Abstract
For women, choosing a facially masculine man as a mate is thought to confer genetic benefits to offspring. Crucial assumptions of this hypothesis have not been adequately tested. It has been assumed that variation in facial masculinity is due to genetic variation and that genetic factors that increase male facial masculinity do not increase facial masculinity in female relatives. We objectively quantified the facial masculinity in photos of identical (n = 411) and nonidentical (n = 782) twins and their siblings (n = 106). Using biometrical modeling, we found that much of the variation in male and female facial masculinity is genetic. However, we also found that masculinity of male faces is unrelated to their attractiveness and that facially masculine men tend to have facially masculine, less-attractive sisters. These findings challenge the idea that facially masculine men provide net genetic benefits to offspring and call into question this popular theoretical framework.
Keywords: mate preference, sexual dimorphism, intralocus sexual conflict, evolution, immunocompetence-handicap principle, good genes, pathogen, health, sexually antagonistic selection, behavior genetics, evolutionary psychology, face perception, human mate selection
A large body of research has shown that women attend to facial masculinity when assessing potential mates. Women tend to show greater preference for facially masculine mates in circumstances thought to increase the relative importance of indirect benefits of mate choice (i.e., genetic benefits to offspring) as opposed to direct benefits of mate choice (e.g., resource provision, protection). For example, women show increased preference for facially masculine men when considering a short-term or extrapair partner (Little, Jones, Penton-Voak, Burt, & Perrett, 2002), during the fertile phase of the menstrual cycle (Gangestad, Thornhill, & Garver-Apgar, 2010; Penton-Voak et al., 1999), when sex drive is high (Welling, Jones, & DeBruine, 2008), when self-perceived attractiveness is high (Little, Burt, Penton-Voak, & Perrett, 2001), and when pathogens are prevalent or health is threatened (DeBruine, Jones, Crawford, Welling, & Little, 2010; Little, DeBruine, & Jones, 2011). The studies just cited focused largely on masculine face shape as opposed to other features, such as shading or texture. The widely accepted interpretation of these findings is that male facial masculinity is a signal of genetic quality (“good genes”) and that women have accordingly evolved to attend to facial masculinity when choosing mates (Gangestad & Simpson, 2000; Little, Jones, & DeBruine, 2011; Roberts & Little, 2008).
Facial masculinity is thought to be an honest signal of genetic quality because of the immunosuppressive effects of testosterone (Folstad & Karter, 1992). The idea is that only men with good innate immune functioning can afford to support the levels of testosterone required to develop masculine facial features (Folstad & Karter, 1992; Zahavi, 1975). Supporting this immunocompetence-handicap hypothesis, research shows that facial masculinity is positively associated with circulating testosterone levels (Penton-Voak & Chen, 2004), and male facial masculinity has been found to correlate positively with both perceived and actual health (Rantala et al., 2012; Rhodes, Chan, Zebrowitz, & Simmons, 2003; Thornhill & Gangestad, 2006). An alternative (or additional) explanation of the relevance of male facial masculinity to genetic quality is the sexy-son hypothesis, according to which the genetic benefits to offspring come in the form of greater attractiveness of male offspring. This situation can create a self-reinforcing runaway effect that exaggerates both the preference and the preferred trait (Fisher, 1915; Huk & Winkel, 2008).
The idea that male facial masculinity signals heritable genetic quality, manifested as immunocompetence and/or sexy sons, has gained broad acceptance (Gangestad & Scheyd, 2005; Gangestad & Simpson, 2000; Little, Jones, et al., 2011; Perrett et al., 1998; Rantala et al., 2012; Roberts & Little, 2008; although see Puts, 2010; Scott, Clark, Boothroyd, & Penton-Voak, 2012). However, this idea depends on two key assumptions that have not been adequately tested. First, it is assumed that male facial masculinity is substantially heritable (i.e., a substantial proportion of the variation is due to additive genetic variation); otherwise, it could not be inherited by offspring and could not signal good genes. Second, it has been assumed that the genes that increase male facial masculinity are not detrimental to female offspring (e.g., by increasing their facial masculinity, which has been shown previously to decrease female attractiveness); otherwise, any genetic benefits to male offspring would be counteracted by the detriment to female offspring (this is termed intralocus sexual conflict; see Bonduriansky & Chenoweth, 2009; Garver-Apgar, Eaton, Tybur, & Thompson, 2011).
Only one previous study has empirically addressed these assumptions (Cornwell & Perrett, 2008), by analyzing ratings of masculinity and attractiveness of the faces in family photographs. However, no objective measures of masculinity were used, and heritability could not be estimated because members of a standard nuclear family equally share both genes and family environment, which are therefore completely confounded. In another study, Mitchem et al. (2013) used facial photos of monozygotic (identical) and dizygotic (nonidentical) twins to distinguish the influence of genes and family environment on facial masculinity and attractiveness; again, however, no objective measures were used. It has been shown previously that subjective ratings of masculinity are based on additional factors other than morphological masculinity, which changes the association with traits such as attractiveness (Scott, Pound, Stephen, Clark, & Penton-Voak, 2010).
In the research reported here, we used geometric morphometrics, the statistical analysis of shape, to objectively quantify the masculinity of facial shape in photographs of a large sample of identical and nonidentical (same-sex and opposite-sex) twins and siblings. Using biometrical modeling, we estimated the heritability of male and female facial masculinity. Finally, we tested for intralocus sexual conflict by assessing the correlation in facial masculinity between opposite-sex twins and siblings, and we investigated the relationship, for each sex, between the objective masculinity and rated attractiveness of the photographs.
Method
Participants
Participants were 1,193 individual twins and 106 of their siblings from 575 families who took part in the Genes for Cognition study and were part of the Brisbane Adolescent Twin Studies (Wright & Martin, 2004). Twins were tested and photographed as close as possible to their 16th birthday (mean age = 16.03 years; SD = 0.47 years), and their siblings were tested and photographed as close as possible to their 18th birthday (mean age = 17.80; SD = 0.46). All participants gave informed written consent, and approval to code and analyze these data was obtained from the Human Research Ethics Committee at QIMR Berghofer.
Photographs
Photographs of participants were taken between 1996 and 2010. In the earliest waves of data collection, photographs were taken using film cameras and later scanned to digital format. Photographs from later waves were taken with digital cameras. Each photograph was taken under standard indoor lighting conditions. Objective measures of masculinity and subjective ratings of masculinity and attractiveness were obtained from these photographs.
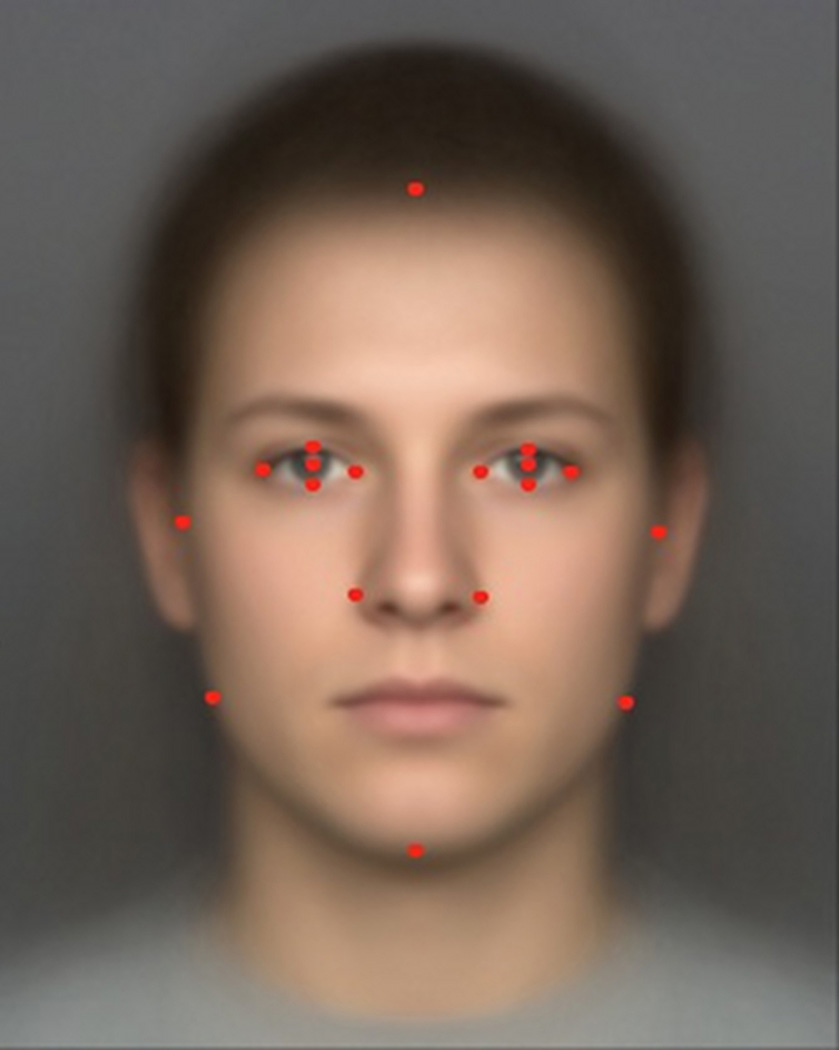
Ten independent raters identified a total of 18 landmarks on each face. Raters were trained for several weeks in hour-long sessions in which landmarks were defined anatomically. Figure 1 shows the location of each landmark. Two raters were randomly chosen for each landmark, and the coordinate for that landmark was calculated as the mean pixel location chosen by these two raters.
Fig. 1.
Facial landmarks (dots) used to compute facial masculinity.
Photographs of participants were not originally taken for shape analysis. Therefore, the photographs varied in ways that could alter the shape information captured by the landmarks (e.g., the participant’s head angle facing the camera or the participant’s facial expression). We assumed that most of this type of variation was idiosyncratic and would therefore simply add error variance rather than biasing the results in any particular direction. However, to avoid the possibility that smiles would bias the measures, we did not use landmarks around the mouth, and we subsequently confirmed that controlling for rated degree of smiling did not affect the results (data not reported here).
Facial masculinity scores
We used geometric morphometrics, the statistical analysis of shape through landmark coordinates, to analyze the faces (Bookstein, 1991; Zelditch, Swiderski, Sheets, & Fink, 2004). Shape is defined as the differences between objects that are not due to translation, size, or rotation, and it therefore encapsulates all other information, such as distances and angles between different landmarks.
To extract shape information from raw facial landmarks, we conducted a generalized Procrustes analysis (Zelditch et al., 2004) on raw x- and y-coordinates. This procedure removes translation effects (position of the object in the shape space) by standardizing all faces to a common shape space, removes size effects by standardizing centroid size to 1, and removes rotational effects by minimizing the summed, squared distances between homologous landmarks across a range of faces. This produces Procrustes coordinates that purely represent shape information. The coordinates are then transformed into shape variables via a principal component analysis. Shape variables are a decomposition of the original Procrustes coordinates and completely maintain the shape information. Shape variables also have the advantage of being compatible with conventional statistical techniques without the need for adjustments. For full details of generalized Procrustes analysis and shape analysis via geometric morphometrics, see Zelditch et al. (2004).
To compute a data-driven single measure of facial masculinity, we conducted a discriminant-function analysis (DFA) with sex as the grouping variable (female = 0, male = 1). DFA produced a discriminant function that was the linear combination of shape variables that best discriminated between male and female landmark configurations. Thus, the discriminant function from this analysis represents the sexual-dimorphism dimension (see Fig. 2 for the distribution of scores on the discriminant function). Related analyses have been used previously to compute data-driven scores of facial masculinity (Gangestad et al., 2010; Scott et al., 2010). The DFA performed on the twins’ data yielded a point-biserial correlation of .66 between participant’s sex and the discriminant score, which was slightly higher than the corresponding value reported in Gangestad et al. (2010). The discriminant function correctly classified the sex of 81% of participants, which is lower than the corresponding value reported in Scott et al. (2010), but their high ratio of predictors to participants (which can cause model overfitting) and lack of cross-validation make it difficult to interpret their very high rate of correct classification.
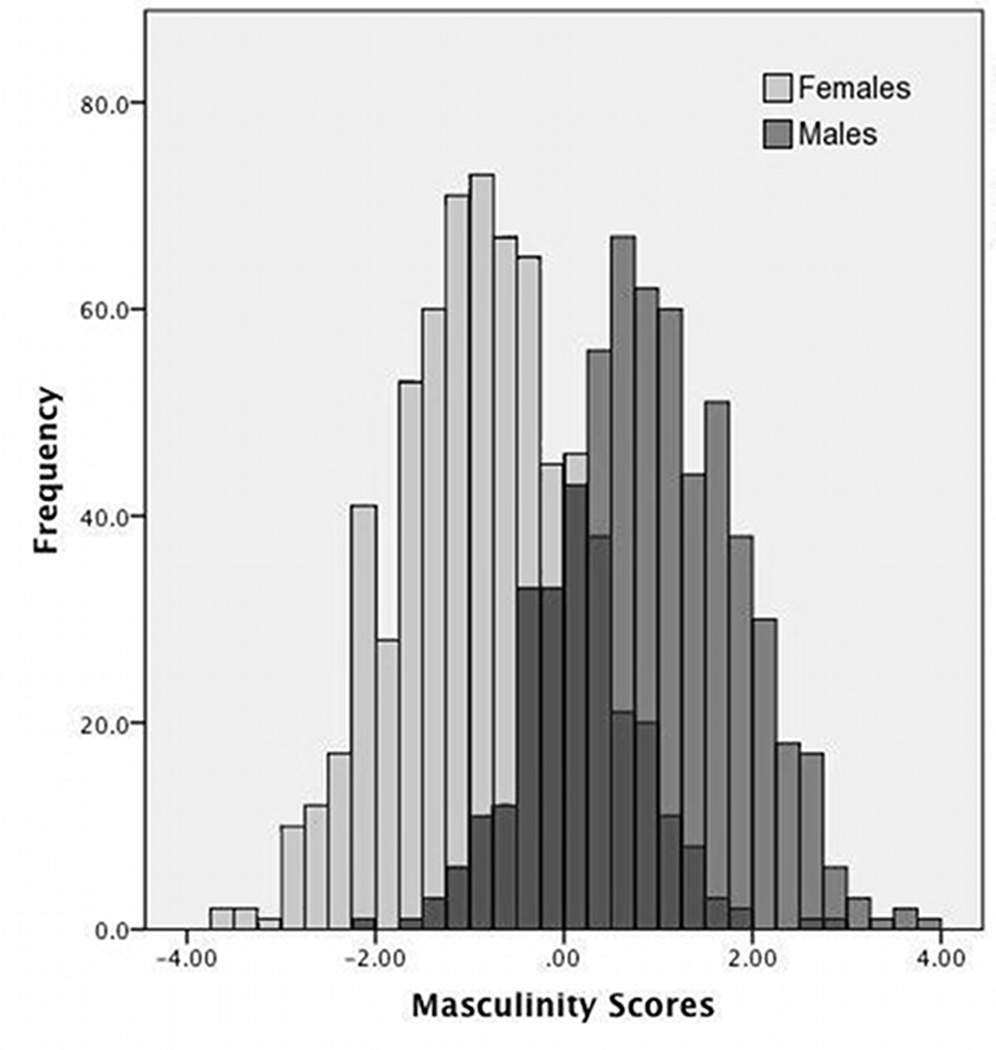
Fig. 2.
Frequency of objective facial masculinity scores from the discriminant-function analysis for males (M = .92; SD = .94) and females (M = −.80; SD = .97), before standardization separately by sex. The purple bars represent overlapping distributions for males and females.
To cross-validate our measure, we applied this same function to the siblings’ data; this yielded a point-biserial correlation between sex and masculinity of .65 and a correct-classification rate of 80%, which indicates that the masculinity measure discriminated between the sexes equally well in the approximately 18-year-old siblings and the approximately 16-year-old twins, further validating our measure.
The discriminant scores were standardized by sex to produce a facial masculinity score for each participant in relation to other participants of the same sex. Five outliers on facial masculinity (≥ ± 3 SD from the mean) were omitted from all analyses; however, an analysis retaining these outliers yielded results virtually identical results to those reported here.
Observer ratings of facial attractiveness and masculinity
Observers also rated the photographs on a number of traits. For this study, we were primarily interested in the attractiveness ratings, but we also analyzed the facial masculinity ratings to check whether facial masculinity scores calculated from landmark coordinates correlated with subjective perceptions of facial masculinity.
We presented the photos in a random order to 8 undergraduate research assistants (4 men and 4 women who were not involved in identifying the facial landmarks) and asked them to rate all faces on attractiveness and facial masculinity. Ratings were given on 7-point scales (for attractiveness, 1 = low attractiveness, 7 = high attractiveness; for masculinity, 1 = very feminine, 7 = very masculine). We did not give raters instructions on how to judge attractiveness, but we did inform them of facial features that are considered to be sexually dimorphic in humans.
Interrater agreement for attractiveness was moderate (intraclass correlation coefficient, = .44, p < .001; α = .87). Averaged scores from male raters and from female raters correlated very highly with the averaged score from all raters (r = .94 for male raters and r = .92 for female raters), so the latter composite score was used for all analyses because it contained substantially less measurement error than the separate scores for male and female raters.
Interrater agreement was low for masculinity (intraclass correlation coefficient = .19; α = .66). Nevertheless, there was still a significant (though modest) correlation between objective and rated masculinity (men: r = .23, p < .001; women: r = .25, p < .001). Objective masculinity was based only on shape and was not associated with ratings of grooming or acne, whereas masculinity ratings were associated with ratings of grooming (women: r = −.44, p < .001; men: r = −.05, p = .29) and acne (women: r = .29, p < .001; men: r = .21, p < .001) and were presumably influenced by cues other than shape, such as skin color and tone, heaviness of brow, and facial hair. Our objective masculinity measure correlated much more strongly with the component of the masculinity ratings that is captured by the landmark-based shape variables (men: r = .53, p < .001; women: r = .57, p < .001) than with the raw masculinity measure. (See the Supplemental Material available online for details of the analysis.) For more detail on the rating process and genetic analyses of observer ratings, see Mitchem et al. (2013).
Statistical analysis
Identical twins share all their genes, whereas nonidentical twins share, on average, half of their segregating genes, and all twins completely share the family environment. Therefore, we were able to partition the variation in scores into three sources: additive genetic (A), shared environmental (C), and residual (E) sources. As is standard for twin-family designs, we conducted maximum-likelihood modeling, which determines the combination of A, C, and E that best matches the observed data (i.e., means, variances, and twin or sibling pair correlations). For further details on the type of twin analysis that we used, see Neale & Cardon (1992) and Posthuma et al. (2003). All analyses were conducted in the Mx software package, Version 1.54a (Neale, Boker, Xie, & Maes, 2006). As is standard in twin modeling, differences between the means and correlations of different zygosity groups were tested by equating the relevant parameters in the model and testing the change in model fit (distributed as χ2) against the change in degrees of freedom (which equals the change in the number of parameters estimated).
Results
Preliminary testing found that mean facial masculinity scores did not significantly differ between identical and nonidentical twins of the same sex, χ2(2, N = y) = 2.48, p = .29. Means of female or male members of same-sex pairs did not differ significantly from means of female or male members of opposite-sex pairs, χ2(2, N = y) = 0.31, p = .85, which suggests that prenatal hormone transfer from one twin to the other had no influence on this trait. Means of twins did not differ significantly from means of other siblings, χ2(2, N = y) = 3.60, p = .17, which suggests that there was nothing unusual about the facial masculinity of twins. Furthermore, the correlations between nonidentical twin pairs (i.e., male-male, female-female, and male-female) did not differ significantly from the correlations between corresponding nontwin sibling pairs, χ2(3, N = y) = 2.18, p = .54, as expected given the equivalent genetic and environmental similarity of nonidentical-twin and sibling pairs; these correlations were equated in subsequent modeling.
There was no significant effect of age on facial masculinity scores in men, χ2(1, N = y) = 0.04, p = .85, or women, χ2(1, N = y) = 0.63, p = .43. Intraclass correlation coefficients are shown in Table 1. Correlations between identical twins were markedly greater than correlations between same-sex nonidentical twins and siblings for both men, χ2(1, N = y) = 11.92, p < .001, and women, χ2(1, N = y) = 4.93, p = .03, which suggests that there is an important genetic component of facial masculinity in both sexes. The estimated proportions of variation in facial masculinity due to genetic and environmental sources are reported in Table 2. For both men and women, approximately half of the variation in facial masculinity was attributed to additive genetic factors, whereas virtually no variation was attributed to shared environmental influences. This finding is consistent with the assumption that variation in facial masculinity is substantially heritable, which is a necessary condition for facial masculinity to serve as a signal for good genes.
Table 1.
Intraclass Correlation Coefficients for Objective Facial Masculinity of Twin and Sibling Pairs
| Zygosity group | r [95% CI] |
|---|---|
| Identical female twins (n = 110 pairs) | .50 [.36, .61] |
| Identical male twins (n = 88 pairs) | .50 [.34, .62] |
| All identical twins | .50 [.39, .59] |
| Nonidentical female twins (n = 113 pairs) | .30 [.11, .45] |
| Female siblings (n = 55 pairs) | .20 [–.16, .46] |
| All nonidentical female twins and siblings | .28 [.11, .42] |
| Nonidentical male twins (n = 93 pairs) | .16 [–.04, .35] |
| Male siblings (n = 39 pairs) | –.09 [–.38, .22] |
| All nonidentical male twins and siblings | .09 [–.08, .26] |
| All nonidentical same-sex twins and siblings | .23 [.10, .35] |
| Nonidentical opposite-sex twins (n = 171 pairs) | .23 [.09, .36] |
| Opposite-sex siblings (n = 120 pairs) | .23 [.04, .39] |
| Opposite-sex twins and siblings | .23 [.12, .33] |
Note: Means and variances were equated across zygosity (within sex). Sibling pairs are not independent (i.e., a nontwin sibling can have a sibling relationship with each member of a twin pair). CI = confidence interval.
Table 2.
Proportions of Variance of Objective Facial Masculinity Estimated to Be Accounted for by A (Additive Genetic), C (Shared Environmental), and E (Residual) Influences
| Participant group | A | C | E |
|---|---|---|---|
| Female | .48 [.11, .61] | .03 [.00, .34] | .49 [.39, .62] |
| Male | .46 [.20, .59] | .00 [.00, .17] | .54 [.41, .71] |
| Overall | .49 [.28, .57] | .00 [.00, .17] | .51 [.43, .61] |
Note: The numbers in square brackets are 95% confidence intervals. Opposite-sex twins contributed to means and variances but not to variance components (i.e., the genetic correlation between opposite-sex twins was left free to vary in the model). The genetic correlation between opposite-sex twins was estimated in the model at .50, the same as the correlation for same-sex nonidentical twins, which implies no sex limitation in facial masculinity (i.e., a perfect genetic correlation, r = 1.0, between male and female facial masculinity).
One of our main goals was to determine the degree to which genes that affect masculinity in men have the same effect in women. The fact that facial masculinity scores were significantly correlated between opposite-sex twins and siblings (r = .23, p < .001; see Table 1) suggests that heritable factors that increase male facial masculinity also increase female facial masculinity. In fact, the correlation between opposite-sex twin and sibling pairs was of the same magnitude to that between same-sex nonidentical twin and sibling pairs, which suggests that the same genes influence male and female facial masculinity (accordingly, modeling showed a genetic correlation of 1.0, p = .02, between the sexes (see Table 2). Masculine female faces were rated as less attractive than feminine female faces by observers (r = −.17, p < .001). This suggests that the heritable factors underlying male facial masculinity reduce female attractiveness. Accordingly, the correlation between brother masculinity and sister attractiveness was negative, r = −.13, p = .03; that is, sisters of more facially masculine men were rated as less facially attractive. Therefore, any genetic benefits to male offspring associated with choosing a facially masculine partner would be offset by reduced attractiveness of female offspring. In contrast, and unsurprisingly, there was no association between sisters’ facial masculinity and their brothers’ facial attractiveness (r = −.02, p = .72).
Furthermore, male facial masculinity was not associated with rated attractiveness (r = .01, p = .84), which calls into question the sexy-sons hypothesis, according to which male facial masculinity is preferred for heritable attractiveness.
Discussion
Despite the large proportion of variation in facial masculinity scores that we estimated to be due to additive genetic influences (.49), our other findings do not support the widely held idea that male facial masculinity is a signal for heritable genetic benefits, for two reasons. First, there was no association between male facial masculinity and rated attractiveness, contrary to the sexy-sons explanation of facial sexual dimorphism. This is by far the largest sample that has been used to assess how natural variation in objective facial masculinity affects individuals’ attractiveness, and the finding accords with previous mixed findings regarding whether male facial masculinity is attractive, unattractive, or neutral (DeBruine, Jones, Smith, & Little, 2010; Perrett et al., 1998; Rhodes, 2006; Scott et al., 2012).
Second, we found that the same genetic factors increased men’s and women’s facial masculinity scores. Combined with the negative association between female facial masculinity and attractiveness, this suggests that the genetic factors increasing male facial masculinity decrease facial attractiveness in female relatives. Accordingly, men who had more masculine faces had sisters with less attractive faces. A sister shares the same proportion (.50) of segregating genes as a daughter, so choosing a facially masculine man as a mate will tend to decrease the attractiveness of resulting daughters. It is possible that yet-to-be-established genetic benefits to sons outweigh these genetic detriments to daughters. However, any such genetic benefits would need to outweigh not only the detriment of masculinity to female facial attractiveness found here, but also perhaps apparent detriments to female fertility (Pfluger, Oberzaucher, Katina, Holzleitner, & Grammer, 2012) and health (Thornhill & Gangestad, 2006).
The existence of facial sexual dimorphism suggests that there have been different selection pressures on male and female facial shape, and that masculine male faces have (or had) a selective advantage of some kind. Our results are difficult to reconcile with the notion that the selective advantage of masculine male faces comes from women’s preference for facially masculine men as a means of obtaining genetic benefits for offspring, but our results do not preclude this type of explanation. For example, it is possible that masculine faces, although not judged as being more attractive by raters overall, are judged as more attractive by women in certain contexts or populations, or by women who are ovulating. Another alternative is that female choice acts not on facial masculinity per se but on correlated traits such as body muscularity or assertive behavioral tendencies.
Moreover, the advantages of male facial masculinity may stem from enhanced fitness from factors that are unrelated to female choice. For example, facially masculine men might gain a survival or reproductive advantage through intrasexual competition by being more robust to physical damage or by signaling formidability and dominance to male competitors (Puts, 2010). In contrast to male facial masculinity, female facial femininity (i.e., low masculinity) is both heritable and associated with attractiveness. Moreover, it is not associated with brothers’ facial attractiveness, so a man who chooses a feminine mate would increase the attractiveness of his daughters with no detriment to his sons’ attractiveness (although there could be disadvantages in terms of body morphology or behavioral assertiveness—the corollary of the caveats already noted). Unlike masculine male faces, feminine female faces are robustly preferred across studies and have been shown to be even more strongly preferred after exposure to pathogen cues and by men with high levels of pathogen sensitivity (Lee et al., 2013; Little, DeBruine, et al., 2011), which perhaps suggests a pathogen-related advantage of feminine faces. All this warrants more research into males’ choice of facially feminine women and the possible direct or indirect (genetic) benefits to offspring.
A potential limitation of our study is that the facial photographs of twins were taken when they were 16 years old, at which time facial masculinity might not yet have fully developed. However, the following observations suggest that the findings would probably hold in an older sample: First, facial dimensions are more than 94% of their adult sizes by age 16 in both men and women (Edwards et al., 2007). Second, there was no mean effect of age on the facial masculinity measure in our study, including among the older siblings. Third, the facial masculinity measure derived from the 16-year-old twins discriminated the sexes equally well in the 18-year-old siblings. Finally, correlations between the twins and older siblings showed the same pattern as correlations within the twins.
Other limitations of our study include standard caveats of the classical twin design.in particular, our biometrical modeling could have overestimated additive genetic effects and underestimated shared environmental and nonadditive genetic effects, because the latter two effects are negatively confounded when they are estimated using only twins (Keller & Coventry, 2005; Keller, Medland, & Duncan, 2010). Future research could overcome this problem by including other members of twins’ families, especially parents.
Assuming that our results are generalizable, how might we explain the findings in light of the aforementioned research showing greater preference for masculine faces in, for example, contexts of disease threat (DeBruine, Jones, Crawford, et al., 2010; Little, DeBruine, et al., 2011)? It has recently been suggested that male facial masculinity may signal direct benefits (Scott et al., 2012) rather than indirect (genetic) benefits. For example, partners that possess markers of good health due to immunocompetence may be preferred because they are less likely to succumb to disease, which would decrease their resource-provisioning ability and increase the likelihood that they will transfer disease to their partners or mutual offspring (Tybur & Gangestad, 2011). Other authors have suggested that male facial masculinity may be a signal for the ability to compete intrasexually for resources or mates (Little, DeBruine, & Jones, 2012; Puts, 2010; Scott et al., 2012). How these various explanations might be distinguished has not been fully resolved (Gangestad & Eaton, 2013; Little, 2013), but the findings reported here call into question the predominant theoretical framework that explains preferences for males with masculine face shape in terms of genetic benefits for offspring.
Supplementary Material
Acknowledgments
We thank our twin sample for their participation; Ann Eldridge, Marlene Grace, Kerrie McAloney, Daniel Park, Maura Caffrey, and Jacob McAloney for photograph collection and processing; David Smyth for information technology support; and Bill von Hippel and Patrik Jern for helpful comments on an earlier draft.
Funding
We acknowledge support from the Australian Research Council (Grants A7960034, A79906588, A79801419, DP0212016, DP0343921, DP0664638, DP1093900, and FT0991360) and the Australian National Health and Medical Research Council (Grants 900536, 930223, 950998, 981339, 983002, 961061, 983002, 241944, 389875, 552485, and 613608). A. J. Lee is supported by an Australian Postgraduate Award, and B. P. Zietsch is supported by a Discovery Early Career Research Award, both from the Australian Research Council.
Footnotes
Author Contributions
The study concept was developed by A. J. Lee, B. P. Zietsch, and M. C. Keller. Data collection was executed by N. G. Martin, M. J. Wright, D. G. Mitchem, and M. C. Keller. A. J. Lee and B. P. Zietsch performed the data analysis and interpretation. A. J. Lee and B. P. Zietsch drafted the manuscript, with critical revisions provided by all other authors. All authors approved the final version of the manuscript for submission.
Declaration of Conflicting Interests
The authors declared that they had no conflicts of interest with respect to their authorship or the publication of this article.
Supplemental Material
Additional supporting information may be found at http://pss.sagepub.com/content/by/supplemental-data
References
- Bonduriansky R, Chenoweth SF. Intralocus sexual conflict. Trends in Ecology & Evolution. 2009;24:280–288. doi: 10.1016/j.tree.2008.12.005. [DOI] [PubMed] [Google Scholar]
- Bookstein FL. Morphometric tools for landmark data. Cambridge, England: Cambridge University Press; 1991. [Google Scholar]
- Cornwell RE, Perrett DI. Sexy sons and sexy daughters: The influence of parents’ facial characteristics on offspring. Animal Behaviour. 2008;76:1843–1853. [Google Scholar]
- DeBruine LM, Jones BC, Crawford JR, Welling LLM, Little AC. The health of a nation predicts their mate preferences: Cross-cultural variation in women’s preferences for masculinized male faces. Proceedings of the Royal Society B: Biological Sciences. 2010;277:2405–2410. doi: 10.1098/rspb.2009.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeBruine LM, Jones BC, Smith FG, Little AC. Are attractive men’s faces masculine or feminine? The importance of controlling confounds in face stimuli. Journal of Experimental Psychology: Human Perception and Performance. 2010;36:751–758. doi: 10.1037/a0016457. [DOI] [PubMed] [Google Scholar]
- Edwards CB, Marshall SD, Qian F, Southard KA, Franciscus RG, Southard TE. Longitudinal study of facial skeletal growth completion in 3 dimensions. American Journal of Orthodontics & Dentofacial Orthopedics. 2007;132:762–768. doi: 10.1016/j.ajodo.2006.01.038. [DOI] [PubMed] [Google Scholar]
- Fisher RA. The evolution of sexual preference. Eugenics Review. 1915;7:184–192. [PMC free article] [PubMed] [Google Scholar]
- Folstad I, Karter AJ. Parasites, bright males, and the immunocompetence handicap. American Naturalist. 1992;139:602–622. [Google Scholar]
- Gangestad SW, Eaton MA. Toward an integrative perspective on sexual selection and men’s masculinity. Behavioral Ecology. 2013;24:594–595. [Google Scholar]
- Gangestad SW, Scheyd GJ. The evolution of human physical attractiveness. Annual Review of Anthropology. 2005;34:523–548. [Google Scholar]
- Gangestad SW, Simpson JA. The evolution of human mating: Trade-offs and strategic pluralism [Target article plus commentaries] Behavioral & Brain Sciences. 2000;23:573–644. doi: 10.1017/s0140525x0000337x. [DOI] [PubMed] [Google Scholar]
- Gangestad SW, Thornhill R, Garver-Apgar CE. Men’s facial masculinity predicts changes in their female partners’ sexual interests across the ovulatory cycle, whereas men’s intelligence does not. Evolution & Human Behavior. 2010;31:412–424. [Google Scholar]
- Garver-Apgar CE, Eaton MA, Tybur JM, Thompson ME. Evidence of intralocus sexual conflict: Physically and hormonally masculine individuals have more attractive brothers relative to sisters. Evolution & Human Behavior. 2011;32:423–432. [Google Scholar]
- Huk T, Winkel WG. Testing the sexy son hypothesis—a research framework for empirical approaches. Behavioral Ecology. 2008;19:456–461. [Google Scholar]
- Keller MC, Coventry WL. Quantifying and addressing parameter indeterminacy in the classical twin design. Twin Research and Human Genetics. 2005;8:201–213. doi: 10.1375/1832427054253068. [DOI] [PubMed] [Google Scholar]
- Keller MC, Medland SE, Duncan LE. Are extended twin family designs worth the trouble? A comparison of the bias, precision, and accuracy of parameters estimated in four twin family models. Behavior Genetics. 2010;40:377–393. doi: 10.1007/s10519-009-9320-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AJ, Dubbs SL, Kelly AJ, von Hippel W, Brooks RC, Zietsch BP. Human facial attributes, but not perceived intelligence, are used as cues of health and resource provision potential. Behavioral Ecology. 2013;24:779–787. [Google Scholar]
- Little AC. Multiple motives in women’s preferences for masculine male faces: Comment on Scott et al. Behavioral Ecology. 2013;24:590–591. [Google Scholar]
- Little AC, Burt DM, Penton-Voak IS, Perrett DI. Self-perceived attractiveness influences human female preferences for sexual dimorphism and symmetry in male faces. Proceedings of the Royal Society B: Biological Sciences. 2001;268:39–44. doi: 10.1098/rspb.2000.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little AC, DeBruine LM, Jones BC. Exposure to visual cues of pathogen contagion changes preferences for masculinity and symmetry in opposite-sex faces. Proceedings of the Royal Society B: Biological Sciences. 2011;278:2032–2039. doi: 10.1098/rspb.2010.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little AC, DeBruine LM, Jones BC. Environment contingent preferences: Exposure to visual cues of direct male-male competition and wealth increase women’s preferences for masculinity in male faces. Evolution & Human Behavior. 2012;34:193–200. [Google Scholar]
- Little AC, Jones BC, DeBruine LM. Facial attractiveness: Evolutionary based research. Philosophical Transactions of the Royal Society B: Biological Sciences. 2011;366:1638–1659. doi: 10.1098/rstb.2010.0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little AC, Jones BC, Penton-Voak IS, Burt DM, Perrett DI. Partnership status and the temporal context of relationships influence human female preferences for sexual dimorphism in male face shape. Proceedings of the Royal Society B: Biological Sciences. 2002;269:1095–1100. doi: 10.1098/rspb.2002.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchem DG, Purkey AM, Grebe NM, Carey G, Garver-Apgar CE, Bates TC, Keller MC. Estimating the sex-specific effects of genes on facial attractiveness and sexual dimorphism. Behavior Genetics. 2013 doi: 10.1007/s10519-013-9627-5. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Mx: Statistical modeling. Richmond: Virginia Commonwealth University; 2006. [Google Scholar]
- Neale MC, Cardon LR. Methodology for genetic studies of twins and families. Boston, MA: Kluwer; 1992. [Google Scholar]
- Penton-Voak IS, Chen JY. High salivary testosterone is linked to masculine male facial appearance in humans. Evolution & Human Behavior. 2004;25:229–241. [Google Scholar]
- Penton-Voak IS, Perrett DI, Castles DL, Kobayashi T, Burt DM, Murray LK, Minamisawa R. Menstrual cycle alters face preference. Nature. 1999;399:741–742. doi: 10.1038/21557. [DOI] [PubMed] [Google Scholar]
- Perrett DI, Lee KJ, Penton-Voak I, Rowland D, Yoshikawa S, Burt DM, Akamatsu S. Effects of sexual dimorphism on facial attractiveness. Nature. 1998;394:884–887. doi: 10.1038/29772. [DOI] [PubMed] [Google Scholar]
- Pfluger LS, Oberzaucher E, Katina S, Holzleitner IJ, Grammer K. Cues to fertility: Perceived attractiveness and facial shape predict reproductive success. Evolution & Human Behavior. 2012;33:708–714. [Google Scholar]
- Posthuma D, Beem AL, de Geus EJC, van Baal GCM, von Hjelmborg JB, Lachine I, Boomsma DI. Theory and practice in quantitative genetics. Twin Research. 2003;6:361–376. doi: 10.1375/136905203770326367. [DOI] [PubMed] [Google Scholar]
- Puts DA. Beauty and the beast: Mechanisms of sexual selection in humans. Evolution & Human Behavior. 2010;31:157–175. [Google Scholar]
- Rantala MJ, Moore FR, Skrinda I, Krama T, Kivleniece I, Kecko S, Krams I. Evidence for the stress-linked immunocompetence handicap hypothesis in humans. Nature Communications. 2012;3 doi: 10.1038/ncomms1696. Article 694. Retrieved from: http://www.nature.com/ncomms/journal/v3/n2/full/ncomms1696.html. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes G. The evolutionary psychology of facial beauty. Annual Review of Psychology. 2006;57:199–226. doi: 10.1146/annurev.psych.57.102904.190208. [DOI] [PubMed] [Google Scholar]
- Rhodes G, Chan J, Zebrowitz LA, Simmons LW. Does sexual dimorphism in human faces signal health? Proceedings of the Royal Society B: Biological Sciences. 2003;270:S93–S95. doi: 10.1098/rsbl.2003.0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts SC, Little AC. Good genes, complementary genes and human mate preferences. Genetica. 2008;132:309–321. doi: 10.1007/s10709-007-9174-1. [DOI] [PubMed] [Google Scholar]
- Scott IML, Clark AP, Boothroyd LG, Penton-Voak IS. Do men’s faces really signal heritable immunocompetence? Behavioral Ecology. 2012;24:579–589. doi: 10.1093/beheco/ars092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott IML, Pound N, Stephen ID, Clark AP, Penton-Voak IS. Does masculinity matter? The contribution of masculine face shape to male attractiveness in humans. PLoS ONE. 2010;5(10):e13585. doi: 10.1371/journal.pone.0013585. Retrieved from http://www.plosone.org/article/info:doi/10.1371/journal.pone.0013585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornhill R, Gangestad SW. Facial sexual dimorphism, developmental stability, and susceptibility to disease in men and women. Evolution & Human Behavior. 2006;27:131–144. [Google Scholar]
- Tybur JM, Gangestad SW. Mate preferences and infectious disease: Theoretical considerations and evidence in humans. Philosophical Transactions of the Royal Society B: Biological Sciences. 2011;366:3375–3388. doi: 10.1098/rstb.2011.0136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welling LLM, Jones BC, DeBruine LM. Sex drive is positively associated with women’s preferences for sexual dimorphism in men’s and women’s faces. Personality and Individual Differences. 2008;44:161–170. [Google Scholar]
- Wright MJ, Martin NG. Brisbane adolescent twin study: Outline of study methods and research projects. Australian Journal of Psychology. 2004;56:65–78. [Google Scholar]
- Zahavi A. Mate selection—a selection for a handicap. Journal of Theoretical Biology. 1975;53:205–214. doi: 10.1016/0022-5193(75)90111-3. [DOI] [PubMed] [Google Scholar]
- Zelditch ML, Swiderski DL, Sheets HD, Fink WL. Geometric morphometrics for biologists: A primer. New York, NY: Elsevier Academic Press; 2004. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.