Abstract
Schwann cells (SCs) have been considered to be one of the most promising cell types for transplantation to treat spinal cord injury (SCI) due to their unique growth-promoting properties. Despite the extensive use as donor cells for transplantation in SCI models, the fate of SCs is controversial due in part to the lack of a reliable marker for tracing the grafted SCs. To precisely assess the fate and temporal profile of transplanted SCs, we isolated purified SCs from sciatic nerves of adult transgenic rats overexpressing GFP (SCs-GFP). SCs-GFP were directly injected into the epicenter of a moderate contusive SCI at the mid-thoracic level at 1 wk post-injury. The number of SCs-GFP or SCs-GFP labeled with Bromodeoxyuridine (BrdU) was quantified at 5 min, 1 d, and 1, 2, 4, 12 and 24 wk after cell injection. Basso, Beattie, and Bresnahan (BBB) locomotor rating scale, footfall error, thermal withdrawal latency, and footprint analysis were performed before and after the SCs-GFP transplantation. After transplantation, SCs-GFP quickly filled the lesion cavity. A remarkable survival of grafted SCs-GFP up to 24 wk post-grafting was observed with clearly identified SC individuals. SCs-GFP proliferated after injection, peaked at 2 wk (26% of total SCs-GFP), decreased thereafter, and ceased at 12 wk post-grafting. Although grafted SCs-GFP were mainly confined within the border of surrounding host tissue, they migrated along the central canal for up to 5.0 mm at 4 wk post-grafting. Within the lesion site, grafted SCs-GFP myelinated regenerated axons and expressed protein zero (P0) and myelin basic protein (MBP). Within the SCs-GFP grafts, new blood vessels were formed. Except for a significant decrease of angle of rotation in the footprint analysis, we did not observe significant behavioral improvements in BBB locomotor rating scale, thermal withdrawal latency, or footfall errors, compared to the control animals that received no SCs-GFP. We conclude that SCs-GFP can survive remarkably well, proliferate, migrate along the central canal, and myelinate regenerated axons when being grafted into a clinically-relevant contusive SCI in adult rats. Combinatorial strategies, however, are essential to achieve a more meaningful functional regeneration of which SCs may play a significant role.
Keywords: Spinal cord injury, Schwann cells, Survival, Proliferation, Migration, Myelination, Functional recovery
Introduction
Spinal cord injury (SCI) results in the destruction of descending motor and ascending sensory pathways leading to permanent damage with limited prospects for spontaneous repair (Bradbury and McMahon, 2006; Thuret et al., 2006). The failure of injured axons to regenerate results from a combination of factors including the lack of permissive growth substrates at lesion sites, the presence of inhibitory molecules that impair axonal outgrowth (Filbin, 2003; Silver and Miller, 2004; Yiu and He, 2006), the lack of neurotrophic support (Bradbury et al., 1999; Iannotti et al., 2003), and the death of oligodendrocytes that induces demyelination of surviving axons (Crowe et al., 1997; Liu et al., 1997; Totoiu and Keirstead, 2005). Since cavity or gap formation is evident following SCI in rats and humans (Oudega and Xu, 2006; Xu and Onifer, 2009), cell transplantation into the lesion site is essential to provide permissive substrates for axonal extension through and beyond it. Different cell types, including neural stem cells (NSCs) (Fujimoto et al., 2012; Sandner et al., 2012), fate-restricted neural and glial precursors (NRPs and GRPs) (Cao et al., 2002; Hill et al., 2004; Mitsui et al., 2005), Schwann cells (SCs) (Deng et al., 2011; Xu et al., 1997; Xu et al., 1995b), olfactory ensheathing glial cells (OEGs) (Feron et al., 2005; Ma et al., 2010), and marrow stroma cells (MSCs) (Ding et al., 2011; Phinney and Isakova, 2005) have been used as donor cells for transplantation after SCI with variable degrees of success. Importantly, genetic modification of transplanted cells could be utilized for gene therapy to enhance morphological and functional restoration (Deng et al., 2011; Girard et al., 2005).
SCs are the myelin-forming cells of the peripheral nervous system, and have been shown to improve functional recovery not only by myelinating axons after transplantation into the injured spinal cord, but also by forming a permissive substrate and producing growth-promoting factors and cell adhesion molecules (Hu et al., 2011; Oudega and Xu, 2006; Xu and Onifer, 2009) for regenerating axons. For example, SCs injected into a 1-wk-old moderate contusive SCI promoted axon regeneration into the transplant and improved hindlimb locomotion (Takami et al., 2002). Our previous studies showed that transplanted SCs in guidance channels successfully bridged the injured spinal cord and promoted axonal regeneration across the lesion gap following SCI (Deng et al., 2011; Xu, 1996; Xu et al., 1997; Xu et al., 1995b). The fate of grafted SCs, however, was not clear and is controversial in the field due in part to the lack of a reliable marker to trace the grafted SCs. For instance, Hill et al. reported that SCs survive poorly within a moderately contusive spinal cord environment. A 7-d delay in transplantation improves their survival, but the majority of SCs still die soon after grafting (Hill et al., 2006).
Here we transplanted SCs isolated from transgenic adult rats expressing green fluorescent protein (SCs-GFP) into a lesion cavity at 1 wk after a moderate contusive SCI at the thoracic level in adult rats to examine the fate and temporal profile of grafted SCs. We also examined migration, proliferation, and apoptosis in these cells. We concluded that grafted SCs-GFP survive well for a considerable time after being grafted into the injured spinal cord. They proliferate, migrate along the central canal, support axonal regeneration, and myelinate regenerated axons within the lesion site. The lack of substantial behavioral recovery after SCs-GFP transplantation emphasizes the need of combinatorial strategies to achieve a more meaningful functional regeneration after SCI.
Materials and Methods
Animals
A total of 4 transgenic adult rats expressing green fluorescent protein (GFP) and 56 female Sprague Dawley (SD) adult rats (Harlan Co; Indianapolis, IN) weighting 200 to 220 g were used in this study. GFP transgenic adult rats (Rosa 26, SD strain, generous gift from George M. Smith, Temple University) were used for isolation and culture of SCs-GFP, whereas the remaining rats were used for SCI and transplantation studies. Among the 56 SD rats, 42 were assigned for assessing the survival, proliferation, migration, and myelination of grafted SCs-GFP, 12 were used for behavior tests, and 2 were used for long-term observation of grafted SCs-GFP. All animal care and surgical interventions were undertaken in strict accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals, Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, National Research Council), and were approved by the Indiana University Institutional Animal Care and Use Committee and Institutional Biosafety Committee.
Isolation and Culture of SCs-GFP
The methods for SCs isolation, purification, and amplification were developed by Morrissey et al. (Morrissey et al., 1991) and described in our previous publications (Deng et al., 2011; Hu et al., 2013; Xu et al., 1995a; Xu et al., 1995b). Briefly, sciatic nerves were obtained from GFP transgenic adult rats anaesthetized with ketamine (87.7 mg/kg; Ben Venue Labs, OH) and xylazine (12.3 mg/kg; Butler, OH) under aseptic conditions. After epineurium and connective tissue were removed, the nerves were cut into 1-mm-long explants. The explants were placed in 35-mm tissue culture dishes (Baxter, Stone Mountain, GA, USA) with low levels (0.8 mL) of Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 10% fetal bovine serum (FBS; Life Technologies, Grand Island, NY, USA). When the outgrowth of migratory cells (predominantly fibroblasts) reached a near-confluent monolayer around the explants (7 d), the explants were transferred to new culture dishes with fresh medium. After 5 to 6 such passages (5–6 wk), the cells that emerged from the explants were primarily SCs. The explants were then transferred to a 35-mm dish containing 1.25 U/mL dispase (Boehringer Mannheim Biochemicals, Indianapolis, IN, USA), 0.05% collagenase (Worthington Biochemicals Corp., Freehold, NJ, USA) and 15% FBS in DMEM for incubation overnight at 37°C in 5% CO2. On the following day, the explants were dissociated and the cells were plated onto poly-L-lysine-coated 100-mm dishes in DMEM/10% FBS. Later, the cultures were re-fed with the same medium supplemented with 20 μg/mL pituitary extract (BTI, Stoughton, MA, USA) and 2 μM forskolin (Sigma, St Louis, MO, USA) for dividing. When the SCs reached confluence, they were rinsed in Ca2+- and Mg2+- free Hanks balanced salt solution (CMF-HBSS; Life Technologies, USA) and briefly treated with 0.05% trypsin (Life Technologies, USA) and 0.02% EDTA (Life Technologies, USA) in CMF-HBSS. Cells were washed twice in DMEM/10% FBS and passed into new dishes at a density of 2 × 106 cells/100 mm dish. The purity of the SCs (Percentage of P75+GFP+ cells in all GFP+ cells) was quantified according to the methods described previously (Xu et al., 1995b). All SCs used in this study were passage (P) 2 cells with a purity of > 98%.
Moderate thoracic contusion injury
Moderate contusion injury was induced by a weight drop device developed at New York University called NYU impactor (Gruner, 1992). A laminectomy was performed at the thoracic vertebrae 8 exposing the dorsal surface of the 9th thoracic spinal cord (T9) underneath without disrupting the dura mater. The exposed spinal cord was injured by dropping a 10.0-g rod from a height of 12.5 mm. The contusion impact height, compression, and velocity were monitored. After injury, a fine dura suture at the epicenter of the injury area was made with 10-0 nylon and the injury was covered with a sterile piece of parafilm (Pechiney Plastic Packaging, Chicago, IL) as landmarks for the secondary surgery and cell injection. The muscles were sutured in layers and the skin was closed with clips. The rats were allowed to recover in a warmed cage with water and food easily accessible. The analgesic buprenorphine hydrochloride (0.01 mg/kg, Reckitt and Colman, Hull, UK) was subcutaneously delivered post-surgery and daily for 2 d.
SCs-GFP transplantation
SC transplantation was performed at 1 wk after SCI. Four hours prior to implantation, fresh DMEM/10% FBS medium containing 20 μg/mL pituitary extract and 2 μM forskolin was added to the culture. SCs-GFP were harvested from culture via trypsinization, centrifuged, re-suspended, and counted (Hill et al., 2007). After counting, SCs-GFP were re-suspended as aliquots in DMEM/10% FBS medium supplemented with 20 μg/mL pituitary extract and 2 μM forskolin and were kept on ice prior to grafting. Rats were re-anesthetized with ketamine and xylazine, as described above, and the injury site was re-exposed. A total of 1×106 SCs-GFP, suspended in 5 μL of culture medium, were directly injected into the lesion cavity, identified by the 10-0 nylon suture on the dura surface. The injection was made using pulled glass capillaries attached to a pneumatic pump device at a speed of 0.1 μL/sec. The injection pipette was kept in place for an additional 3 min to minimize leakage upon withdrawal. After the injection, the muscle and the skin were closed in layers and animals received postoperative care according to our standard protocol (Xu et al., 1997; Xu et al., 1995a; Xu et al., 1995b). Injury-only controls received 5 μL of culture medium injections. All animals received a daily subcutaneous injection of cyclosporine A (CsA, 10mg/kg; Sigma) starting 3 d prior to the transplantation which was continued until the end of the experiments. In 2 rats that survived for 52 wk (~1 yr) after SCs-GFP transplantation, the rats received CsA treatment for only the first 3 months.
Pulse-labeling of the proliferating SCs following transplantation
To assess the proliferation of grafted SCs, one day before the termination of animals at each time point (except for the group sacrificed at 5 min post-transplantation), animals received 4 intraperitoneal injections of 5-bromo-2′-deoxyuridine (BrdU, Sigma, St. Louis, MO; 100 mg/kg/injection) at every 6 h.
Histology
At indicated time points post-transplantation (Fig. 1), animals were deeply anesthetized with 60 mg/kg pentobarbital and perfused transcardially with 100 mL heparinized saline followed by 4% paraformaldehyde (PFA) in phosphate buffer (PB; 0.01M, pH 7.4). The spinal cord segments containing the injury/transplant epicenter were removed, post-fixed in the same perfusion fixative for overnight at 4°C, and cryoprotected in 30% sucrose in phosphate-buffered saline (PBS; 0.01M, pH 7.4) for another 5–7 d. A 2-cm-long spinal cord segment centered at the injury/transplant epicenter was dissected out. The cord segments were embedded in Tissue-Tek and sectioned longitudinally or transversely (a subset) at 20 μm in 5 sets on a cryostat (Leica CM 1950). Sections were mounted onto gelatin-subbed slides and stored at −20°C for immunohistological analyses and quantification of grafted SCs-GFP.
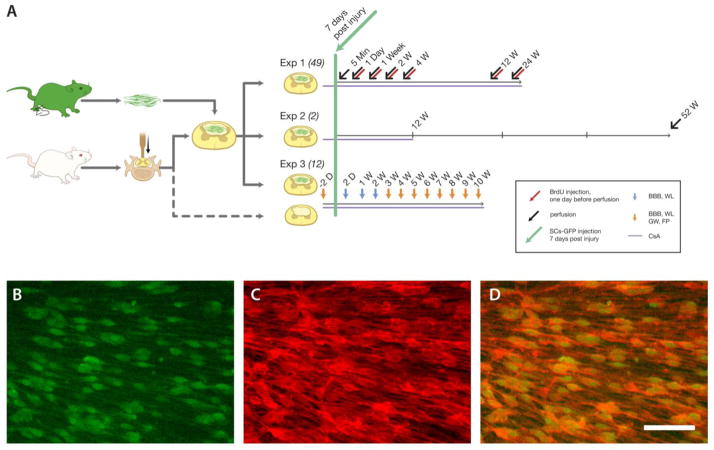
Fig. 1.
Experimental design and Schwann cells overexpressing green fluorescent protein (SCs-GFP) culture in vitro. A: Schwann cell isolated from the sciatic nerves of the adult transgenic rats strongly expressed GFP and expanded to form a confluence layer in culture. One week after spinal cord injury, these cells were transplanted into the epicenters of injured spinal cords and continued to observe the survival, proliferation, apoptosis, migration, and myelination of SCs (Exp. 1), the long-term fate of SCs (Exp. 2) and behavioral changes over time (Exp. 3). Immunocytochemistry showed that over 98% of these SCs-GFP cells (B, green) express P75 neurotrophic factor receptor (P75NTR, C, red), a specific marker for Schwann cells (D). Scale bars = 20 μm.
Immunocytochemistry
For in vitro experiments, cells in culture were fixed in 4% PFA for 20 min prior to blocking/permeabilization for immunocytochemistry with mouse anti-p75NTR antibody (Cell Signaling Technology, Danvers, MA) overnight followed by the rhodamine-conjugated donkey anti-mouse IgG (1:200; Jackson ImmunoResearch Lab, West Grove, PA) for 1 h at 37°C. Mouse IgG control sera were used at the same concentration as the corresponding primary antibodies to establish the specificity of staining.
For in vivo experiments, every 5th sagittal section (100 μm apart) was immunochemically stained as previously described (Liu et al., 2008). The following primary antibodies were used: rabbit anti-glial fibrillary acidic protein to identify astrocytes (GFAP, 1:400; Chemicon, Temecula, CA), mouse anti-SMI-31 to recognize the phosphorylated neurofilament epitope of axons (1:200, Sigma), mouse anti-ED-1 to identify activated microglia/macrophages (1:400, Sigma), and mouse RECA-1 (R&D Systems, Minneapolis, MN, USA) to recognize blood vasculature. A mix of the monoclonal rabbit anti-Neurofilament 200 antibody (1:200, Sigma) and one of the following antibodies: goat anti myelin protein zero (P0, 1:400) and mouse anti-myelin basic protein (SMI-94, 1:1,000, Covance), were used to determine the type of myelination on axons within the graft region. After the primary antibodies, sections were incubated with the corresponding secondary antibodies including AMCA-conjugated donkey anti-rabbit IgG, rhodamine-conjugated donkey anti-mouse IgG, or rhodamine-conjugated donkey anti-goat IgG (1:200; all from Jackson ImmunoResearch Lab) alone or in combination according to the primary antibodies used. After staining, the slides were rinsed with PBS and mounted with Gel/Mount aqueous mounting media contained with Hoechst 33342, a fluorescent nuclear dye.
For BrdU staining, a commercially available in situ BrdU incorporation assay was used. Briefly, sections were treated with 1 N HCl for 40 min at 37°C to denature the DNA prior to the use of primary rabbit anti-BrdU antibody (1:100; Sigma) overnight at 4°C and then secondary antibody (rhodamine-conjugated donkey anti-rabbit IgG; 1:200; Jackson ImmunoResearch Lab) at room temperature for 2 h. The slides were rinsed with PBS and mounted with Gel/Mount aqueous mounting media containing Hoechst 33342.
To determine whether transplanted SCs underwent apoptosis, the terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay was performed using an In Situ Cell Death Detection Kit (Roche, Penzberg, Germany). In brief, after incubation in permeabilization solution containing 0.1% triton X-100 in 0.1% sodium citrate for 2 min, the slides were washed twice with PBS and incubated with 50 μL of TUNEL reaction solution for 1 h in a humidified chamber at 37 °C in the dark. The slides were rinsed with PBS and mounted with Gel/Mount aqueous mounting media containing Hoechst 33342.
Quantification of SCs-GFP survival, proliferation and apoptosis
Complete sets of every 5th section (20 μm thick, 100 μm apart) of the spinal cord segments containing grafted SCs-GFP were processed. Fluorescent images were acquired with a Zeiss Axiovert 200M fluorescent microscope in Z-stack mode and were deconvoluted with the Axiovision software package (Carl Zeiss) to obtain near-confocal quality optical sections. Merged images were post-processed using ImageJ (NIH, Bethesda, MD) to quantify the total number of SCs-GFP per 20 μm-thick section. Only GFP+ SCs that contained a clear nuclear dye Hoechst 33342 were recognized and counted. All BrdU+ SCs-GFP, representing proliferating grafted SCs, were photographed and counted manually in each 20 μm-thick section. Likewise, all TUNEL+ SCs-GFP, representing grafted SCs that underwent apoptosis, were counted manually in each 20 μm-thick section. The estimated total number of GFP+ SCs was calculated as: (the number of GFP+ SCs per section) x (the number of total sections per set) x (the number of sets). The estimated total number of BrdU+ SCs-GFP and TUNEL+ SCs-GFP was calculated in the same manner. The percentage of BrdU+ or TUNEL+ SCs-GFP in all grafted SCs-GFP at different time points was calculated by dividing the total number of BrdU+ or TUNEL+ SCs-GFP by the total number of SCs-GFP.
Assessment of maximum distance of SC migration
To assess the migratory ability of transplanted SCs-GFP within the injured spinal cord, all longitudinal sections that contained transplanted SCs-GFP in one set of sections were examined under an Olympus BX60 microscope. We measured the longest distance (mm) that the grafted SCs-GFP migrated from the rostral or caudal lesion border demarcated by the GFAP-labeled astrocytes on the border.
Behavioral testing
Rats for the behavioral assessments were randomly assigned to 2 groups: the control group received injections of culture medium alone and the transplantation group received injections of SCs-GFP. The Basso, Beattie, and Bresnahan (BBB) open-field locomotor rating scale was used to assess hindlimb locomotor recovery according to the previously published work (Basso et al., 1995). The tests were performed by two examiners who were blinded to the animals’ treatments at 1 d pre-injury (baseline, BL), 2 d after injury, and 1, 2, 3, 4, 5, 6, 7, 8, 9, and 10 wk after injections of cells or medium. Animals with a minimum BBB score of 12 were further analyzed and included in the footprint and grid-walking analyses from the 3rd wk after cell transplantation.
Footprint analysis was performed at different time points using a procedure described previously (Hu et al., 2013). The hindlimbs of the rats were dipped in nontoxic blue dyes and the animals walked on a paper-covered narrow runway (100 cm long and 7 cm wide). To perform the measurements, the first and last 15 cm of the prints were excluded to avoid analyzing the beginning and end of the limb movements. The angle of rotation (AOR) was assessed by measuring the angle between two lines connecting the third toe and the stride line at the center of the paw pad. The base of support was determined by measuring the distance between the central pads on the hind paws (DBF). Stride length (SL) was determined by measuring the linear distance between corresponding successive points of contact of the same foot. Paw length (PL) was determined by measuring the longest paw print. Toe spread (TS) was determined by measuring the distance between first and fifth toes based on the largest value attained for each side. The trial was repeated 3 times and the results were averaged for each rat.
Grid-walk analysis was performed to assess the deficits in descending motor functions (Metz et al., 2000) in a manner complementary to the footprint. The rats were allowed to traverse over a wire mesh grid (2.5 × 2.5 cm2 grid spaces). Animals were required to walk for at least 30 s on this device up to a 3-min test period during which the total number of hindlimb movements and the number of footfalls of the hindlimb were counted, respectively, by two examiners blind to the experiment. One footfall was defined when the hindlimb paw protruded entirely through the grid with all toes and heel extended below the wire surface. Data were expressed as the percentage of footfalls over the total number of hindlimb movement. For each session, the average number of paw falls of each rat was taken from 3 trials.
Thermal sensitivity was assessed by measuring latency to paw withdrawal from a noxious heat source, as described by Hargreaves et al (Hargreaves et al., 1988). Rats were allowed to acclimate within acrylic glass enclosures on a clear glass plate maintained at 30°C. A radiant heat source was focused through the glass plate onto the plantar surface of the both hind paws. Withdrawal latency was measured using a motion detector that shut off the heat stimulus and a timer on withdrawal of the paw. A maximal cutoff of 20 s was used to prevent tissue damage. Thermal sensitivity was measured at baseline, 2 d after SCI, and weekly post-cell transplantation.
Statistical analysis
One-way ANOVA followed by the Fisher’s least significant difference (LSD) post hoc test was used to determine statistical differences of the average number of SCs-GFP+ within the lesion site of a moderately contused spinal cord between animals sacrificed at different time points. However, in case of unequal variance (F test), a nonparametric analysis (Kruskal–Wallis test followed by Mann–Whitney test) was used. Pearson’s Chi-square test was used to determine statistical differences of the percentage of BrdU+, or TUNEL+ SCs-GFP between animals terminated at different time points. A mixed factorial (repeated measures) ANOVA followed by the Tukey-Kramer test was used for comparison of weekly functional recovery (BBB and footprint) after injury. Differences were accepted to be statistically significant at P < 0.05*, <0.01** and <0.001***. All errors are given as standard deviation of mean.
Results
Characterization of SCs-GFP in Vitro
Schwann cells isolated from transgenic adult rats expressing GFP (SCs-GFP) showed similar growth properties to SCs isolated from wild type adult rats. These cells exhibited spindle morphology and swirling cell pattern with parallel cell alignment when cultured on poly-L-lysine. Over 98% of these SCs expressed P75NTR, a marker for Schwann cells (Fig. 1). Since the purpose of this study was to accurately assess SC survival, it was important to precisely establish the number of cells transplanted. A previous study, however, showed that the actual average number of SCs that passed through the glass needle was only about 56.5% of the intended number of SCs (Hill et al., 2007). To eliminate this error from the calculation of percentage of surviving SCs, the actual number of cells present immediately after injury (5 min post-transplantation), instead of the intended number of cells, was considered the baseline value (100%) of grafted SCs-GFP.
Survival of grafted SCs-GFP up to 24 wk post-grafting in vivo
In all rats, the SCs-GFP transplant could be easily recognized under a fluorescence microscope because these cells expressed strong green fluorescence signal even at 1 y after transplantation. Morphological analysis revealed changes in the appearance of grafted SCs-GFP over time (Fig. 2) as they integrated into the lesion site. At 5 min (Fig. 2A, H) after transplantation, SCs-GFP cells within the lesion site were spherical in shape. SCs with a bipolar shape, a sign of integration into the host tissue, were found in the transplant margins at 1 d (Fig. 2B, I) and throughout the transplant at 1 wk (Fig 2C, J). Between week 2 and week 24, SCs-GFP further elongated along the longitudinal axis of the spinal cord and arranged in an orderly fashion (Fig. 2D/K, E/L, 2F/M, and G/N). At 5 min post-transplantation, an average of 8.12×105 cells/graft was observed. Although a significant decrease of the percent total number of SCs-GFP was detected at 1 d after grafting ((6.42±0.52)×105, compared to 5 min, t=4.67, p=0.0012), a significant recovery ((7.81±0.76) ×105, compared to 1 d, t=3.29, p=0.011) of the number of SCs-GFP was observed at 2 wk after transplantation (compare to 5 min, t=0.8, p=0.44). Thereafter, the cell number slightly decreased over a prolonged period of time and, at 24 wk after transplantation, (6.21±0.56)×105 grafted cells survived at the lesion site (compared to 2 wk, t=4.15, p=0.002) (Fig. 2O).
Fig. 2.
Survival of grafted Schwann cells overexpressing green fluorescent protein (SCs-GFP) up to 24 wk post-transplantation in vivo. Representative fluorescence photomicrographs of longitudinal sections of the spinal cord showing that SCs-GFP survived remarkably well at 5 min (A), 1 d (B), 1 wk (C), 2 wk (D), 4 wk (E), 12 wk (F), and 24 wk (G) post-transplantation. The surviving SCs-GFP were evenly distributed at the lesion site and were surrounded by GFAP+ host tissue (stained in red). H–N shows the detailed morphology of SCs-GFP at different post-transplantation time points corresponding to A–F. Note that when SCs were initially grafted into the lesion cavity, they exhibited rounded morphology (H). These cells extended their processes (I) and arranged in an orderly manner (J–N) likely in accordance with the growth of axons into the graft region. O: Quantification of the number of SCs-GFP at different time points shows the remarkable survival of these cells up to 24 wk post-transplantation. *, p<0.05, **, p<0.01. Scale bar: A–G, 200 μm; H–N, 20 μm.
Proliferation of Grafted SCs-GFP in Vivo
To determine whether SC proliferation was a contributing factor to the total number of survived SCs-GFP within the lesion site of a moderately contused spinal cord, we examined and quantified the proliferation rate of SCs-GFP by BrdU pulse-labeling of proliferating SCs. One day after grafting, 5.47±0.84% of the grafted SCs-GFP was BrdU positive (Fig. 3B). This proliferative rate increased slightly to 9.54±1.23% at 1 wk after injection (Fig. 3C, χ2=24708, p<0.001) and reached its peak at 25.91±2.21% at 2 wk post-grafting (Fig. 3D, χ2=402136, p<0.001), corresponding to the highest number of total SCs-GFP after cell injection. The SCs-GFP proliferation decreased markedly at 4 wk post-grafting (6.13±0.49%, χ2=815, p<0.001, Fig. 3E). At 12 (0.07±0.00%, χ2=80570, p<0.001, Fig. 3F) or 24 wk post-grafting, few, if any, proliferating SCs-GFP were observed (Fig. 3H).
Fig. 3.
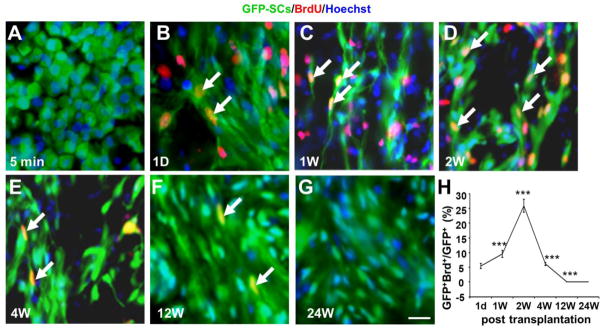
Proliferation of grafted Schwann cells overexpressing green fluorescent protein (SCs-GFP) at different time points post-transplantation. An intraperitoneal injection of 100 mg/kg BrdU was performed every 6 h, 1 d before animal perfusion. A–G: Proliferation of SCs-GFP (double labeled with BrdU & GFP, arrows) was observed as early as 1 d post-transplantation and it reached the maximum at 2 wk post-transplantation. H: The proliferation rate of SCs-GFP (GFP+BrdU+Hoechst+/GFP+Hoechst+) was about 5.47±0.84% at 1 d. Compared to day 1 this proliferation rate increased 1 wk (9.54±1.23%, χ2=24708, p<0.001), peaked at 2 wk (25.91±2.21, χ2=402136, p<0.001), decreased at 4 wk (6.13±0.49%, χ2=815, p<0.001), and stopped at 12 wk (0.07±0.00%, χ2=80570, p<0.001) and 24 wk (E) post-transplantation. Scale bar = 25 μm.
Lack of substantial apoptosis of grafted SCs-GFP in vivo
Apoptosis-induced cell death is an important possible mechanism for the loss of grafted cells. Although a few SCs-GFP were identified to be positively stained for TUNEL at 5 min post-grafting (0.17±0.06%, Fig. 4A, H), we did not observe substantial apoptosis of grafted SCs-GFP over a period of 24 wk post-grafting. Very few SCs-GFP cells were found to be apoptotic (TUNEL+) at 1 d post-grafting (0.03±0.02%, χ2=230819, p<0.001, Fig. 4B, H,) and no apoptotic cells were found between 1 wk (Fig. 4C) to 24 wk (Fig. 4D–G). These results strongly suggest that substantial apoptotic cell death did not occur in SCs-GFP after being grafted into the injured spinal cord, revealing another important contributing factor to the survival of SCs-GFP within the lesion site.
Fig. 4.
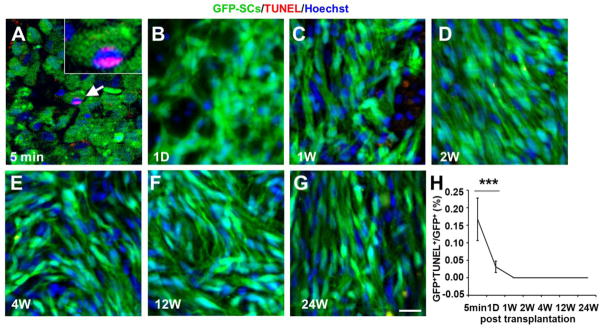
Apoptosis of grafted Schwann cells overexpressing green fluorescent protein (SCs-GFP) at different time points post-transplantation. A–G: Very few grafted SCs-GFP were underwent apoptosis. A representative TUNEL+ Schwann cell (GFP+) was shown at 5 min post-transplantation (A, arrow). H: Percent apoptosis of grafted SCs at different time points. Very few SCs-GFP were found to be apoptotic at 5 min (0.17%) and 1 d (0.03%) post-transplantation. No TUNEL+ cells were detected between 1 wk and 24 wk post-transplantation. Scale bar = 25 μm.
Migration of grafted SC cells after transplantation
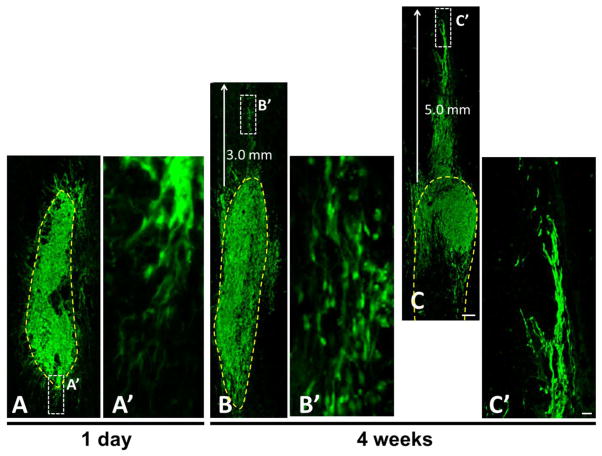
Migration of SCs to host tissue may facilitate axonal growth from the graft into the host spinal cord. As early as 1 day following transplantation, SCs-GFP attempted to elongate from the lesion border (yellow dashed line) into the host tissue (Fig. 5A). Four weeks after grafting, SCs-GFP migration from the lesion border into the host tissue was found only in the rostral and/or caudal directions. In some cases, the SCs-GFP migrated into the host spinal cord for a longest distance of 3.0 mm (Fig. 5B) to 5.0 mm (Fig. 5C), often along enlarged central canal.
Fig. 5.
Migration of grafted Schwann cells overexpressing green fluorescent protein (SCs-GFP) post-transplantation. A: At 1 d post-transplantation, GFP-SCs formed radiating processes attempting to extend from the graft into the host spinal cord. A′ is a high magnification of boxed area in A clearly showing the migratory attempt of SCs-GFP. B,C: 4 wk post-transplantation, GFP-SCs were mainly confined within a well-defined graft-host interface that surrounded the grafted cells. At the rostral graft-host interface, migration of SCs-GFP from the lesion border (yellow dashed line) into the host tissue was found for a distance of 3.0 mm (B) and 5.0 mm (C). B′ and C′, are higher magnification of boxed areas in B and C. Scale bar: A–C, 200 μm; A′–C′, 20 μm.
Myelination by grafted SCs in vivo
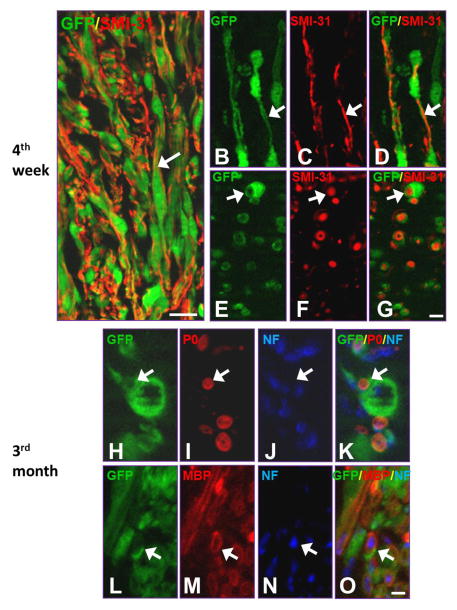
Except for the formation of a permissive substrate and provision of growth-promoting factors and cell adhesion molecules for regenerating axons, grafted SCs can myelinate regenerated axons. At 4 wk after transplantation, grafted SCs-GFP exhibited a close association with SMI-31+ axons at the lesion site (Fig. 6A). Longitudinal (Fig. 6B–D) and cross sectional (Fig. 6E–G) confocal images clearly show that grafted SCs-GFP twined around and/or wrapped SMI-31+ axons that had grown into that region. Three months after transplantation, triple fluorescence staining showed that protein zero-positive (P0+, Fig. 6H–K) and myelin basic protein-positive (MBP+, Fig. 6L–O) mature myelin was formed by SCs-GFP cells. Interestingly, with the increase of P0 and MBP signals, the fluorescence intensity of GFP decreased, making the blurry observation of the co-localization of P0/GFP and MBP/GFP.
Fig. 6.
Myelination of grafted Schwann cells overexpressing green fluorescent protein (SCs-GFP) post-transplantation. A: Immunofluorescence staining demonstrated that grafted SCs-GFP were in close association with axons growing into the graft at 4 wk post-transplantation (arrow). B–D: A longitudinal section shows that a SC established a one-on-one relationship with a single axon immunostained with SMI-31+ (arrows). E–G: A cross section shows that numerous SCs (arrows) wrapped axons (SMI-31+) within the graft region. H–O: Myelinated axons expressed myelin markers P0 (H–K, arrows) and MBP (L–O, arrows) at 3 mo post-transplantation. Scale bar: A, 40 μm; B–G, 20 μm; H–O, 10 μm.
Blood vessel formation after SCs-GFP transplantation
Blood supply to the graft and growing axons at the lesion site are critical for meaningful transplantation-mediated repair. We therefore examined the newly formed blood vessels among different groups. In the group that received no cell transplantation, contusive SCI resulted in the development of a well-defined large cystic cavity at the lesion site at 3 mo post-grafting. REAC+ blood vessels distributed within the host tissue surrounding the cavity and no REAC+ blood vessels were found within the lesion cavity (Fig. 7A, C). When SCs-GFP were transplanted into the lesion site, a few REAC+ blood vessels could be found in the SCs-GFP graft as early as 1 wk post-transplantation (Fig. 7F) and maintained throughout all time points up to 24 wk post-transplantation (Fig. 7G–J). These vessels might play important roles in providing substantial nutritional support for the grafted SCs.
Fig. 7.
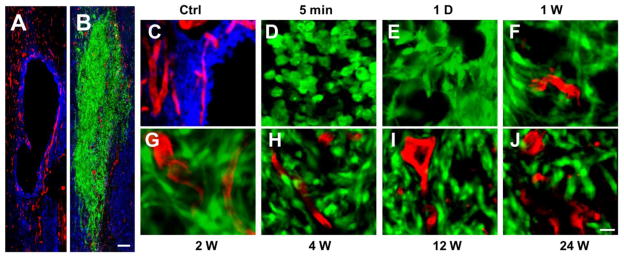
Blood vessel supply to the Schwann cells (SCs) transplants. A: In a control case that received a contusive spinal cord injury (SCI) without SCs-GFP transplantation, a well-defined cystic cavity was formed at 3 mo post-injury and REAC+ blood vessels were observed only in host tissues surrounding the lesion cavity. B: In contrast, many REAC+ blood vessels were found within the SCs-GFP graft at 3 mo post-transplantation. C–J: High magnifications show the REAC+ blood vessel staining at the lesion border in a control case (C), the lack of blood vessels within the graft at 5 min (D) and 1 d (E) post-transplantation, the first appearance of blood vessels within the graft at 1 wk post-transplantation (F), and increased blood vessels were observed at 2 (G), 4 (H), 12 (I), and 24 (J) wk post-transplantation. Scale bar: A–B, 200 μm; C–J, 20 μm.
Locomotor and sensory assessments after SCs-GFP transplantation
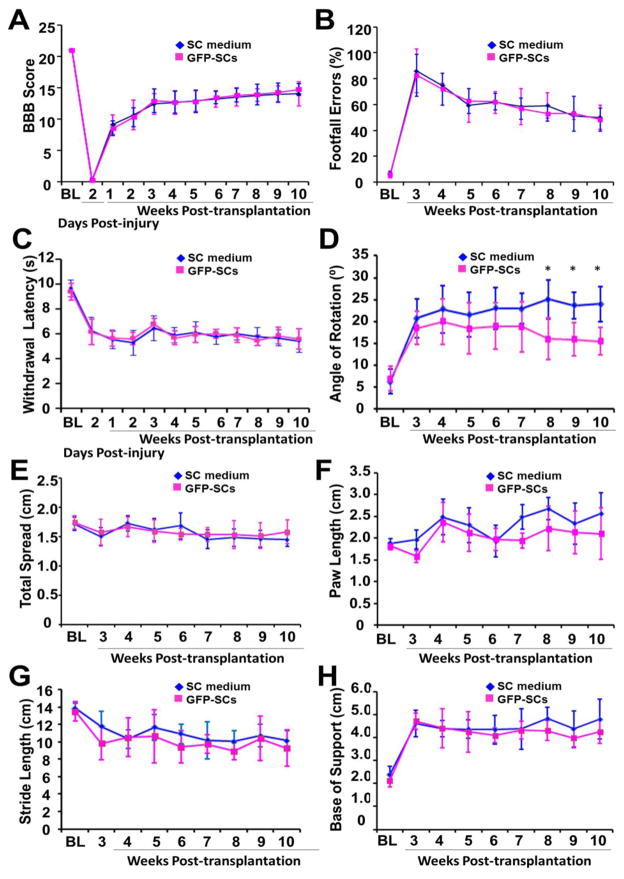
Twelve rats were randomly selected for behavior testing after SCI and then were randomly assigned to 2 groups including the SCs-GFP transplantation and non-transplantation (medium only) groups. All animals exhibited a gradual improvement in hindlimb locomotor function, measured using the BBB locomotor rating scale, during the 10-wk period after cell transplantation (Fig. 8A). In 2 experimental groups, most rats exhibited frequent to consistent weight-supported plantar stepping and occasional (BBB score of 12) to frequent (BBB score of 13) forelimb–hindlimb coordination at 3 wk when they were eligible for grid walking and footprint tests. However, up to 10 wk post-transplantation, we did not observe significant differences in BBB locomotor rating scale (Fig. 8A), footfall errors (Fig. 8B), and paw withdraw latency (Fig. 8C) between the 2 groups. Although we also did not observe significant difference in total spread (Fig. 8E), paw length (Fig. 8F), stride length (Fig. 8G), and base of support (Fig. 8H) of the foot print analysis between the 2 groups, we did find an improvement in angle of rotation of the SCs-GFP transplantation group compared to the control group after 8 wk post-transplantation (Fig. 8D, p<0.05).
Fig. 8.
Locomotor and sensory outcomes after Schwann cells overexpressing green fluorescent protein (SCs-GFP) transplantation. A–C: Up to 10 wk post-transplantation, no statistically significant differences in BBB locomotor rating scale (A), footfall errors (B) and paw withdrawal latency (C) were found between the SCs-GFP transplantation and control groups. D–H: For footprint analysis, significant improvement between the 2 groups was found only in the angle of rotation (D) but not in the total spread (E), paw length (F), stride length (G), and base of support (H). *, p<0.05.
Long-term survival of grafted SCs-GFP: a need for immunosuppression
We have observed remarkable survival of grafted SCs-GFP up to 24 wk post-transplantation with the application of the immunosuppressive drug CsA. To verify the importance of immunosuppression for long-term survival of grafted SCs, CsA immunosuppression of 2 animals was withdrawn at 3 mo after the SCs-GFP transplantation and the animals were allowed to survival for additional 9 mo (i.e., up to 1 y after transplantation). We observed that numerous GFP+ SCs survived and filled in the lesion cavity in an orderly orientation (Fig. 9A, B) indicating long-term survival of grafted SCs, at least for 1 y in rats, can be achieved. Notably, ample ED1+ macrophages (Fig. 9E) were found, not only in areas around the transplant but also in the adjacent host tissue and for considerable distances particularly in the rostral and caudal directions. These macrophages were GFP+ (Fig. 9C, F) indicating that they might have phagocytized exogenously grafted SCs-GFP to become fluorescent green and then migrated out from the lesion site. These results clearly indicated that consistent immunosuppression is required for long-term survival of grafted SCs-GFP and for preventing secondary damage induced by invasion of macrophages and/or activation of microglia.
Fig. 9.
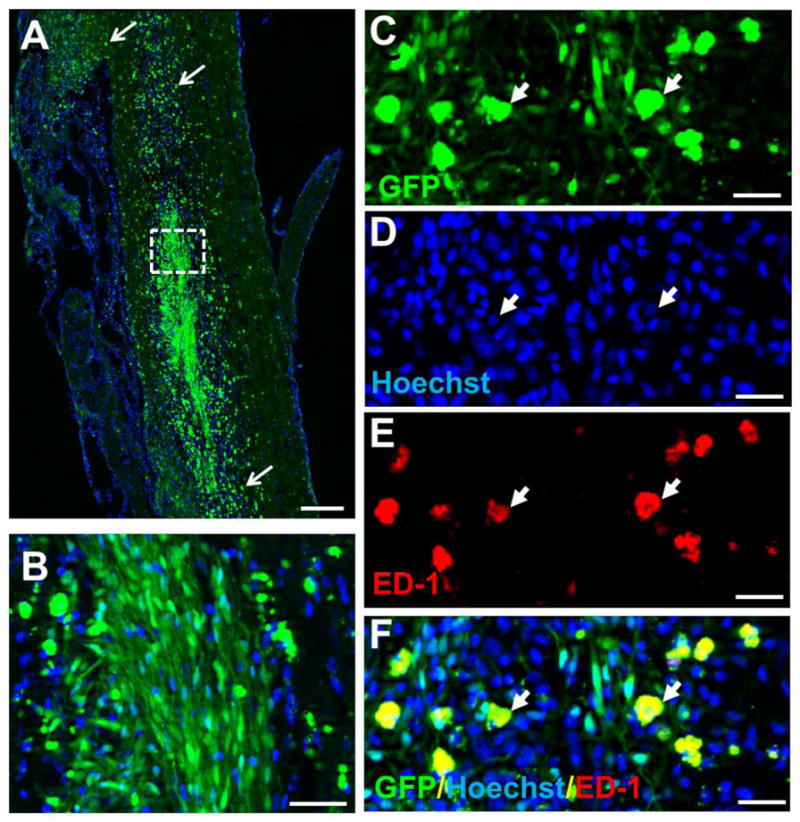
Survival of grafted Schwann cells overexpressing green fluorescent protein (SCs-GFP) up to 1 y. A–B: Survival of SCs-GFP at 1 y post-transplantation. In these animals, the immunosuppressant cyclosporine A (CsA) was used for the first 3 mo and then withdrawn. Numerous SCs-GFP survived up to 1 y, which could be appreciated at higher magnification (B) of the boxed area of A. Numerous GFP-labeled phagocytes were also found remote to the graft site (arrows, A) indicating that macrophages might have engulfed degenerated SCs-GFP and migrated away from the graft site. C–F: Triple labeling of GFP (arrowheads, C), hoechst (arrowheads, D) and ED1+ (arrowheads, E, a marker for macrophages) confirmed that macrophages engulfed debris of degenerated SCs-GFP and then became fluorescent green. Scale bar: A, 500 μm; B, 100 μm; C–F, 50 μm.
Discussion
In the present study, SCs-GFP were transplanted into the spinal cord at 1 wk after a moderate contusive SCI. We found a remarkable survival of grafted SCs-GFP up to 1 y post-grafting. The grafted SCs-GFP proliferated, peaked at 2 wk post-transplantation, and gradually decreased at 4 wk and thereafter. SCs-GFP migration from the lesion border into the host tissue was found only in the rostral and/or caudal directions and the longest distance of SCs migration reached 5.0 mm, which occurred along the enlarged central canal. Grafted SCs-GFP myelinated axons within the lesion site and expressed P0 and MBP at 12 wk post-transplantation. Except for a significant decrease of angle of rotation in footprint analysis, however, no significant behavioral improvements were observed in BBB locomotor rating scale, thermal withdrawal latency, footfall errors, and other parameters of foot print analysis in the SCs-GFP transplanted group as compared to the non-transplanted group. We conclude that grafted SCs-GFP survive, proliferate, migrate (to a limited extent in rostrocaudal orientation), and myelinate regenerated axons within the lesion site. Combining SC transplantation with other optimal strategies, however, is essential to achieve a more meaningful functional regeneration after SCI. This study has, for the first time, provided direct evident that long-term survival of grafted SCs is possible and practicable in a model of contusive SCI.
Survival of grafted cells is extremely important for cell transplantation-based therapy. The key finding of the current study was that grafted SCs-GFP survived remarkably well for at least 24 wk (approximately 6 mo) post-grafting. The longest survival was observed at 1 y post-transplantation even without using an immunosuppressant in the last 9 mo. Our observation was different from previous studies by Hill et al. reporting a 78% reduction in SC number within the first week after SC transplantation predominantly by necrosis (Hill et al., 2010; Hill et al., 2007). When comparing these studies to ours, four evident differences exist. First, the current study used passage 2 SCs for transplantation whereas the previous studies used passage 3 SCs. Second, the current study used two SC mitogens, i.e. forskolin and pituitary extract in the culture and in the transplantation medium, whereas the previous studies used three mitogens, i.e. forskolin, pituitary extract, and heregulin in the culture. Third, in the current study, all animals received a daily subcutaneous injection of CsA starting at 3 d prior to the transplantation and continued until the end of the experiments; whereas, in the previous study, the application of an immunosuppressive drug was not mentioned (Hill et al., 2007). Lastly, in the current study, SCs were isolated from adult transgenic rats expressing GFP, whereas, in the previous studies, SCs were isolated from Fisher 344 rats transduced with lentiviruses expressing GFP. While all grafted SCs in our case expressed GFP, only a subpopulation of SCs in their case might express GFP depending on the transduction efficiency of the virus. Therefore, different survival prognoses of grafted SCs may be caused by the fact that we counted all SCs including proliferating SCs that were GFP positive, whereas, they counted only lenti-GFP transduced SCs, leaving untransduced SCs not counted. Although the transduction efficiency in the SC cultures was > 99% as reported, it was difficult to know what percentage of SCs remained GFP positive in vivo especially after several days and weeks of survival at the lesion site.
It should be noted that ongoing cytotoxic processes within the contusion environment are harmful to the grafted cells. These processes include excitotoxicity, oxidative stress, inflammation, proteolysis, and anoxic damage, which are all components of progressive secondary injury (Amar and Levy, 1999; Tator and Fehlings, 1991). It is possible that in the Hill et al. study, lentiviral-transfected SCs might be more vulnerable to these damaging processes compared to non-transfected SCs, which then led to a decrease in proliferation and an increase in their sensitivity to these cytotoxic events. Additionally, cell-free lentiviral vectors might affect the microenvironment surrounding the grafted SCs to increase the complexity of assessment. Finally, we cannot exclude the possibility that SCs derived from GFP rats in our case may have unknown alternative properties that make their survival in the lesion area feasible. Additionally, examination of strategies to enhance transplant survival in other models has demonstrated that the survival of transplanted cells is affected by not only post-graft interval and the site of grafting, but also by how cells are prepared before grafting (Sortwell, 2003). In the current study we treated the SCs-GFP culture medium with fresh mitogens at 4 h before cell collection. This approach may be advantageous to ensure that the cells were at optimal growth condition to resist injury-induced inflammatory environment after being transplanted into the lesion site.
Necrosis and apoptosis are two main contributors to death of transplanted cells. Although we did not examine the necrotic death profile of SCs-GFP post transplantation, our TUNEL staining showed that only a few grafted SCs-GFP underwent apoptosis 1 d after transplantation with no detected apoptosis thereafter. The low occurrence of apoptosis post-transplantation could be a critical factor and supporting evidence that the majority of grafted SCs-GFP survived well for a prolonged period following transplantation. Contrast to the present study, lentiviral transduction of SCs underwent heavy necrosis, and apoptosis especially, within 1 wk following transplantation (Hill et al., 2007). This is not surprising because vector-related toxicity caused by the envelope protein has been reported by several studies (Power et al., 2004; Yang et al., 2007).
Proliferation of grafted SCs could be another important contributing factor for the presence of a large quantity of SCs-GFP at the lesion site after transplantation. Here we clearly demonstrated, for the first time, that grafted SCs were highly proliferative before they differentiated and myelinated axons at the lesion site. Fresh medium containing 10% FBS and 2 mitogens, pituitary extract and forskolin, were applied to the culture system prior to and during cell collection. This might boost the initial proliferation of SCs-GFP that we observed at 1 d after transplantation. Mostly impressively, the proliferation rate reached as high as 26% at 2 wk post-transplantation (Fig. 3C, G). This was consistent with the higher number of SCs-GFP found at 2 wk post-transplantation (Fig. 2O), indicating that proliferation contributed to an overall increase in the total number of SCs-GFP at this time point. The cell number increased by proliferation might also compensate for the cell loss induced by necrosis and/or apoptosis. The dynamic balance between cell survival and death might finally lead to a stable SCs-GFP cell number over a prolonged post-transplantation period. It should be noted that the time course of SCs-GFP proliferation occurred in a time window when host axons vigorously grew into the transplant. In a previous study, when SCs were seeded into a semipermeable guidance channel which was then transplanted into a right-sided spinal hemisection, anterogradely traced propriospinal axons grew into the graft prior to 2 d post-transplantation and approached the caudal host-graft interface at 14 d (Hsu and Xu, 2005), correlating well with the time course of SCs-GFP proliferation seen in the present study.
To maintain a long term survival of grafted cells, reestablishment of blood supply to regenerative tissue is essential. We therefore examined whether or not new blood vessels were formed within the graft environment. We found that new blood vessels, immunolabeled with REAC, grew into the SC graft as early as 1 wk after transplantation and integrated well with the graft tissue throughout the entire survival period that was studied (24 wk, Fig. 7). The newly formed blood vessels may provide nutrition and trophic support for the survival of grafted SCs-GPF and axons within this region.
For allogeneic SC transplantation, an immune response to grafted cells exists and administration of an immunosuppressant could enhance the survival of grafted allogeneic SCs (Hill et al., 2006). We confirmed this observation in two ways. First, pretreatment and daily application of an immunosuppressant CsA led to remarkable SCs survival for a considerable period of time (up to 24 wk) after transplantation. In the CsA-treated spinal cord, only a small number of macrophages were found surrounding the transplants. Secondly, stopping the application of CsA after the 3rd month in the 1 y survival group resulted in massive invasion of ED1+ microphages within and surrounding the SCs-GFP graft. The phagocytic macrophages, containing engulfed debris of SCs-GFP (therefore became fluorescent green), migrated from the original graft site into the host tissue and evenly distributed throughout the host spinal cord close to the lesion (Fig. 9A). In addition, the marker label on the SCs in the Hill study was human placental alkaline phosphatase which could have engendered an immune response. Indeed, survival of these SCs was enhanced by immunosuppression with cyclosporine (Hill et al., 2006) The transgenic GFP label of SCs in the current study may have been much less immunogenic. These results emphasize the importance of using immunosuppressive agents in cell-based therapies in the preclinical and clinical settings.
SCs-GFP grafts decreased the angle of rotation of hindlimbs. This finding is in correlation with our observations that SC grafts resulted in a higher number of propriospinal or supraspinal axons regenerating into SCs-GFP grafts and SCs-GFP myelinated these regenerating or sprouted axons at the lesion site. It was shown previously that hindlimb recovery after an incomplete SCI depends on the number of spared and regenerated descending axons from brainstem nuclei and cerebral cortex (Basso et al., 1996; Saruhashi and Young, 1994) and from local propriospinal axons (Helgren and Goldberger, 1993). Interestingly, although decreased angle of rotation of hindlimb was observed in this study, we did not observe significant differences in BBB score, footfall error, paw withdrawal latency, total spread, paw length, stride length, and base of support between the SCs-GFP transplantation and non-transplantation groups. These results indicate that transplantation of SCs into the lesion gap may not be sufficient to promote substantial recovery of function. Additional strategies, including the activation of SCs prior to the transplantation or combining SC transplantation with other novel strategies such as increasing neuronal intrinsic capability for regeneration or exercise training, are required to promote meaningful functional recovery.
Just recently, the Miami Project to Cure Paralysis at University of Miami Miller School of Medicine received permission from the Food and Drug Administration (FDA) to begin a Phase I clinical trial to evaluate the safety of transplanting human autologous SCs to treat patients with spinal cord injuries. This is the only FDA-approved cell therapy-based clinical trial for sub-acute SCI in the United States (Xu, 2012). This study is certainly an important step towards applying cell-based therapies for patients with SCIs. Based on the findings of the present study, we believe the approved autologous Schwann cells transplantation trial is a practicable and promising cell based therapy. To achieve meaningful functional recovery, it is likely that SC transplantation can be combined with other optimal strategies such as co-transplantation with other cell candidates (Hu et al., 2013), reconstruction of growth-promoting pathways through and beyond a lesion gap (Deng et al., 2013), or induction of intrinsic regenerative capacity of axons to regenerate (Liu et al., 2010).
Highlights.
We assessed the fate and temporal profile of grafted Schwann cells (SCs) overexpressing GFP in a contusive spinal cord injury (SCI).
Grafted SCs showed a remarkable survival up to 24 wk post-grafting.
Grafted SCs proliferated and the proliferation peaked at 2 wk post-transplantation.
Grafted SCs facilitated new blood vessels formation within the lesion site.
Acknowledgments
We thank Ms. Lili Zhang for her assistance on animal behavior tests and Ms. Patti Raley, a medical editor, for her critical reading of the manuscript. This work was supported in part by National Institutes of Health (NIH) 1R01 NS052290, NS052290-06S1, NS059622, NS050243, the Indiana Clinical and Translational Sciences Institute (CTSI) Collaboration in Biomedical/Translational Research (CBR/CTR) Pilot Program Grants (Grant #RR025761) from the NIH, the Indiana Spinal Cord and Brain Injury Research Foundation (ISCBIRF, A70-3-079793) and Mari Hulman George Endowment Funds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amar AP, Levy ML. Pathogenesis and pharmacological strategies for mitigating secondary damage in acute spinal cord injury. Neurosurgery. 1999;44:1027–1039. doi: 10.1097/00006123-199905000-00052. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Khemani S, King VR, Priestley JV, McMahon SB. NT-3 promotes growth of lesioned adult rat sensory axons ascending in the dorsal columns of the spinal cord. Eur J Neurosci. 1999;11:3873–3883. doi: 10.1046/j.1460-9568.1999.00809.x. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, McMahon SB. Spinal cord repair strategies: why do they work? Nat Rev Neurosci. 2006;7:644–653. doi: 10.1038/nrn1964. [DOI] [PubMed] [Google Scholar]
- Cao QL, Howard RM, Dennison JB, Whittemore SR. Differentiation of engrafted neuronal-restricted precursor cells is inhibited in the traumatically injured spinal cord. Exp Neurol. 2002;177:349–359. doi: 10.1006/exnr.2002.7981. [DOI] [PubMed] [Google Scholar]
- Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med. 1997;3:73–76. doi: 10.1038/nm0197-73. [DOI] [PubMed] [Google Scholar]
- Deng LX, Deng P, Ruan Y, Xu ZC, Liu NK, Wen X, Smith GM, Xu XM. A novel growth-promoting pathway formed by GDNF-overexpressing Schwann cells promotes propriospinal axonal regeneration, synapse formation, and partial recovery of function after spinal cord injury. J Neurosci. 2013;33:5655–5667. doi: 10.1523/JNEUROSCI.2973-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng LX, Hu J, Liu N, Wang X, Smith GM, Wen X, Xu XM. GDNF modifies reactive astrogliosis allowing robust axonal regeneration through Schwann cell-seeded guidance channels after spinal cord injury. Exp Neurol. 2011 doi: 10.1016/j.expneurol.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Yan Q, Ruan JW, Zhang YQ, Li WJ, Zeng X, Huang SF, Zhang YJ, Wang S, Dong H, Zeng YS. Bone marrow mesenchymal stem cells and electroacupuncture downregulate the inhibitor molecules and promote the axonal regeneration in the transected spinal cord of rats. Cell Transplant. 2011;20:475–491. doi: 10.3727/096368910X528102. [DOI] [PubMed] [Google Scholar]
- Feron F, Perry C, Cochrane J, Licina P, Nowitzke A, Urquhart S, Geraghty T, Mackay-Sim A. Autologous olfactory ensheathing cell transplantation in human spinal cord injury. Brain. 2005;128:2951–2960. doi: 10.1093/brain/awh657. [DOI] [PubMed] [Google Scholar]
- Filbin MT. Myelin-associated inhibitors of axonal regeneration in the adult mammalian CNS. Nat Rev Neurosci. 2003;4:703–713. doi: 10.1038/nrn1195. [DOI] [PubMed] [Google Scholar]
- Fujimoto Y, Abematsu M, Falk A, Tsujimura K, Sanosaka T, Juliandi B, Semi K, Namihira M, Komiya S, Smith A, Nakashima K. Treatment of a Mouse Model of Spinal Cord Injury by Transplantation of Human iPS Cell-derived Long-term Self-renewing Neuroepithelial-like Stem Cells. Stem Cells. 2012 doi: 10.1002/stem.1083. [DOI] [PubMed] [Google Scholar]
- Girard C, Bemelmans AP, Dufour N, Mallet J, Bachelin C, Nait-Oumesmar B, Baron-Van Evercooren A, Lachapelle F. Grafts of brain-derived neurotrophic factor and neurotrophin 3-transduced primate Schwann cells lead to functional recovery of the demyelinated mouse spinal cord. J Neurosci. 2005;25:7924–7933. doi: 10.1523/JNEUROSCI.4890-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruner JA. A monitored contusion model of spinal cord injury in the rat. J Neurotrauma. 1992;9:123–128. doi: 10.1089/neu.1992.9.123. [DOI] [PubMed] [Google Scholar]
- Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- Helgren ME, Goldberger ME. The recovery of postural reflexes and locomotion following low thoracic hemisection in adult cats involves compensation by undamaged primary afferent pathways. Exp Neurol. 1993;123:17–34. doi: 10.1006/exnr.1993.1137. [DOI] [PubMed] [Google Scholar]
- Hill CE, Guller Y, Raffa SJ, Hurtado A, Bunge MB. A calpain inhibitor enhances the survival of Schwann cells in vitro and after transplantation into the injured spinal cord. J Neurotrauma. 2010;27:1685–1695. doi: 10.1089/neu.2010.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill CE, Hurtado A, Blits B, Bahr BA, Wood PM, Bartlett Bunge M, Oudega M. Early necrosis and apoptosis of Schwann cells transplanted into the injured rat spinal cord. Eur J Neurosci. 2007;26:1433–1445. doi: 10.1111/j.1460-9568.2007.05771.x. [DOI] [PubMed] [Google Scholar]
- Hill CE, Moon LD, Wood PM, Bunge MB. Labeled Schwann cell transplantation: cell loss, host Schwann cell replacement, and strategies to enhance survival. Glia. 2006;53:338–343. doi: 10.1002/glia.20287. [DOI] [PubMed] [Google Scholar]
- Hill CE, Proschel C, Noble M, Mayer-Proschel M, Gensel JC, Beattie MS, Bresnahan JC. Acute transplantation of glial-restricted precursor cells into spinal cord contusion injuries: survival, differentiation, and effects on lesion environment and axonal regeneration. Exp Neurol. 2004;190:289–310. doi: 10.1016/j.expneurol.2004.05.043. [DOI] [PubMed] [Google Scholar]
- Hsu JY, Xu XM. Early profiles of axonal growth and astroglial response after spinal cord hemisection and implantation of Schwann cell-seeded guidance channels in adult rats. J Neurosci Res. 2005;82:472–483. doi: 10.1002/jnr.20662. [DOI] [PubMed] [Google Scholar]
- Hu J, Zhou J, Li X, Wang F, Lu H. Schwann cells promote neurite outgrowth of dorsal root ganglion neurons through secretion of nerve growth factor. Indian J Exp Biol. 2011;49:177–182. [PubMed] [Google Scholar]
- Hu JG, Wang XF, Deng LX, Liu NK, Gao X, Chen JH, Zhou FC, Xu XM. Co-transplantation of glial restricted precursor cells and Schwann cells promotes functional recovery after spinal cord injury. Cell Transplant. 2013 doi: 10.3727/096368912X661373. [DOI] [PubMed] [Google Scholar]
- Iannotti C, Li H, Yan P, Lu X, Wirthlin L, Xu XM. Glial cell line-derived neurotrophic factor-enriched bridging transplants promote propriospinal axonal regeneration and enhance myelination after spinal cord injury. Exp Neurol. 2003;183:379–393. doi: 10.1016/s0014-4886(03)00188-2. [DOI] [PubMed] [Google Scholar]
- Liu K, Lu Y, Lee JK, Samara R, Willenberg R, Sears-Kraxberger I, Tedeschi A, Park KK, Jin D, Cai B, Xu B, Connolly L, Steward O, Zheng B, He Z. PTEN deletion enhances the regenerative ability of adult corticospinal neurons. Nat Neurosci. 2010;13:1075–1081. doi: 10.1038/nn.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XZ, Xu XM, Hu R, Du C, McDonald JW, Dong HX, Wu YJ, Fan GS, Jacquin MF, Hsu CY, Choi DW. Neuronal and glial apoptosis after traumatic spinal cord injury. J Neurosci. 1997;17:5395–5406. doi: 10.1523/JNEUROSCI.17-14-05395.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Wang X, Lu CC, Kerman R, Steward O, Xu XM, Zou Y. Repulsive Wnt signaling inhibits axon regeneration after CNS injury. J Neurosci. 2008;28:8376–8382. doi: 10.1523/JNEUROSCI.1939-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma YH, Zhang Y, Cao L, Su JC, Wang ZW, Xu AB, Zhang SC. Effect of neurotrophin-3 genetically modified olfactory ensheathing cells transplantation on spinal cord injury. Cell Transplant. 2010;19:167–177. doi: 10.3727/096368910X492634. [DOI] [PubMed] [Google Scholar]
- Metz GAS, Merkler D, Dietz V, Schwab ME, Fouad K. Efficient testing of motor function in spinal cord injured rats. Brain Res. 2000;883:165–177. doi: 10.1016/s0006-8993(00)02778-5. [DOI] [PubMed] [Google Scholar]
- Mitsui T, Shumsky JS, Lepore AC, Murray M, Fischer I. Transplantation of neuronal and glial restricted precursors into contused spinal cord improves bladder and motor functions, decreases thermal hypersensitivity, and modifies intraspinal circuitry. J Neurosci. 2005;25:9624–9636. doi: 10.1523/JNEUROSCI.2175-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey TK, Kleitman N, Bunge RP. Isolation and functional characterization of Schwann cells derived from adult peripheral nerve. J Neurosci. 1991;11:2433–2442. doi: 10.1523/JNEUROSCI.11-08-02433.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudega M, Xu XM. Schwann cell transplantation for repair of the adult spinal cord. J Neurotrauma. 2006;23:453–467. doi: 10.1089/neu.2006.23.453. [DOI] [PubMed] [Google Scholar]
- Phinney DG, Isakova I. Plasticity and therapeutic potential of mesenchymal stem cells in the nervous system. Curr Pharm Des. 2005;11:1255–1265. doi: 10.2174/1381612053507495. [DOI] [PubMed] [Google Scholar]
- Power C, Zhang K, van Marle G. Comparative neurovirulence in lentiviral infections: The roles of viral molecular diversity and select proteases. J Neurovirol. 2004;10(Suppl 1):113–117. doi: 10.1080/753312762. [DOI] [PubMed] [Google Scholar]
- Sandner B, Prang P, Rivera FJ, Aigner L, Blesch A, Weidner N. Neural stem cells for spinal cord repair. Cell Tissue Res. 2012 doi: 10.1007/s00441-012-1363-2. [DOI] [PubMed] [Google Scholar]
- Saruhashi Y, Young W. Effect of mianserin on locomotory function after thoracic spinal cord hemisection in rats. Exp Neurol. 1994;129:207–216. doi: 10.1006/exnr.1994.1162. [DOI] [PubMed] [Google Scholar]
- Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5:146–156. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- Sortwell CE. Strategies for the augmentation of grafted dopamine neuron survival. Front Biosci. 2003;8:s522–532. doi: 10.2741/1096. [DOI] [PubMed] [Google Scholar]
- Takami T, Oudega M, Bates ML, Wood PM, Kleitman N, Bunge MB. Schwann cell but not olfactory ensheathing glia transplants improve hindlimb locomotor performance in the moderately contused adult rat thoracic spinal cord. J Neurosci. 2002;22:6670–6681. doi: 10.1523/JNEUROSCI.22-15-06670.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;75:15–26. doi: 10.3171/jns.1991.75.1.0015. [DOI] [PubMed] [Google Scholar]
- Thuret S, Moon LD, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7:628–643. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- Totoiu MO, Keirstead HS. Spinal cord injury is accompanied by chronic progressive demyelination. J Comp Neurol. 2005;486:373–383. doi: 10.1002/cne.20517. [DOI] [PubMed] [Google Scholar]
- Xu XM. Roles of Schwann cells in the repair and regeneration of the central nervous system. Chinese J Neuroanat. 1996;12:291–302. [Google Scholar]
- Xu XM, Chen A, Guenard V, Kleitman N, Bunge MB. Bridging Schwann cell transplants promote axonal regeneration from both the rostral and caudal stumps of transected adult rat spinal cord. J Neurocytol. 1997;26:1–16. doi: 10.1023/a:1018557923309. [DOI] [PubMed] [Google Scholar]
- Xu XM, Guenard V, Kleitman N, Aebischer P, Bunge MB. A combination of BDNF and NT-3 promotes supraspinal axonal regeneration into Schwann cell grafts in adult rat thoracic spinal cord. Exp Neurol. 1995a;134:261–272. doi: 10.1006/exnr.1995.1056. [DOI] [PubMed] [Google Scholar]
- Xu XM, Guenard V, Kleitman N, Bunge MB. Axonal regeneration into Schwann cell-seeded guidance channels grafted into transected adult rat spinal cord. J Comp Neurol. 1995b;351:145–160. doi: 10.1002/cne.903510113. [DOI] [PubMed] [Google Scholar]
- Xu XM, Onifer SM. Transplantation-mediated strategies to promote axonal regeneration following spinal cord injury. Respir Physiol Neurobiol. 2009;169:171–182. doi: 10.1016/j.resp.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SH, Agca Y, Cheng PH, Yang JJ, Agca C, Chan AW. Enhanced transgenesis by intracytoplasmic injection of envelope-free lentivirus. Genesis. 2007;45:177–183. doi: 10.1002/dvg.20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yiu G, He Z. Glial inhibition of CNS axon regeneration. Nat Rev Neurosci. 2006;7:617–627. doi: 10.1038/nrn1956. [DOI] [PMC free article] [PubMed] [Google Scholar]