Abstract
The biologic agents vary considerably in terms of their long-term duration of effect. Using the definitions provided by the National Psoriasis Foundation Medical Board, the objective of this review was to compare all biologic agents with respect to time to relapse and potential for rebound. Overall, alefacept had the longest off-treatment benefit (29.9 weeks in Psoriasis Area and Severity Index [PASI] 75 responders), followed by ustekinumab (22 weeks), infliximab (19.5 weeks), adalimumab (18 weeks), etanercept (12.1 weeks in PASI 50 responders), and, lastly, efalizumab (9.6 weeks). Rebound was reported commonly for efalizumab (14%) and, extremely rarely, for etanercept (0.002%).
Keywords: biologic agents, relapse, rebound
INTRODUCTION
Psoriasis is an inflammatory, hyperproliferative skin disorder with systemic manifestations, affecting 2% to 3% of the U.S. population. Because of its chronic nature and variable course, it is responsible for causing significant patient morbidity. Currently no available therapies are curative, and generally the disease sooner or later recurs on discontinuation of therapy. Given that, the biologic agents have received considerable attention over the years because of demonstrated efficacy and potentially better long-term safety profile with respect to major organ toxicity. However, although all currently approved biologics are immunosuppressants, their chemical compositions and mechanisms of action differ, and thus their individual long-term benefits vary considerably.
The National Psoriasis Foundation Medical Board defined key outcome measures for these long-term therapeutic effects so that clinical trial results could be compared.1 Two of the key terms addressed were time to relapse and rebound. Relapse can be applied only to responders, those who attained a 50% or better improvement in Psoriasis Area and Severity Index (PASI) score from baseline (i.e., PASI 50). Time to relapse is therefore the time from the end of treatment to when 50% improvement in baseline PASI is lost in responders (i.e., time to loss of PASI 50). Rebound is defined as a PASI score 125% or greater of baseline or change in morphology (generalized pustular, erythrodermic, or increased inflammatory psoriasis) within 3 months of stopping treatment.1
The objective of this review is to evaluate the long-term impact of biologic agents on disease state using the Foundation medical board’s definitions published in the Archives of Dermatology.1 Reviewing published clinical trials, a comparison of all biologic agents and their respective duration of treatment response are presented.
METHODS
A search of PubMed was conducted using two different search strategies. First, a search for clinical trials using the term psoriasis and the names of biologic agents (alefacept, efalizumab, etanercept, infliximab, adalimumab, and ustekinumab) was conducted. Second, a search for studies using the names of biologic agents and the terms duration of therapeutic effect, (time to) relapse, remission, and rebound was also conducted. Only studies where off-treatment responses (duration of therapeutic effect and remission, time to relapse, and incidence of rebound) were clearly defined were included in the comparison. Although efalizumab has been discontinued, we thought it important to include it in this review to provide as comprehensive an assessment as possible for this class of therapeutic agents (i.e., biologics).
RESULTS
Thirteen publications fit the selection criteria and were clinical trials (randomized, double-blind, or open-label) evaluating biologic agents with reference to relapse and rebound. No consistent data on duration of therapeutic effect and remission could be found. Studies included patients at least 18 years old with stable, chronic plaque psoriasis affecting at least 10% body surface area and evaluated patients receiving biologic monotherapy.
Many of the studies reported data according to varying definitions of relapse, not in accordance with the strict definitions provided by the NSF MAB. In an effort to compare off-treatment responses to the various biologic agents, these results were still included and classified as “time to relapse” with qualifying factors noted.
Alefacept (Amevive)
Ellis and colleagues (2001) conducted a phase II clinical trial with 249 participants, who received intravenous alefacept (0.025, 0.075, or 0.150 mg/kg) or placebo weekly for 12 weeks, with off-treatment follow-up for at least 12 weeks. Twelve weeks after stopping treatment, 47% of patients in the 0.025 mg/kg group, 63% of patients in the 0.075 mg/kg group, and 42% of patients in the 0.150 mg/kg group had maintained at least a PASI 50. For those who achieved a Physician Global Assessment (PGA) of “clear” or “almost clear,” the median time to re-treatment (criteria for re-treatment not clearly defined) was 306 days. Twelve weeks after initial treatment ended, 24% of patients still had a PGA status of “clear” or “almost clear,” despite not having treatment during the follow-up period. No cases of rebound were encountered.2
Gordon and Langley (2003) presented a double-blind extension study of a previous phase III trial of 131 patients receiving intramuscular alefacept (10 or 15 mg) or placebo weekly for 12 weeks, followed by a minimum of 12-week treatment-free follow-up. In those receiving 15 mg intramuscular alefacept and achieving PASI 75 at any point in the study, the median time to loss of PASI 50 was 209 days in 54 patients. In those who achieved a PGA status of “clear” or “almost clear,” the median duration to loss of PASI 50 was 245 days in 40 patients. No rebound was encountered after discontinuation of treatment.3
Efalizumab (Raptiva)
Carey and colleagues (2006) presented a summary of pooled data from various phase III trials. Patients received treatment for 12 to 24 weeks, with an off-treatment observation period following. In PASI 75 responders, median time to loss of PASI 50 was 67 days. Rebound was experienced in 14% of patients (188 of 1,316).4
Several other studies have also demonstrated this rebound phenomenon on discontinuation of efalizumab. In the initial clinical trial conducted by Genentech for licensing, rebound was identified in 13% (152 of 1,201) of patients, with patients experiencing PASI 125 or change in morphology to guttate, erythrodermic, or pustular psoriasis.5 Tsai (2007) conducted an open-label study of 49 patients receiving 1 mg/kg subcutaneous efalizumab for 12 weeks, followed by an observation period. Rebound was seen in 18% of patients (8 of 45).6
Etanercept (Enbrel)
In a phase III trial conducted by Gordon and colleagues (2006), 652 patients received subcutaneous etanercept (25 mg weekly, 25 mg twice weekly, or 50 mg twice weekly) or placebo for 12 weeks. For the subsequent 12 weeks, patients receiving drug were continued on the same dose and placebo-treated patients were switched to subcutaneous etanercept 25 mg twice weekly. Following the 24-week double-blind phase, patients who achieved PASI 50 underwent an off-treatment period until relapse, and those who did not achieve PASI 50 received open-label treatment with etanercept. Of 409 patients who participated in the off-treatment phase of the study, 347 patients relapsed and required retreatment. Median time to loss of PASI 50 in PASI 50 responders was 85 days (range, 70–91 days). Median time to loss of PASI 50 in PASI 75 responders was 91 days. One patient of 409 experienced rebound. At the 60-week conclusion of the study with 36 weeks of off-treatment time, 11% of patients were in remission.7
Moore and associates (2007) conducted an open-label study of 2,546 patients who received 50 mg twice weekly for 12 weeks, followed by continuous or interrupted therapy with 50 mg once weekly for the next 12 weeks. Interrupted therapy was defined as re-treatment if PGA score was 2 or less (scale: 0 = clear, 1 = almost clear, 2 = mild, 3 = moderate, 4 = marked, 5 = severe). Median time to loss of PGA responder status was 39.6 days. At the conclusion of the study at week 24, after 12 weeks off treatment, 36.1% of patients (331 of 917 patients who were week 12 responders in the interrupted arm) did not require further treatment. After 12 weeks of interrupted therapy, 32.2% (n= 1,272) had maintained a PGA status of “clear” or “almost clear.”8
Infliximab (Remicade)
In a phase II study conducted by Gottlieb and colleagues (2003), 33 patients were given placebo or infliximab (5 or 10 mg/kg) at 0, 2, and 6 weeks during the initial induction phase. Inflixmab treatment groups and placebo nonresponders were then re-randomized at week 10 into the open-label extension phase, in which patients received open label infliximab (5 or 10mg/kg) at weeks 10, 12, and 16. Placebo responders were followed up until relapse (loss of 50% improvement in PASI score achieved at week 10) and then given the same three-dose induction regimen of infliximab, with their study visits reset to 0, 2, and 6 weeks following the initial 12 weeks. Thus, there were two different groups: infliximab treatment groups receiving two induction regimens and the placebo group receiving one induction regimen. All patients responding to treatment (at least 50% improvement in PASI) were followed up until relapse for another 26 weeks and offered single-dose re-treatment infusions on relapse. Nine of 22 (41%) patients who initially responded to infliximab relapsed and required retreatment. Time to relapse was 6 to 18 weeks from the end of treatment; 57% of patients maintained at least PASI 50, and 50% maintained at least PASI 75 at week 26. The authors therefore suggest that infliximab can induce clearing of psoriasis for up to 20 weeks after initial treatment. No rebound was reported in the study.9
In a subsequent phase II study, also conducted by Gottlieb and colleagues (2004), 259 patients received intravenous infliximab (3 or 5 mg/kg) or placebo at 0, 2, and 6 weeks. At week 26, patients with a PGA score of 3 or greater were eligible for re-treatment. Improvements in response were observed up until 10 to 14 weeks, after which PASI scores began to increase toward placebo levels. At week 26, 72.2% of patients (114 of 158 who completed the 30-week study) required re-treatment. Based on the figure provided by the authors (reproduced in Figure 1), the median time to loss of PASI 50 can be estimated to be 19.5 weeks (range, 16–23 weeks) after treatment. The authors concluded that a maintenance dosing of infliximab every 8 weeks would be appropriate to maintain a meaningful clinical response.10
Figure 1.
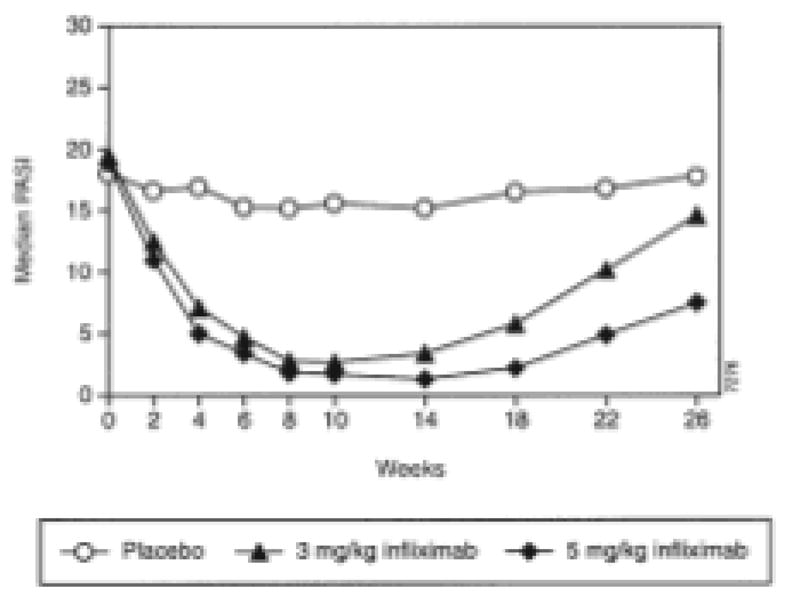
Median Psoriasis Area and Severity Index (PASI) with infliximab treatment at weeks 0, 2, and 6.
(Adapted with permission from Gottlieb AB, Evans R, Li S, Dooley LT, Guzzo CA, Baker D, et al. Infliximab induction therapy for patients with severe plaque-type psoriasis: a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2004;51:534–42.)
Menter and associates (2007) conducted a randomized, double-blind trial of 835 patients who received placebo or infliximab (3 or 5 mg/kg) at 0, 2, and 6 weeks, followed by either maintenance every 8 weeks of dosing of the same original dose or as needed treatment from week 14 through week 46. Patients in the placebo group crossed over to infliximab 5 mg/kg at weeks 16, 18, and 22 and every 8 weeks thereafter. In the as-needed group, among the PASI 75 responders, time to loss of PASI 75 was 14 to 22 weeks after stopping infliximab. Re-treatment was initiated when improvement in PASI score was less than 75%; the most common interval between infusions was 4 to 8 weeks. No rebound was experienced.11
The literature has suggested that a specific chemical property of infliximab progressively diminishes its duration of therapeutic effect over time. Because infliximab is a chimeric protein (75% human and 25% mouse), it is immunogenic and associated with increased concern regarding the development of anti-infliximab antibodies. In a phase III trial conducted by Reich and colleagues (2005),12 maintenance of therapeutic response was related to having stable serum infliximab concentrations and was greater for antibody-negative patients than for antibody- positive patients. Additionally, because of the development of antibodies, many patients undergoing long-term treatment (>1 year) develop the phenomenon of “dosage creep,” in which an increased dose or frequency interval is required to maintain the therapeutic response and overcome the neutralizing antibodies.13
In a phase III study, 78% of patients (n =150) achieved PASI 75 at week 10 with 5 mg/kg of infliximab administered every 8 weeks. However, at week 50, despite continued infliximab dosing every 8 weeks, only 54.5% of patients maintained a PASI 75. Similarly, 73.0% of patients (n = 148) achieved PASI 75 at week 10 with 3 mg/kg of infliximab administered as needed. At week 50, only 25.4% of patients maintained a PASI 75. Therefore, this loss of efficacy depends on dosage and dosing regimen (i.e., continuous infusions every 8 weeks vs. as-needed dosing).14
Antibodies to other biologics, specifically etanercept (a dimeric human and mouse fusion protein) and adalimumab (a human monoclonal antibody), have also been detected but are not associated with a decreased clinical response in psoriasis.15
Adalimumab (Humira)
Blum and associates (2005) conducted a phase II clinical trial of 136 patients who received placebo or 80 mg subcutaneous adalimumab at weeks 0 and 1, 40 mg subcutaneously weekly from weeks 2 to 12, and 40 mg subcutaneously every other week or placebo through week 24, with follow-up until week 76. Time to loss of PASI 50 in the adalimumab group was between 12 and 24 weeks. No rebound was noted.16
Menter and co-workers (2008) published a phase III clinical trial of 1,212 patients who received placebo at week 0, followed by placebo every other week until week 15 or 80 mg of subcutaneous adalimumab at week 0, followed by 40 mg subcutaneously every other week until week 15. Patients who achieved a PASI 75 response at week 16 were eligible to enter the open-label phase of the study. From weeks 17 to 33, all subjects received 40 mg of subcutaneous adalimumab every other week. At week 33, PASI 75 responders were eligible to enter period C of the study, in which patients either received placebo or adalimumab until week 52. During weeks 33 to 52, 28% of patients receiving placebo lost adequate response (defined as loss of PASI 50 and a 6-point increase in PASI score from week 33), compared with 5% continued on adalimumab. The time course and median time to of loss of PASI 50 were not specified in the study. The authors noted that the rate at which relapse occurred was significantly faster in the placebo group compared with that of the treatment group. No rebound was observed.17
Ustekinumab (Stelara)
Leonardi and colleagues (2008) conducted a phase III clinical trial comprising 766 patients. At weeks 0 and 4, patients received 45 or 90 mg of ustekinumab or placebo, followed by the maintenance dosing every 12 weeks up to week 40. Patients receiving placebo crossed over to ustekinumab at week 12 and continued dosing every 12 weeks until week 40. After week 40, patients either received placebo or continued with the same dosing of ustekinumab. In the group of patients receiving placebo after week 40, the median improvement in PASI scores declined from 96% at week 40 to 40% at week 64. The authors only reported time to loss of PASI 75 (15 weeks), based on the figure provided, so we estimated the median time to loss of PASI-50 to be approximately 62 weeks into the study, representing a 22-week interval from the end of treatment (Figure 2). No rebound was reported.18
Figure 2.
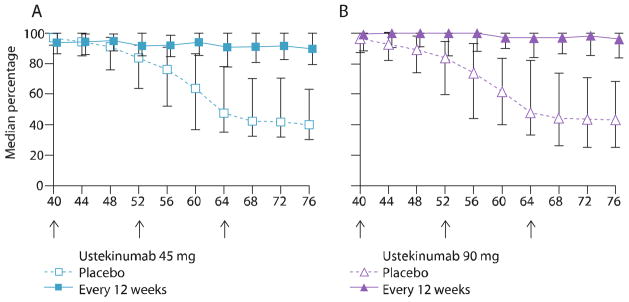
Median percentage improvement in Psoriasis Area and Severity Index (PASI) score from baseline at weeks 40 to 76 for ustekinumab (45 and 90 mg)
(Adapted with permission from Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y, et al; PHOENIX 1 study investigators. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial [PHOENIX 1]. Lancet. 2008;371:1665–74.)
DISCUSSION
In this review, all biologic therapies for chronic plaque psoriasis were compared in terms of time to relapse and possible rebound (Table 1). No data on duration of remission were available. One limitation of the study was that because many of the clinical trials were completed before the standardized definitions of these measures being published,1 many studies reported efficacy data using other definitions. Furthermore, trials varied in their study design, treatment regimen, off-treatment period, and endpoints for time to relapse. These studies were therefore difficult to interpret within this review but were still included for comparison. Another limitation of the study was the lack of head-to-head comparison between biologics.
Table 1.
Comparison of Time to Relapse and Rebound for Biologic Agents
| Biologic Agent | Treatment | Duration of Treatment | Time to Relapse (Loss of PASI 50) | Rebound |
|---|---|---|---|---|
| Alefacept (n = 131) | IM (15 mg) alefacept | 12 wk with 12-wk treatment-free follow-up | 29.9 wk in PASI 75 responders3 | None reported |
| Efalizumab (n = 1,316) | Pooled data; treatment regimens not specified | 12–24 wk of treatment, with treatment-free observation period | 9.6 wk in PASI 75 responders4 | 14% (188/ 1,316)4 |
| Etanercept (n = 652) | SC etanercept (25 mg weekly, 25 mg twice weekly, 50 mg twice weekly) | 12–24 wk of treatment, with treatment-free follow-up until relapse | 12.1 wk in PASI 50 responders7 | 0.002% (1/409)7 |
| Infliximab (n = 259) | IV infliximab (3 or 5 mg/kg) | Treatment at 0, 2, 6 wk, with follow- up until wk 30. Nonresponders were re-treated at week 26 | 19.5 wk for all patients10** | None reported |
| Adalimumab (n = 136) | SC adalimumab (80 mg at week 0 and 1, 40 mg SC weekly from weeks 2–12, 40 SC or placebo every other week through wk 24) | 12 wk open-label followed by 12 wk blinded with follow-up to 76 wk | 18 wk16 | None reported |
| Ustekinumab (n= 766) | 45 or 90 mg at wk 0 and 4 and every 12 wk thereafter until wk 40 | 28–40 wk of treatment, with follow-up until wk 76 | 22 wk in all patients18** | None reported |
Data for infliximab and ustekinumab were extrapolated from graphs provided in the original study.
IM = intramuscularly; IV = intravenously; PASI = Psoriasis Area and Severity Index; SC = subcutaneously
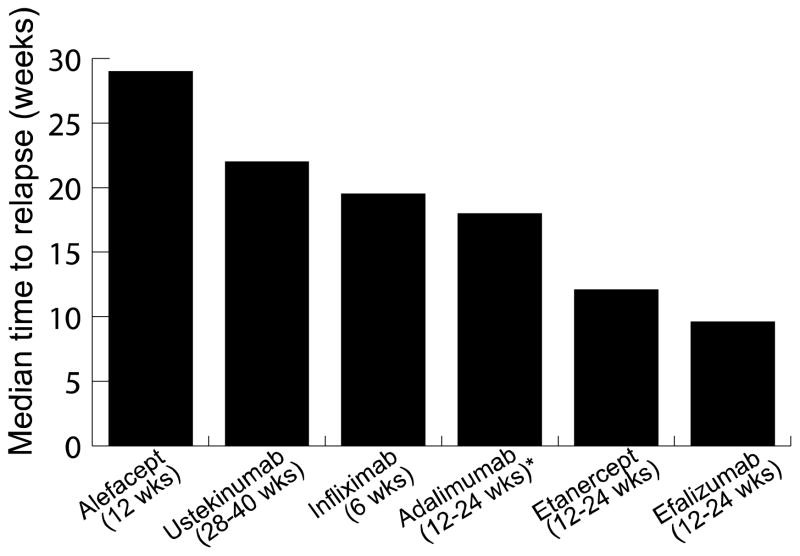
In reference to time to relapse, alefacept had the longest clinical benefit after drug was stopped, followed by ustekinumab, infliximab, adalimumab, and etanercept, and lastly efalizumab. One caveat for alefacept and efalizumab is that the time to relapse was measured only for PASI 75 responders. The time to loss of PASI 50 for ustekinumab and infliximab was based on estimations from graphs provided in the studies. It should also be noted that treatment courses for each biologic agent varied, from 6 to 40 weeks of treatment. The treatment duration could have impacted the time to relapse, as many biologics are known to produce a sustained or improved response with longer treatment durations (Figure 3). It should also be noted that the total number of subjects studied varied greatly among the biologics: alefacept (n = 380), efalizumab (n = 2,566), etanercept (n = 3,198), infliximab (n = 1,277), adalimumab (n = 1,348), and ustekinumab (n = 766). This variation could have also impacted data obtained on time to replase and incidence of rebound.
Figure 3.
Comparison of biologic agents and their respective time to relapse.
Results for alefacept,3 ustekinumab,18 efalizumab,4 and etanercept7 were based on phase III data. Infliximab10 was based on phase II data. Note: Graph is based on varying definitions of time to relapse.
*Dosing used for adalimumab not usual dosing used in clinical practice
Regarding rebound, efalizumab had the most reported cases, with four studies describing negative effects of abrupt discontinuation of therapy with subsequent rebound. Efalizumab has since been discontinued because of an association with progressive multifocal leukoencephalopathy. In the literature search, only one case of rebound associated with discontinuation of etanercept was found. No cases of rebound were reported for the other biologic agents (see Table 1).
CONCLUSION
The biologic agents vary considerably in terms of duration of effect. Overall, in responders, alefacept has demonstrated the longest off-treatment benefit in clinical trials and efalizumab the shortest. Time to relapse is an important consideration when determining choice of biologic treatment, and such information can be valuable to the patient and physician when deciding between therapies. Research in off-treatment response to biologic agents should be an ongoing area of study. This is the first review to compare ustekinumab, the new biological agent that may soon become available for commercial use, and adalimumab with the other biologic agents. We recommend that future trials, with head-to-head comparisons, endpoints using the established definitions of effect duration published by the National Psoriasis Foundation Medical Board, and study designs with similar on- and off-treatment durations, should be conducted to obtain more standardized and clinically reliable data. However, this review is useful to assist practicing dermatologists who have to make day-to-day clinical decisions despite the lack of ideal (i.e., head-to-head) comparison data.
Footnotes
Consultant
Advisory Board
Investigator,
Speaker,
Advisory board
Dr. Koo: Amgen,1,2,3 Abbott,1,2,3 Astellas,1,2,3 Biogen,1,2,3 Photomedics,1,3 Teikoku,1,3 Ranbaxy,1 Warner Chilcott,1,3 Stiefel,1,3 Leo Pharmaceutical,1,2,3 Galderma2
Disclosures
Monique Kamaria has no conflicts of interest to report.
References
- 1.Gordon KB, Feldman SR, Koo JY, Menter A, Rolstad T, Krueger G. Definitions of measures of effect duration for psoriasis treatments. Arch Dermatol. 2005;141:82–4. doi: 10.1001/archderm.141.1.82. [DOI] [PubMed] [Google Scholar]
- 2.Ellis CN, Krueger GG Alefacept Clinical Study Group. Treatment of chronic plaque psoriasis by selective targeting of memory effector T lymphocytes. N Engl J Med. 2001;345:248–55. doi: 10.1056/NEJM200107263450403. [DOI] [PubMed] [Google Scholar]
- 3.Gordon KB, Langley RG. Remittive effects of intramuscular alefacept in psoriasis. J Drugs Dermatol. 2003;2(6):624–8. [PubMed] [Google Scholar]
- 4.Carey W, Glazer S, Gottlieb AB, Lebwohl M, Leonardi C, Menter A, et al. Relapse, rebound, and psoriasis adverse events: an advisory group report. J Am Acad Dermatol. 2006;54:S171–81. doi: 10.1016/j.jaad.2005.10.029. [DOI] [PubMed] [Google Scholar]
- 5.Genentech, Inc. Biologic License Application. [Accessed July 2009];Dermatologic and Ophthalmic Drugs Advisory Committee Meeting: Raptiva (Efalizumab) 2003 Sep 9; Available at: http://www.fda.gov/ohrms/dockets/ac/03/briefing/3983B1_01_Genentech-Raptiva.pdf.
- 6.Tsai TF, Liu MT, Liao YH, Licu D. Clinical effectiveness and safety experience with efalizumab in the treatment of patients with moderate-to-severe plaque psoriasis in Taiwan: results of an open-label, single-arm pilot study. J Eur Acad Dermatol Venereol. 2008;22:345–52. doi: 10.1111/j.1468-3083.2007.02430.x. [DOI] [PubMed] [Google Scholar]
- 7.Gordon KB, Gottlieb AB, Leonardi CL, Elewski BE, Wang A, Jahreis A, et al. Clinical response in psoriasis patients discontinued from and then reinitiated on etanercept therapy. J Dermatol Treat. 2006;17:9–17. doi: 10.1080/09546630500472838. [DOI] [PubMed] [Google Scholar]
- 8.Moore A, Gordon KB, Kang S, Gottlieb A, Freundlich B, Xia HA, et al. A randomized, open-label trial of continuous versus interrupted etanercept therapy in the treatment of psoriasis. J Am Acad Dermatol. 2007;56:598–603. doi: 10.1016/j.jaad.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 9.Gottlieb AB, Chaudhari U, Mulcahy LD, Li S, Dooley LT, Baker DG. Infliximab monotherapy provides rapid and sustained benefit for plaque-type psoriasis. J Am Acad Dermatol. 2003;48:829–35. doi: 10.1067/mjd.2003.307. [DOI] [PubMed] [Google Scholar]
- 10.Gottlieb AB, Evans R, Li S, Dooley LT, Guzzo CA, Baker D, et al. Infliximab induction therapy for patients with severe plaque-type psoriasis: a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2004;51:534–42. doi: 10.1016/j.jaad.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 11.Menter A, Feldman SR, Weinstein GD, Papp K, Evans R, Guzzo C, et al. A randomized comparison of continuous vs. intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2007;56:31 e1–15. doi: 10.1016/j.jaad.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Reich K, Nestle FO, Papp K, Ortonne JP, Evans R, Guzzo C, et al. EXPRESS study investigators. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet. 2005;366:1367–74. doi: 10.1016/S0140-6736(05)67566-6. [DOI] [PubMed] [Google Scholar]
- 13.Koo J, Khera P. Update on the mechanisms and efficacy of biologic therapies for psoriasis. J Dermatol Sci. 2005;38:75–87. doi: 10.1016/j.jdermsci.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 14.Gottlieb A, Felman S, Weinstein G, et al. Infliximab for the Treatment of Psoriasis: PASI-75 response. Poster presented at: American Academy of Dermatology (AAD) 64th Annual Meeting; March, 2006; San Francisco, CA. [Google Scholar]
- 15.Emi Aikawa N, de Carvalho JF, Artur Almeida Silva C, Bonfa E. Immunogenicity of anti-TNF-alpha agents in autoimmune diseases. Clin Rev Allergy Immunol. 2009 Jun 30; doi: 10.1007/s12016-009-8140-3. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 16.Blum R, Lebwohl M, Wong V, Gottlieb A, Luo A, Hoffman R. Durability of treatment response in patients with moderate to severe psoriasis following withdrawal from or a dose reduction in adalimumab therapy. Poster presented at: American Academy of Dermatology; February 18–22, 2005; New Orleans, LA. [Google Scholar]
- 17.Menter A, Tyring SK, Gordon K, Kimball AB, Leonardi CL, Langley RG, et al. Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. J Am Acad Dermatol. 2008;58:106–15. doi: 10.1016/j.jaad.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 18.Leonardi CL, Kimball AB, Papp KA, Yeilding N, Guzzo C, Wang Y, et al. PHOENIX 1 study investigators. Efficacy and safety of ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with psoriasis: 76-week results from a randomised, double-blind, placebo-controlled trial (PHOENIX 1) Lancet. 2008;371:1665–74. doi: 10.1016/S0140-6736(08)60725-4. [DOI] [PubMed] [Google Scholar]