Abstract
Previously we showed that thyroid hormone (T3) regulated the Herpes Simplex Virus Type -1 (HSV-1) gene expression and replication through its nuclear receptor TR via histone modification and chromatin remodeling in a neuroblastoma cell line neuro-2a cells (N2a). This observation suggested that T3 regulation may be neuron-specific and have implication in HSV-1 latency and reactivation. In this study, our in vitro latency/reactivation model demonstrated that removal of T3 can de-repress the HSV-1 replication and favor reactivation. Transfection studies and infection assays indicated that HSV-1 thymidine kinase (TK), a key viral gene during reactivation, was repressed by TR/T3 in cells with neuronal origin but not in non-neuronal cells. Additional studies showed that RCC1 (Regulator of Chromosome Condensation 1) was sequestered but efficiently detected upon viral infection in N2a cells. Western blot analyses indicated that addition of T3 repressed the RCC1 expression upon infection. It is likely that diminution of RCC1 upon infection in neuronal cells under the influence of TR/T3 may lead to repression of viral replication/gene expression thus promote latency. Together these results demonstrated that TR/T3 mediated regulation is specific to neuronal cells and differential chromosome condensation may play a critical role in this process.
Keywords: HSV-1, Thyroid hormone, Chromosome, Neuron, Latency
Introduction
HSV-1 is responsible for a number of infectious complications in humans (Lopez Garcia, Enriquez [1-2]. After a brief period of primary acute infection, the virus may launch a dormant infection in the sensory neuron of trigeminal ganglia for life and reactivation occurs over the different areas of lips collectively called cold sores or fever blisters [3]. HSV-1 can often lead to other clinical symptoms at different sites such as cornea (herpes keratitis) [4], finger and nail (herpetic whitlow)[5], skin (herpes dermatitis) [6], etc. The virus may result in lethal complications, for example, herpes encephalitis. Approximately 10% of viral encephalitis derived from HSV-1 [7] and reactivation from the latency in the trigeminal ganglia seemed to be playing a critical role although the molecular mechanisms leading to HSV encephalitis remained vague [8].
A variety of scenarios have been suggested to be involved in the establishment of HSV-1 latency and momentary reactivation [9]. Of all these mechanisms, hormonal regulation/imbalance was discussed but not extensively studied [10-13]. For example, thyroid hormone (TH) was shown to play a role in regulating HSV-1 key genes and participate in latency/reactivation but detailed molecular pathways are still unclear [14-16].
TH is produced from the thyroid gland and there are two products, thyroxine (T4) and triiodothyronine (T3), and the latter is more potent and responsible for the majority of biological effects [17]. T3 employs its function via its nuclear receptors (TRs), which are transcription factors usually dimerized with other nuclear receptors [18]. Two major TR isoforms were characterized. TR-α1 is broadly expressed with high level of expression in cardiac and skeletal muscles. TR-β1, however, is predominately produced in brain, liver and kidney [19]. It has been shown that both liganded and unliganded TRs are involved in gene regulation.
In addition to TH effects, roles of repressive chromatin/chromosome condensation on latent HSV-1 genome raised significant interest during the past decade [20-26]. The present investigation focused on the interaction of T3, TRs, and chromosome condensation during HSV-1 gene regulation in neuronal cells. The gene regulation of thymidine kinase (TK) was analyzed in neuroblastoma cells N2a and N2aTRβ (N2a cell constitutively expressing TR β). Our results indicated that T3/TR regulated the TK promoter activity and this regulatory effect is neuronal-specific with participation of chromosome condensation.
Methods
Viruses, cell lines, and culture conditions
The neuroblastoma cell line N2a (mouse; ATCC® CCL-131™) and SK-N-MC (human; ATCC® HTB-10™) were obtained from the American Type Culture Collection (ATCC) and cultured in DMEM/F12 medium supplemented with 10% charcoal-treated fetal bovine serum (FBS).
N2aTRβ cell was a generous gift from Dr. Robert Denver (University of Michigan, Ann Arbor, MI) and was maintained under the same conditions of N2a. Vero cells and HEK293 were cultured in 10% FBS supplemented with DMEM according to ATCC. All cells were cultured at 37°C with 5% CO2. HSV-1 strain 17Syn+-EGFP was a gift from Dr. Gus Kousoulas at the Louisiana State University [27]. T3 was acquired from Sigma (St. Louis, MO).
Plasmids and introduction of point mutation
The pRL-TK vector was procured from Promega (Madison, WI; Cat#: TB240) and it contains HSV-1 TK promoter driving luciferase as reporter. Transfection efficiency control was achieved by using Monster Green® Fluorescent Protein phMGFP Vector (Promega, Cat#: E6421). Normalization was accomplished by using plasmid pICP4-SEAP containing reporter secreted alkaline phosphatase (SEAP) driven by ICP4 promoter [25]. Site-directed mutagenesis at the TK TRE was essentially described previously [15].
Transfection
For non-neuronal cells, plasmids were transfected using lipofectame 2000 and DNA was mixed in Opti- MEM medium to allow complex formation according to the manufacturer (Life Technology, CA). N2a and N2aTRβ cells were transfected using Nucleofector II from Amaxa (Cat#: AAD-1001S, Gaithersburg, MD) for high efficiency of transfection (protocol essentially described by the manufacturer) using Kit V (Amaxa, Cat#: VCA-1005) and the protocol number was T-024. Plasmid phMGFP was cotransfected as a control.
Reporter assays
Transfected cells were harvested at 48 hr after transfection and the protocol was essentially described by the manufacturer (Promega, WI). Promoter activity was analyzed by measuring the luminescence over a 10-sec interval with a 2-sec delay on the 20/20n Luminometer (Turner Biosystem, Sunnyvale, CA). The results were presented as the fold induction of the reporter plasmid in the presence or absence of T3 (100 nM). The SEAP (Secreted Alkaline Phosphtase) assay was used as an internal control [23]. All experiments were performed with triplicate and repeated at least twice.
Infection
The N2a and Vero cells were plated in 35 mm dish a day before infection. On the day of infection the medium was removed and the cells were washed with PBS and then infected with 250 μl of medium containing 1 MOI of virus. At the 1hpi medium was replaced by fresh medium.
Antibodies
Anti-RCC1 is a mouse monoclonal antibody recognizing amino acids 122-421 purchased from Santa Cruz Biotechnology (Cat#: sc-55559) with 1:500 dilution for Western blot analyses. Anti-Ran is a rabbit polyclonal antibody obtained from Abcam (Cat#: ab4781). Anti-GFP is a rabbit antibody from Sigma-Aldrich (Cat#: G1544). Anti-α Tubulin antibody was procured from Calbiochem (Cat#: CP06). It is a mouse monoclonal antibody. All these antibodies used for Western blot analyses or immunofluorescent assays had the dilution factors performed according to the manufacturer's suggestions.
Immuno-fluorescence
Cells were treated based on the experimental needs a day before the test. To obtain the best results, approximately 20,000/well were placed in a multi-chamber slide (Cat# 354104 BD Falcon). After infection cells were washed once with 2 ml PBS for 5 min and fixed with 100% methanol at −20°C. Chamber slides were further incubated with 2% blocking serum followed by incubation with primary antibody overnight at 4°C. Fluorescent conjugated secondary antibody (Invitrogen cat# A21424) was used at RT for 1 hr. To finish, fluorescent mounting medium containing DAPI was applied for microscopy. The detection of EGFP, red fluorescence, and DAPI staining were recorded by an Olympus fluorescence microscope IX71 equipped with a digital camera photo apparatus DP71.
Western blotting
Cell extract was subjected to gel electrophoresis and transferred to membrane using iBlot® Gel Transfer Device (Life Technology, Cat#: IB1001). Anti-RCC1 was added (Cat#: sc-55559) with 1:500 dilution and 1:1000 for anti-GFP (Cat#: G1544). Anti-α-Tubulin, as an internal control was used at a dilution of 1:10,000. The chemi-luminescent signal was detected by Bio-Rad Chemi-docl XRS imaging systems (Hercules, CA). Our established protocol was described [28].
T3 removal experiments
N2a and N2aTRβ cells were prepared approximately 50% on a poly-D-lysine coated 6-well plate with 100 nM T3. Fresh T3 were refreshed every two days. On day 6, infection was performed at moi of 1. At 5 dpi, the media was collected and cells were washed with 1 ml of PBS followed by the addition of 1 ml of fresh media with or without T3 for 48 h. at the end of the experiments, the media were collected and subjected to plaque assays for replication analyses.
Plaque assays
Media collected from negative controls and infected cells were subjected to plaque assays by infecting Vero cells. Cell monolayers at 100% confluency in 24-well plates were incubated with 200 |il of media for 45 min on a rocking platform at different dilutions followed by addition of 1 ml fresh media to each well for 48 h. At the end the infected cells were rinsed with PBS and treated with crystal violet (PML Microbiologicals, Wilsonville, OR) for 20 min followed by washing with water. Plaques were counted in each triplicated well and the probability was measured by a Student's paired t-Test with a two-tailed distribution using Microsoft Excel.
Results
Removal of T3 reversed the hormone-mediated repression of HSV-1 replication
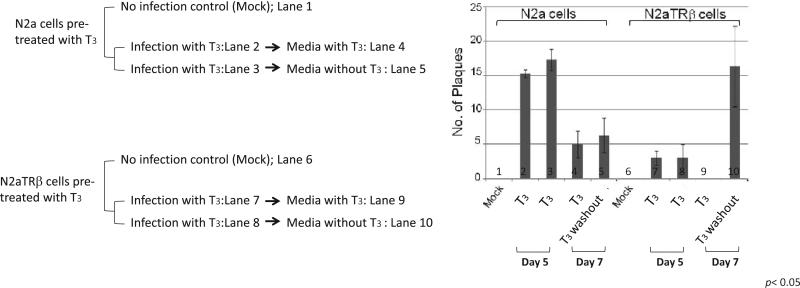
The regulatory effect of T3 on viral replication was tested using our in vitro latency cell culture model. The results showed that on Day 5 while T3 is present, the liganded TR exerted stronger inhibitory effects on the viral replication (Figure 1, compare bar 2, 3 and 7, 8). Subsequent observation indicated that viral replication continued to decrease after 7 dpi, particularly in the presence of T3 (Figure 1, compare bar 4 and 9). Fluorescent microscopy detected similar numbers of cells producing green fluorescent signals confirming that the number of infected cells were equivalent (data not shown). Interestingly, the replication increased drastically when the hormone was washed out only when the TR is present (Figure 1, compare bar 4, 5, 9, and 10). These results demonstrated that T3/TR repressed HSV-1 replication in neuronal cells. This inhibition is reversible since hormone washout can restore the replication capability.
Figure 1. T3 washout increased the release of infectious HSV-1 in N2aTRβ cells.
A. Scheme of infection and T3 treatment.
B. Cells were infected with HSV-1 at moi of 1 in the presence of T3. At Day 5 post-infection the media were removed/collected and fresh media with or without T3 were added. At Day 7 post-infection the media were collected and subjected to plaque assays. Lane 1 and 6: no infection control; Lane 2, 3, 7, and 8: infection with thyroid hormone T3 and the plaque assays using media collected on Day 5; Lane 4 and 9: plaque assays performed using media collected on wells from Lane 2 and 7, respectively; Lane 5 and 10: plaque assays performed using media collected on wells from Lane 3 and 8, respectively.
Regulation of HSV-1 TK by T3 and TR was specific to neuronal cells
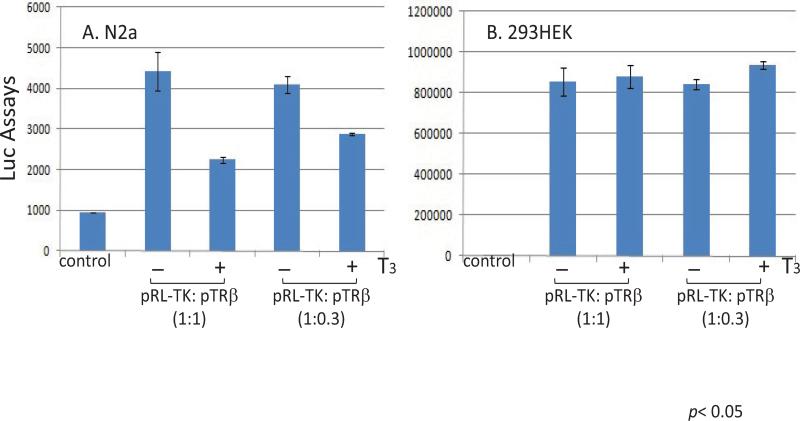
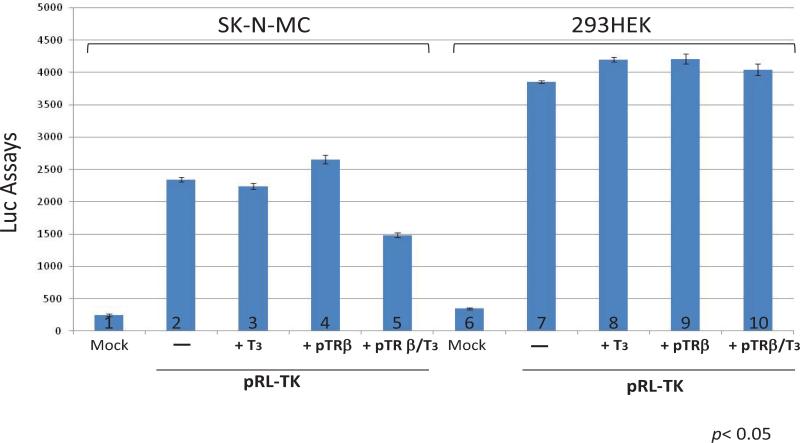
To test this hypothesis, neuronal cells n2a and non-neuronal cells 293HEK were co-transfected with pRL-TK and TRβ expression vector pTRβ in the absence or presence of T3 followed by reporter assays. The Luc assays data revealed a dose-dependent repression on TK promoter activity in the presence of T3 only in n2a cells but not in HEK293, which is a non-neuronal, human embryonic kidney cells (Figure 2A and 2B). this hypothesis was further characterized by transfecting a human neuronal cells SK-N-MC and the results confirmed the previous observation that hormone-mediated repression was limited to cells with neuronal origin (Figure 3). It has been suggested that HEK293 exhibited several neuronal makers [29] and this observation would argue our hypothesis. In our opinion, this T3/TR mediated regulation is likely due to a complex scenario involving not only neuronal factors but also differentiation condition since HEK293 never displayed an obvious differentiation under standard culture condition. It is unclear why a human embryonic kidney cell line would reveal neuronal makers but continues to proliferate without differentiation, a signature of neurons. We also found other non-neuronal cells such as Vero showed no T3/TR regulation on HSV-1 TK (unpublished data). N2a cells, nevertheless, was reported to unveil a differentiation morphology and physiology in the presence of TRβ and T3 (Lebel JM, et al. [30]). Together these results indicated that neuronal cellular environment as well as differentiation status and specific epigenetic control may contribute to this novel regulation. TK is not required for lytic infection. However, it is essential for viral replication in neurons to generate dNTP since it is in a resting state [15]. Besides, viral replication, although occurred later during the lytic infection, is critical for efficient α and β expressions in neurons during reactivation [31]. Therefore TK has been proposed to promote a transcription and replication during reactivation [32]. This hypothesis was further supported by in vivo studies that TK was one of the first genes to be produced [33] and TK-null mutant exhibited poor α and β expression during reactivation [34]. Hence we proposed that TK regulation by T3/TR played a vital role during viral reactivation and T3 level was crucial to control global HSV-1 gene expression and regulate viral latency.
Figure 2. TR/T3 repressed TK in mouse neuronal cells.
A. Co- transfection of pTRβ and pRL-TK in mouse N2a cell line regulated TK expression in presence of T3.
B. HEK 293 cells co- transfection of pTRβ and pRL-TK with or without T3 showed no regulation of TK activity from Luciferase assay.
Figure 3. Human neuronal cells exhibited TK regulation by TR/T3.
Transient co-transfection of pRL-TK and pTRβ in human neuronal cells SK-N-MC produced an inhibitory effect on TK promoter activity in the presence of T3. This regulation was not seen in HEK293 cells by Luciferase Assay.
Participation of chromosomal condensation in the T3-mediatd regulation on HSV-1 replication
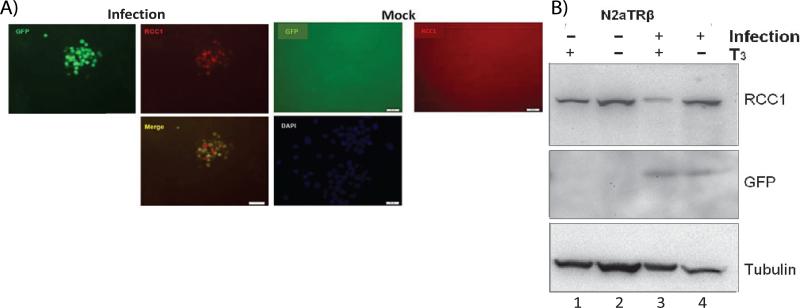
It is known that HSV-1 replicated poorly in the absence of RCC1 since temperature-sensitive cells lacking RCC1 blocked HSV-1 replication [35-36] is a signaling molecule responsible for the regulation of chromosomal condensation, sensing non-replicating DNA, and generating the inhibitory signal to halt mitosis. Loss of function causes premature/inappropriate chromatin condensation, and thus inhibits gene expression [37]. Therefore we investigated the role of RCC1 using our in vitro n2a cell culture model by immuno-fluorescent assays. The results showed that RCC1 coexist with cells that were infected, cells without infection exhibited no RCC1 signal (Figure 4A). However, Western blot analyses indicated that RCC1 was present in cells regardless the status of infection but T3 treatment reduced the level of RCC1 (Figure 4B). The level of RCC1 was not affected when the virus infected Vero cells but drastically reduced during infection of N2a cells in the presence of TR/T3 (data not shown). It is not understood why RCC1 was not seen from the immuno-fluorescent assays. The possible explanation is that RCC1 is a nuclear protein which may not be efficiently assessable during immunofluorescent assays but the infection disrupted the subcellular organization thus made the RCC1 available. It is also likely that RCC1 was sequestered within the cellular compartment and HSV-1 infection induced post-translational modification on the RCC1 and exposed the RCC1.
Figure 4. TR/T3 modulated RCC1 expression profile during infection.
A. RCC1 was detected in N2aTRβ cells upon infection. Scale bar: 50 μm.
B. Western blot analyses showed that RCC1 was present in N2aTRβ cells without infection. TR/T3 decreased the RCC1 detection upon infection.
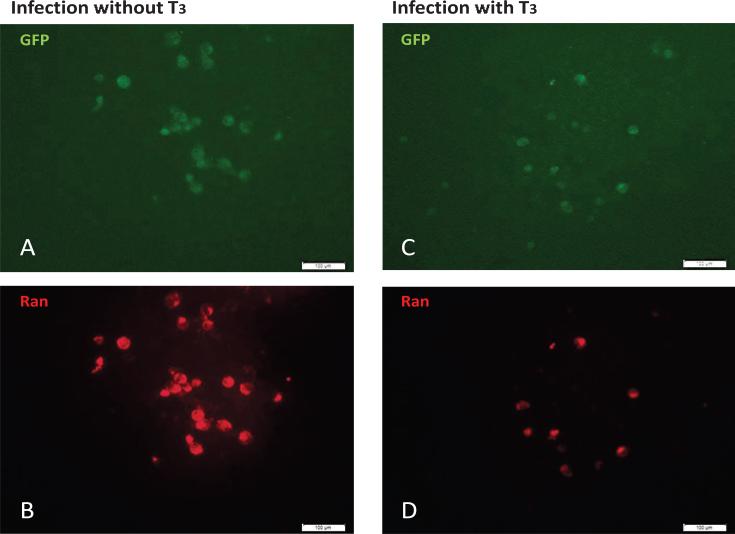
In addition, RCC1 functions as a Guanine nucleotide exchange factor to control the trafficking of the nuclear G protein Ran (RAs-related Nuclear protein). Ran is required for the translocation of cellular cargo such as RNA and proteins through the nuclear pore complex and also participated in the regulation of DNA synthesis and cell cycle progression [38]. Based on this information, it is likely that TR/T3 influenced the chromatin structure of HSV-1 in neuronal cells by affecting the level of RCC1/Ran complex and thus controlled mitosis, DNA replication, and cargo trafficking between nucleus and cytoplasm. To further study this novel hypothesis, we examine the Ran status in our model in the presence or absence of T3. The results of immuno-fluorescent assays indicated that when the T3 was removed and viral replication was active, the infected cells emitted green (virus) and red (Ran) fluorescence with strong intensity (Figure 5A and 5B). Nonetheless while T3 was available and the viral replication was repressed, the infected cells were decreased with weaker Ran signal (Figure 5C and 5D). Together these observations suggested that RCC1/Ran is beneficial to viral replication, promotes lytic infection of virus, and halts host replication. TR/T3 may repress viral gene expression/replication via reduction of RCC1/Ran expression/availability and thus keep the viral DNA in circular and promotes latency.
Figure 5.
Ran expression was diminished in N2aTRβ cells upon infection by TR/T3. Infection of N2aTRβ cells with T3 exhibited weaker Ran expression. Bar: 100 μm.
In conclusion, our results showed that thyroid hormone exert a novel negative regulation on HSV-1 gene TK and it required TR and neuronal environment. More studies are underway to investigate the roles of chromatin condensation, histone modification, chromatin remodeling, chromatin insulation in the TR/T3 mediated HSV-1 gene regulation and replication in neuronal environment.
Acknowledgment
We thank Dr. Timothy Foster of LSUHSC in New Orleans and Dr. Gus Kousoulas from LSU Baton Rouge, LA for the 17Syn+-EGFP virus. Our gratitude also goes to Dr. Robert Denver from University of Michigan in Ann Arbor, MI for the N2aTRβ cell line. We are grateful for the assistance of the editorial staff at University of Maryland Eastern Shore. This project is supported by NIH R01NS081109 to SVH. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS/NIH.
Footnotes
Citation: Chen F, Palem J, Balish M, Figliozzi R, Ajavon A, et al. (2014) A Novel Thyroid Hormone Mediated Regulation of HSV-1 Gene Expression and Replication is Specific to Neuronal Cells and Associated with Disruption of Chromatin Condensation. SOJ Pharm PharmSci, 1(1), 7.
References
- 1.Lopez Garcia FR, Enriquez Ascarza R, Rodriguez Martinez JC, Sirvent Pedreno AE. Interferon therapy for herpes simplex virus infection in a 70 years old patient. An Med Interna. 2002;19:600–601. [PubMed] [Google Scholar]
- 2.Taylor TJ, Brockman MA, McNamee EE, Knipe DM. Herpes simplex virus. Front Biosci. 2002;7:752–764. doi: 10.2741/taylor. [DOI] [PubMed] [Google Scholar]
- 3.Everett RD. ICP0, a regulator of herpes simplex virus during lytic and latent infection. Bioessays. 2000;22:761–770. doi: 10.1002/1521-1878(200008)22:8<761::AID-BIES10>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 4.Shah A, Farooq AV, Tiwari V, Kim MJ, Shukla D. HSV-1 infection of human corneal epithelial cells: receptor-mediated entry and trends of re-infection. Mol Vis. 2010;16:2476–2486. [PMC free article] [PubMed] [Google Scholar]
- 5.Usatine RP, Tinitigan R. Nongenital herpes simplex virus. Am Fam Physician. 2010;82:1075–1082. [PubMed] [Google Scholar]
- 6.Higaki S, Inoue Y, Yoshida A, Maeda N, Watanabe H, et al. Case of bilateral multiple herpetic epithelial keratitis manifested as dendriform epithelial edema during primary Kaposi's varicelliform eruption. Jpn J Ophthalmol. 2008;52:127–129. doi: 10.1007/s10384-007-0514-6. [DOI] [PubMed] [Google Scholar]
- 7.Martinez PA, Diaz R, Gonzalez D, Oropesa L, Gonzalez R, et al. The effect of highly active antiretroviral therapy on outcome of central nervous system herpesviruses infection in Cuban human immunodeficiency virus-infected individuals. J Neurovirol. 2007;13:446–451. doi: 10.1080/13550280701510088. [DOI] [PubMed] [Google Scholar]
- 8.Goel N, Mao H, Rong Q, Docherty JJ, Zimmerman D, et al. The ability of an HSV strain to initiate zosteriform spread correlates with its neuroinvasive disease potential. Arch Virol. 2002;147:763–773. doi: 10.1007/s007050200024. [DOI] [PubMed] [Google Scholar]
- 9.Hsia SC, Bedadala GR, Balish MD. Effects of thyroid hormone on HSV-1 gene regulation: implications in the control of viral latency and reactivation. Cell Biosci. 2011;1:1–24. doi: 10.1186/2045-3701-1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garza HH, Jr, Hill JM. Effect of a beta-adrenergic antagonist, propranolol, on induced HSV-1 ocular recurrence in latently infected rabbits. Curr Eye Res. 1997;16:453–458. doi: 10.1076/ceyr.16.5.453.7051. [DOI] [PubMed] [Google Scholar]
- 11.Hardwicke MA, Schaffer PA. Differential effects of nerve growth factor and dexamethasone on herpes simplex virus type 1 oriL- and oriS-dependent DNA replication in PC12 cells. J Virol. 1997;71:3580–3587. doi: 10.1128/jvi.71.5.3580-3587.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Noisakran S, Halford Veress WP, Veress L, Carr DJ. Role of the hypothalamic pituitary adrenal axis and IL-6 in stress-induced reactivation of latent herpes simplex virus type 1. J Immunol. 1998;160:5441–5447. [PubMed] [Google Scholar]
- 13.Marquart M, Bhattacharjee P, Zheng X, Kaufman H, Thompson H, et al. Ocular reactivation phenotype of HSV-1 strain F(MP)E, a corticosteroid-sensitive strain. Curr Eye Res. 2003;26:205–209. doi: 10.1076/ceyr.26.3.205.14890. [DOI] [PubMed] [Google Scholar]
- 14.Bedadala GR, Pinnoji RC, Palem JR, Hsia SC. Thyroid hormone controls the gene expression of HSV-1 LAT and ICP0 in neuronal cells. Cell Res. 2010;20:587–598. doi: 10.1038/cr.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsia SC, Pinnoji RC, Bedadala GR, Hill JM, Palem JR. Regulation of herpes simplex virus type 1 thymidine kinase gene expression by thyroid hormone receptor in cultured neuronal cells. J Neurovirol. 2010;16:13–24. doi: 10.3109/13550280903552412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Varedi M, Moattari A, Amirghofran Z, Karamizadeh Z, Feizi H. Effects of hypo- and hyperthyroid states on herpes simplex virus infectivity in the rat. Endocr Res. 2013;39:50–55. doi: 10.3109/07435800.2013.808208. [DOI] [PubMed] [Google Scholar]
- 17.Deme D, Pommier J, Nunez J. Kinetics of thyroglobulin iodination and of hormone synthesis catalysed by thyroid peroxidase. Role of iodide in the coupling reaction. Eur J Biochem. 1976;70:435–440. doi: 10.1111/j.1432-1033.1976.tb11034.x. [DOI] [PubMed] [Google Scholar]
- 18.Tsai MJ, O'Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- 19.Flamant F, Baxter JD, Forrest D, Refetoff S, Samuels H, et al. International Union of Pharmacology. LIX. The pharmacology and classification of the nuclear receptor superfamily: thyroid hormone receptors. Pharmacol Rev. 2006;58:705–711. doi: 10.1124/pr.58.4.3. [DOI] [PubMed] [Google Scholar]
- 20.Kubat NJ, Amelio AL, Giordani NV, Bloom DC. The herpes simplex virus type 1 latency-associated transcript (LAT) enhancer/rcr is hyperacetylated during latency independently of LAT transcription. J Virol. 2004;78:12508–12518. doi: 10.1128/JVI.78.22.12508-12518.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kubat NJ, Tran RK, McAnany P, Bloom DC. Specific histone tail modification and not DNA methylation is a determinant of herpes simplex virus type 1 latent gene expression. J Virol. 2004;78:1139–1149. doi: 10.1128/JVI.78.3.1139-1149.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amelio AL, McAnany PK, Bloom DC, et al. A chromatin insulatorlike element in the herpes simplex virus type 1 latency-associated transcript region binds CCCTC-binding factor and displays enhancer-blocking and silencing activities. J Virol. 2006;80:2358–2368. doi: 10.1128/JVI.80.5.2358-2368.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bedadala GR, Pinnoji RC, Hsia SC. Early growth response gene 1 (Egr-1) regulates HSV-1 ICP4 and ICP22 gene expression. Cell Res. 2007;17:546–555. doi: 10.1038/cr.2007.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen Q, Lin L, Smith Sheryl, Huang Jing, Berger Shelley L, et al. A CTCF-dependent chromatin boundary element exists between the LAT and ICP0 promoters in the HSV-1 genome. J Virol. 2007;10:5192–5201. doi: 10.1128/JVI.02447-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinnoji RC, Bedadala GR, George B, Holland TC, Hill JM, et al. Repressor element-1 silencing transcription factor/neuronal restrictive silencer factor (REST/NRSF) can regulate HSV-1 immediate-early transcription via histone modification. Virol J. 2007;4:56. doi: 10.1186/1743-422X-4-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knipe DM, Cliffe A. Chromatin control of herpes simplex virus lytic and latent infection. Nat Rev Microbiol. 2008;6:211–221. doi: 10.1038/nrmicro1794. [DOI] [PubMed] [Google Scholar]
- 27.Foster TP, Rybachuk GV, Kousoulas KG. Expression of the enhanced green fluorescent protein by herpes simplex virus type 1 (HSV-1) as an in vitro or in vivo marker for virus entry and replication. J Virol Methods. 1998;75:151–160. doi: 10.1016/s0166-0934(98)00107-4. [DOI] [PubMed] [Google Scholar]
- 28.Bedadala GR, Palem JR, Graham L, Hill JM, McFerrin HE, et al. Lytic HSV-1 infection induces the multifunctional transcription factor Early Growth Response-1 (EGR-1) in rabbit corneal cells. Virol J. 2011;8:262. doi: 10.1186/1743-422X-8-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaw G, Morse S, Ararat M, Graham FL. Preferential transformation of human neuronal cells by human adenoviruses and the origin of HEK 293 cells. FASEB J. 2002;16:869–871. doi: 10.1096/fj.01-0995fje. [DOI] [PubMed] [Google Scholar]
- 30.Lebel JM, Dussault JH, Puymirat J. Overexpression of the beta 1 thyroid receptor induces differentiation in neuro-2a cells. Proc Natl Acad Sci U S A. 1994;91:2644–2648. doi: 10.1073/pnas.91.7.2644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nichol PF, Chang JY, Johnson EM, Jr, Olivo PD. Herpes simplex virus gene expression in neurons: viral DNA synthesis is a critical regulatory event in the branch point between the lytic and latent pathways. J Virol. 1996;70:5476–5486. doi: 10.1128/jvi.70.8.5476-5486.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tal-Singer R, Peng C, Ponce De Leon M, Abrams WR, Banfield BW, et al. Interaction of herpes simplex virus glycoprotein gC with mammalian cell surface molecules. J Virol. 1995;69:4471–4483. doi: 10.1128/jvi.69.7.4471-4483.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tal-Singer R, Lasner TM, Podrzucki W, Skokotas A, Leary JJ, et al. Gene expression during reactivation of herpes simplex virus type 1 from latency in the peripheral nervous system is different from that during lytic infection of tissue cultures. J Virol. 1997;71:5268–5276. doi: 10.1128/jvi.71.7.5268-5276.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kosz-Vnenchak M, Jacobson J, Coen DM, Knipe DM. Evidence for a novel regulatory pathway for herpes simplex virus gene expression in trigeminal ganglion neurons. J Virol. 1993;67:5383–5393. doi: 10.1128/jvi.67.9.5383-5393.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Umene K, Nishimoto T. Replication of herpes simplex virus type 1 DNA is inhibited in a temperature-sensitive mutant of BHK-21 cells lacking RCC1 (regulator of chromosome condensation) and virus DNA remains linear. J Gen Virol. 1996;77:2261–2270. doi: 10.1099/0022-1317-77-9-2261. [DOI] [PubMed] [Google Scholar]
- 36.Strang BL, Stow ND. Blocks to herpes simplex virus type 1 replication in a cell line, tsBN2, encoding a temperature-sensitive RCC1 protein. J Gen Virol. 2007;88:376–383. doi: 10.1099/vir.0.82417-0. [DOI] [PubMed] [Google Scholar]
- 37.Dasso M. RCC1 in the cell cycle: the regulator of chromosome condensation takes on new roles. Trends Biochem Sci. 1993;18:96–101. doi: 10.1016/0968-0004(93)90161-f. [DOI] [PubMed] [Google Scholar]
- 38.Pemberton LF, Paschal BM. Mechanisms of receptor-mediated nuclear import and nuclear export. Traffic. 2005;6:187–198. doi: 10.1111/j.1600-0854.2005.00270.x. [DOI] [PubMed] [Google Scholar]