, filarial worms, Schistosoma species, liver and lung flukes, Cryptococcus neoformans, and Coccidioides immitis [16].
Among these varied etiologies, there was unfortunately less consideration given to the likelihood of a nematode larval infection in a densely urban setting, including B. procyonis. Indeed, when B. procyonis was first proposed as an etiology of OLM [17], it was downplayed by others who stated “several patients with [OLM] lived in highly urban centers in the northeast and midwest, making exposure to raccoons highly unlikely” [14, 15]. This was and still is untrue, based on the present case, other cases from urban localities, and the well-known occurrence of raccoons in some of our largest cities. Urban raccoon populations carrying Baylisascaris have been documented recently in metropolitan Atlanta, Georgia; Portland, Oregon; Chicago, Illinois; the Twin Cities metroplex in Minnesota; Orange County, California; and Toronto, Canada [3,4,13,25]. In such urban settings where raccoons occur and leave feces, B. procyonis eggs are extremely hardy, infective for years in the soil, and are resistant to destruction by most chemicals and environmental conditions [13]. As much as raccoons have been documented rummaging through garbage cans, their feces may also contaminate sidewalks, front porches, small yards, and local parks in cities.
Since B. procyonis is not a neurotropic parasite, but an accidental invader of the CNS, a relatively low percentage of larvae (est. 5–7%) actually enters the CNS following oral infection [10]. Hence, humans will not normally develop clinical CNS disease during accidental contaminative ingestion of low numbers of eggs [8,13,20]. However, neurological symptoms may indeed manifest when the ingested larval load is significant, as when infants engage in pica or geophagia of soil or other materials contaminated with raccoon feces and B. procyonis eggs [8,13,20]. The number of larvae entering the CNS is what determines the clinical severity of B. procyonis NLM, and the clinical syndrome may vary from subtle to severe [8, 20].
Once the CNS is infected with B. procyonis larvae, patients with NLM typically present with acute eosinophilic meningoencephalitis. Mechanical damage to the CNS caused by larval migration is compounded by the host inflammatory response, consisting largely of eosinophils, which is neither protective nor curative, but no doubt damaging [11, 19]. From a clinical and diagnostic perspective, one of the most serious problems with B. procyonis NLM is the lag time in development of clinical signs. Even in heavy infections, it typically takes two to three weeks before enough CNS damage occurs so that clinical signs are manifested and medical assistance is sought; by then the ensuing damage and complications may be very difficult to deal with [8,9,13,20,19,5, 24].
After being suspected clinically, the definitive diagnosis of B. procyonis NLM is dependent on the demonstration of anti-B. procyonis antibodies by ELISA in serum and CSF coupled to negative serology for Toxocara spp. and other agents. Western blotting is also very useful for separating infections with B. procyonis from Toxocara spp., which is known to cross-react in the B. procyonis screening ELISA [2]. Although elevated serum isohemagglutinins caused by cross-reactions between larval glycoproteins and human blood group antigens are not specific for B. procyonis, they do provide an additional clue, as they do for toxocariasis. Stool studies for ova and parasites are not helpful as B. procyonis does not complete its life cycle in humans [6].
With this infection, waiting for serologic results may unduly delay the initiation of treatment with albendazole (25–50 mg/kg daily, or 400 mg twice daily, each for 10–20+ days), and early empirical treatment is critical for hope of better outcome [8,12,13,20]. In animal studies, the greatest protection was seen when treatment was started within 1–3 days of infection and extended for 10 days [7,13, 18]. Unfortunately, except in cases of recognized exposure and probable infection, in most cases treatment initiation with albendazole will be delayed due to the lag time in development of clinical signs [8,20,12]. Corticosteroids have been used to mitigate the effects of eosinophil degranulation as well as the inflammation that occurs in the setting of larvicidal anthelmintic treatment.
Despite treatment with albendazole and corticosteroids, the prognosis for B. procyonis NLM is guarded to poor with or without treatment. However, with early and aggressive treatment or in cases of low infection with acute manifestations, several patients have been stabilized or have shown improvement [1, 23]. Most other survivors, however, have been left in a persistent vegetative state or with severe residual deficits [8,13,20]. Thus, early consideration and empiric treatment are both imperative for the best hope of maintaining neurological function in the affected patient [8,12,20]. In cases of eosinophilic meningoencephalitis of unknown etiology, particularly in children, it is recommended that B. procyonis be strongly considered and that treatment with albendazole and steroids be initiated promptly, while awaiting results of diagnostic tests and environmental sampling. Time is clearly of the essence in the treatment of this infection, and treatment can always be suspended later pending other results.
Acknowledgments
The authors would like to thank Dr. S. Dangoudoubiyam of Purdue University for performing the Baylisascaris serology. F. S. Machado was supported by Conselho Nacional de Desenvolvimento Cientfico e Tecnolgico (CNPq) and PRPq-UFMG.
References
- 1.Chun CS, Kazacos KR, Glaser C, Bardo D, Dangoudoubiyam S, Nash R. Global neurological deficits with Baylisascaris encephalitis in a previously healthy teenager. Ped Infect Dis J. 2009;28:925–927. doi: 10.1097/INF.0b013e3181a648f1. [DOI] [PubMed] [Google Scholar]
- 2.Dangoudoubiyam S, Kazacos KR. Differentiation of larva migrans caused by Baylisascaris procyonis and Toxocara species by Western blotting. Clin Vaccine Immunol. 2009;16:1563–1568. doi: 10.1128/CVI.00251-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eberhard ML, Nace EK, Won KY, Punkosdy GA, Bishop HS, Johnston SP. Baylisascaris procyonis in the metropolitan Atlanta area. Emerg Infect Dis. 2003;9:1636–1637. doi: 10.3201/eid0912.020795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans RH. Baylisascaris procyonis (Nematoda: Ascaridoidea) eggs in raccoon (Procyon lotor) latrine scats in Orange County, California. J Parasitol. 2002;88:189–190. doi: 10.1645/0022-3395(2002)088[0189:BPNAEI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 5.Fox AS, Kazacos KR, Gould NS, Heydemann PT, Thomas C, Boyer KM. Fatal eosinophilic meningoencephalitis and visceral larva migrans caused by the raccoon ascarid Baylisascaris procyonis. N Eng J Med. 1985;312:1619–1623. doi: 10.1056/NEJM198506203122507. [DOI] [PubMed] [Google Scholar]
- 6.Garcia LS. Diagnostic Medical Parasitology. Washington, DC: ASM Press; 2007. Baylisascaris procyonis; pp. 294–298. [Google Scholar]
- 7.Garrison RD. master’s thesis. West Lafayette, IN, USA: Purdue University; 1996. Evaluation of anthelmintic and corticosteroid treatment in protecting mice (Mus musculus) from neural larva migrans due to Baylisascaris procyonis. [Google Scholar]
- 8.Gavin PJ, Kazacos KR, Shulman ST. Baylisascariasis. Clin Microbiol Rev. 2005;18:703–718. doi: 10.1128/CMR.18.4.703-718.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gavin PJ, Kazacos KR, Tan TQ, Brinkman WB, Byrd SE, Davis AT, Mets MB, Shulman ST. Neural larva migrans caused by the raccoon roundworm Baylisascaris procyonis. Pediatr Infect Dis J. 2002;21:971–975. doi: 10.1097/00006454-200210000-00017. [DOI] [PubMed] [Google Scholar]
- 10.Kazacos KR. Raccoon ascarids as a cause of larva migrans. Parasitol Today. 1986;2:253–255. doi: 10.1016/0169-4758(86)90010-4. [DOI] [PubMed] [Google Scholar]
- 11.Kazacos KR. Visceral, ocular, and neural larva migrans. In: Connor DH, Chandler FW, Schwartz DA, Manz HJ, Lack EE, editors. Pathology of Infectious Diseases. Vol. 2. Stamford, CT: Appleton and Lange; 1997. pp. 1459–1473. [Google Scholar]
- 12.Kazacos KR. Protecting children from helminthic zoonoses. Contemp Pediatr. 2000;17(Suppl):1–24. [Google Scholar]
- 13.Kazacos KR. Baylisascaris procyonis and related species. In: Samuel WM, Pybus MJ, Kocan AA, editors. Parasitic Diseases of Wild Mammals. 2nd ed. Ames, IA: Iowa State University Press; 2001. pp. 301–341. [Google Scholar]
- 14.Kazacos KR, Raymond LA, Kazacos EA, Vestre WA. The raccoon ascarid. a probable cause of human ocular larva migrans. Ophthalmology. 1985;92:1735–1743. doi: 10.1016/s0161-6420(85)34100-3. [DOI] [PubMed] [Google Scholar]
- 15.Lewis RA. Discussion of [14] Ophthalmology. 1985;92:1743–1744. [Google Scholar]
- 16.Long SS. Principles and Practice of Pediatric Infectious Diseases. New York: Churchill Livingstone; 2008. [Google Scholar]
- 17.Mets MB, Noble AG, Basti S, Gavin P, Davis AT, Shulman ST, Kazacos KR. Eye findings of diffuse unilateral subacute neuroretinitis and multiple choroidal infiltrates associated with neural larva migrans due to Baylisascaris procyonis. Am J Ophthalmol. 2003;135:888–890. doi: 10.1016/s0002-9394(02)01539-8. [DOI] [PubMed] [Google Scholar]
- 18.Miyashita M. Prevalence of Baylisascaris procyonis in raccoons in Japan and experimental infections of the worm to laboratory animals. J Urban Living Hlth Assoc. 1993;37:137–151. (Japanese). [Google Scholar]
- 19.Moertel CL, Kazacos KR, Butterfield JH, Kita H, Watterson J, Gleich GJ. Eosinophil-associated inflammation and elaboration of eosinophil-derived proteins in 2 children with raccoon roundworm (Baylisascaris procyonis) encephalitis. Pediatrics. 2001;108:E93. doi: 10.1542/peds.108.5.e93. [DOI] [PubMed] [Google Scholar]
- 20.Murray WJ, Kazacos KR. Raccoon roundworm encephalitis. Clin Infect Dis. 2004;39:1484–1492. doi: 10.1086/425364. [DOI] [PubMed] [Google Scholar]
- 21.New York City Department of Health and Mental Hygiene. Health advisory #8: Baylisascariasis (raccoon roundworm) infection with severe outcome identified in two New York City children. [Accessed April 18, 2010];2009 Apr 9; http://www.nyc.gov/html/doh/downloads/pdf/cd/2009/09md08.pdf.
- 22.Page LK, Anchor C, Luy E, Kron S, Larson G, Madsen L, Kellner K, Smyser TJ. Backyard raccoon latrines and risk for Baylisascaris procyonis transmission to humans. Emerg Infect Dis. 2009;15:1530–1531. doi: 10.3201/eid1509.090128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pai PJ, Blackburn BG, Kazacos KR, Warrier RP, Bégué RE. Full recovery from Baylisascaris procyonis eosinophilic meningitis. Emerg Infect Dis. 2007;13:928–930. doi: 10.3201/eid1306.061541. [DOI] [PubMed] [Google Scholar]
- 24.Rowley HA, Uht RM, Kazacos KR, Sakanari J, Wheaton WV, Barkovich AJ, Bollen AW. Radiologic-pathologic findings in raccoon roundworm (Baylisascaris procyonis) encephalitis. Am J Neuroradiol. 2000;21:415–420. [PMC free article] [PubMed] [Google Scholar]
- 25.Yeitz JL, Gillin CM, Bildfell RJ, DeBess EE. Prevalence of Baylisascaris procyonis in raccoons (Procyon lotor) in Portland, Oregon, USA. J Wildl Dis. 2009;45:14–18. doi: 10.7589/0090-3558-45.1.14. [DOI] [PubMed] [Google Scholar]