Abstract
Objectives
To assess the role of dopamine agonist therapy and deep brain stimulation on reflection impulsivity in non-demented patients with Parkinson’s disease (PD).
Methods
We recruited 61 PD patients, 20 treated with L-dopa in combination with a dopamine agonist, 14 taking L-dopa monotherapy, a further 16 PD patients with bilateral subthalamic nucleus deep brain stimulation treated with L-dopa in combination with a dopamine agonist, and 11 PD patients with bilateral subthalamic nucleus deep brain stimulation taking L-dopa but not a dopamine agonist. Results were compared with 18 healthy controls. Patients who had evidence of impulsive compulsive behaviour were excluded.
Reflection impulsivity was assessed with the beads task, which is a validated information sampling task.
Results
All patients treated with a dopamine agonist gathered significantly less information and made more irrational decisions than all other groups regardless of whether they had surgical treatment.
Conclusions
Our results imply that dopamine agonist therapy but not deep brain stimulation lead to “reflection impulsivity“ in PD.
Keywords: Parkinson’s disease [165], deep brain stimulation [292], neuropsychology [199]
Introduction
Although L-dopa remains the most efficacious drug in Parkinson’s disease (PD), younger patients are often first treated with dopamine agonists (DA). Increased awareness of an association between DA and impulsive compulsive behaviours (ICBs) has led to a reconsideration of this approach.[1] In a subgroup of advanced PD patients, sufficient motor control cannot be achieved with oral anti-Parkinson medication, and deep brain stimulation (DBS) of the subthalamic nucleus (STN) may be required.[2] The effect of DBS on pre-existing ICBs is still not clear with some studies reporting amelioration[3-5] and others deterioration of ICBs following DBS.[6-8] Similarly neuropsychological tests done in PD patients with DBS showed impairment in decision making with increase impulsive choice[9] and loss chasing behaviour[10] in some studies, whilst others found an improvement in learning behaviour.[11] In this study we have assessed the role of dopamine agonists and the role of deep brain stimulation in “reflection impulsivity” by using the beads task, which is a validated information sampling task.[12] Reflection impulsivity is different to ‘motor’ impulsivity, the inability to stop a movement and to ‘waiting’ impulsivity, the inability to delay an action.[13] Poor information sampling on the beads task has been also reported in patients with schizophrenia.[14–15] We have previously reported early decision or ‘jumping to conclusions’ in PD patients with and without ICBs on this task and found that PD patients with ICBs resembled substance abusers, whereas PD patients without an ICB performed similarly to pathological gamblers.[16] Thus this task has a high sensitivity to detect underlying impulsivity and has been therefore chosen for this study.[16]
Given that PD patients taking DA but without ICBs performed as poorly as non PD pathological gamblers on this task, we speculated that DA might be responsible reflection impulsivity. Thus we predicted that PD patients on DA (PD+DA) would gather significantly less information and make more irrational choices than PD patient without DA treatment (PD-DA). We have also speculated that the PD-DA group would perform similarly to healthy volunteers, since DA but not L-dopa is the main risk factor for ICBs in PD. Further we also hypothesized that DBS alone would not impair decision making on the beads task but thought that those patients who had DBS and were taking a DA would perform worse than all other groups, given reports that ICBs settle only when DA are completely weaned off.[5]
Methods
Patients
All patients were recruited from the National Hospital for Neurology and Neurosurgery London, and fulfilled the Queen Square Brain Bank criteria for the diagnosis of PD.[17] We recruited in total 61 PD patients. Twenty PD patients were taking L-dopa in combination with a DA (PD+DA) and 14 were on L-dopa therapy but were never treated with a DA or had been off the DA for at least 14 months (PD-DA). Twenty seven PD patients had undergone bilateral STN DBS, 16 were taking L-dopa in combination with a DA (DBS+DA), and 11 were on L-dopa without a DA (DBS-DA).
L-dopa equivalent units (LEU - Table 1) were calculated as described previously.[18] Results were compared with 18 healthy volunteers. Participants who scored under 26/30 points on the Mini-Mental state examination (MMSE) were excluded. Patients with a current or past history of an ICB assessed in a semi-structured interview using accepted diagnostic criteria for pathological gambling [19], compulsive shopping [20], compulsive sexual behaviour [21] and punding [18] were excluded. All patients were tested in their “on” state as we are interested in group trait effects not interactions between group and medication. Group trait effects on medication are the most clinically relevant, as this is the primary state the patients are in.
Table 1.
Demographic information on tested groups.
| Controls | PD-DA | PD+DA | DBS-DA | DBS+DA | t statistic χ 2 or F-statistic | p-value | |
|---|---|---|---|---|---|---|---|
| Participants(no.) | 18 | 14 | 20 | 11 | 16 | ||
| Gender (male) | 15 | 11 | 19 | 10 | 11 | χ 2=5.1 | 0.27 |
| Age (yrs) | 58.9±12.8 | 67.2±7.5 | 64.3±5.2 | 57.0±7.0 | 59.1±11.6 | F=3.0 | 0.023 |
| Age PD of diagnosis | 61.1±2.2 | 53.2±1.8 | 42.5±2.3 | 43.7±2.2 | F=15.9 | <0.001* | |
| PD Disease duration (yrs) | 6.2±3.8 | 11.1±7.0 | 14.4±5.0 | 15.6±6.0 | F=7.3 | <0.001* | |
| DBS (yrs) | 3.4±3.3 | 3.6±2.2 | t=0.2 | 0.8 | |||
| Education (yrs) | 13.6±3.2 | 14.8±3.1 | 14.5±2.5 | 13.9±2.8 | 13.7±2.8 | F=0.4 | 0.75 |
| LEU dose(mg/day) | 511±321 | 854.2±356 | 739.2±409 | 771.4±337 | F=2.6 | 0.058 | |
| DA dose (LEU) | 216.0±109 | 204.9±97.1 | t=0.3 | 0.7 | |||
| UPDRS on | 16.0±2.3 | 19.4±11.6 | 16.5±4.4 | 17.6±4.4 | F=0.5 | 0.6 | |
| DA duration (yrs) |
Standard protocol approvals and patient consents
All patients had attended the Specialist Movement Disorders Clinic at the National Hospital for Neurology and Neurosurgery, London, UK. Informed consent was obtained from all participants, and the study had local ethics committee approval.
Beads task
The beads task requires the subjects to guess from which of two cups beads are being drawn. One of the cups contains primarily green beads, with fewer blue beads, and one of the cups contains primarily blue beads with fewer green beads. For each sequence of bead draws, the computer program selects one of the cups, and begins presenting beads to the participant. Thus, the trial begins with the presentation of a single bead, either green or blue. After seeing the bead the subject is asked whether they want to guess which cup is being drawn from (either the predominantly blue cup, or the predominantly green cup) or whether they would like to see another bead before making their decision. If they decide to guess a cup, they are told whether or not they were correct. If they decide to draw another bead, they are shown another bead drawn from the same cup, and again presented with the choice of either guessing an cup, or drawing additional beads. They could draw up to 10 beads in each trial. There were 4 blocks of 3 trials each. In two of the blocks the bead ratios in the urns were 60/40 and in two of the blocks the beads ratios were 80/20. Further, in two of the blocks the participants lost 10 points for being wrong, and in two of the blocks they lost 0 points. They always won 10 points for being right, and they were charged 0.2 units for each additional bead drawn.
The examiner (AD) also brought 2 actual cups labeled with “green” and “blue” - the blue cup contained more blue than green beads and vice versa for the green cup. The researcher explained the task and performed a few draws to make sure that all participants understood the rules before the computerized test was started. The best strategy in the beads task is to draw more beads and gather more evidence before deciding. Further details can be found elsewhere. [16]
Statistics
For the behavioural variables we performed analyses using a generalized linear model (SPSS). Beads ratio (80/20 or 60/40) and loss condition (loss, no loss), DA (yes/no), DBS (yes/no) were modeled as fixed factors and age was included as a covariate. Subject was a random factor nested under DBS and DA. Demographic variables (Table 1) were analysed using ANOVA, t-test or χ 2 tests where appropriate and as indicated.
Results
Demographic and clinical features
There were significant differences in age and age at onset across the 4 groups as shown in Table 1.
Beads task
The patient data, excluding controls, were analysed using a generalized linear model that included DBS (yes/no), DA (yes/no), beads ratio and loss as factors. For number of draws before making a decision there was a significant effect of DA (Wald χ2=11.4, p=0.001) and beads ratio (Wald χ2=34.8, p<0.001). There were no effects of loss condition (Wald χ2=0.06, p=0.797), DBS (Wald χ2=0.6, p=0.422) and no interaction of DBS and DA (Wald χ2=0.03, p=0.852) or beads ratio and loss condition (Wald χ2=1.2, p=0.259). Neither age (Wald χ2=1.51, p = 0.220) nor age of disease onset (Wald χ2=0.61, p = 0.434) were significant covariates. We also performed pairwise comparison between all groups including controls (Table 2, Figure 1A). PD+DA and DBS+DA both drew significantly less than controls, PD-DA and DBS-DA patients (all p<0.001).
Table 2.
Pair-wise comparisons between groups for number of draws. All p-values shown are uncorrected. Values equal or less than 0.01 (highlighted in bold) are significant. All p-values are for main effect of group.
| Group (χ2, p-value) | PD-DA | DBS+DA | DBS-DA | Controls |
|---|---|---|---|---|
|
| ||||
| PD+DA | ||||
| Draws | 101.6, p<0.001 | 1.19, p=0.275 | 84.6 p<0.001 | 87.3, p<0.001 |
| Opposite colour | 11.1, p=0.001 | 0.33, p=0.564 | 9.1, p=0.002 | 13.2, 0<0.001 |
|
| ||||
| PD-DA | ||||
| Draws | 71.5, p<0.001 | 2.1, p=0.142 | 0.3, p=0.561 | |
| Opposite colour | 12.5, p<0.001 | 0.145, p=0.703 | 0.64, p=0.421 | |
|
| ||||
| DBS+DA | ||||
| Draws | 75.3, p<0.001 | 63.6, p<0.001 | ||
| Opposite colour | 6.1, p=0.012 | 9.1, p=0.003 | ||
|
| ||||
| DBS-DA | ||||
| Draws | 5.1, p=0.023 | |||
| Opposite colour | 0.002, p=0.969 | |||
Figure 1.
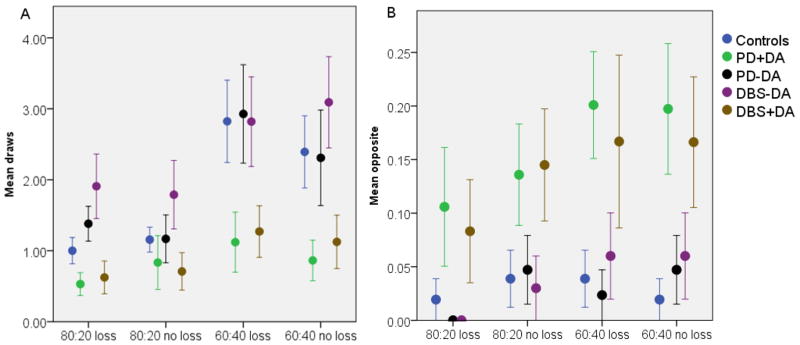
Figure 1A: Average drawing behaviour per condition of different groups (left). One bead is always shown before the participant must make a decision, so total beads seen are mean draws plus one. 1B: Number of times participants chose the opposite colour (right).
Next we examined the number of times participants picked the less likely cup given the information they had at the time of their choice, termed “irrational decisions” (Figure 1B). Comparing the patients groups we found a significant effect of DA (Wald χ2=13.8, p<0.001) and beads ratio (Wald χ2=4.3, p=0.039). Age (Wald χ2=0.01, p=0.917) and age of disease onset (Wald χ2=0.5, p=0.821) were modelled as covariates but they were not significant. In pair-wise comparisons (Table 2) PD+DA made irrational decisions more than PD-DA, DBS-DA and healthy controls (p 0.002). Similarly DBS+DA patients made significantly more irrational choices than DBS-DA (p=0.012) and PD-DA and controls (p< 0.003).There was no effect of loss condition (Wald χ2=3.3, p=0.068), DBS (Wald χ2=0.03, p=0.854) and no interaction of DBS and DA (Wald χ2=0.3, p=0.561). Finally, we found that there were no significant correlations between either the mean draws per trial (r = −0.106, p=0.422) or irrational choices and LEU dose (r=0.155, p=0.238).
Discussion
Impulsivity in general is referred to as “a behaviour that is performed with little or inadequate forethought”. [22] Here we assessed one aspect of cognitive impulsivity, termed “reflection impulsivity” to assess whether STN-DBS causes jumping to conclusion behaviour. Both DA medication[23] and DBS have been reported to increase impulsivity in PD[9], but the effect of STN-DBS and DA treatment on “reflection impulsivity” has not been studied yet.
This study directly compared PD patients with and without DA treatment and with and without STN-DBS on a test of reflection impulsivity. Patients treated with a DA gathered less evidence and made significantly more irrational choices than patients not treated with a DA. STN-DBS had no effect in this task. In addition, patients not taking a DA did not differ from controls in information sampling, whereas those that were treated with a DA performed significantly worse.
Previous studies have suggested that the most important factor for impulsive decision making is the total amount of dopaminergic medication including a combination of DA and L-dopa[24] and our prediction that DBS in combination with a DA would further increase reflection impulsivity was not confirmed. Reduction in ICBs after DBS has been observed in patients where it was possible to make substantial reductions in dopaminergic medication.[5–25] When dopaminergic reduction is not possible after DBS it has been suggested that electrode misplacement outside the STN may be one explanation as well as persistent dopamine dysregulation.[5]
In our study, total LEU doses were not significantly different between PD+DA and PD patients treated with DBS and yet only those treated with a DA made impulsive choices. Increased temporal discounting, the preference of a smaller immediate over a delayed but higher reward has been observed in PD patients without ICBs who were treated with a DA. Discounting in these patients was not affected by current medication state, raising the possibility that DA therapy may induce persistent long term behavioural changes.[26]
Our results expand on previous studies showing no impairment in decision making and risk taking in drug naïve PD patients[27] and suggest that L-dopa alone or in combination with STN-DBS does not cause increased reflection impulsivity. Whereas L-dopa alone or in combination with a MAO-inhibitor have been shown not to increase the risk of pathological gambling[28], DA have been a shown to change reward learning. Drug-naïve PD patients who had intact learning from negative feedback but impaired learning from positive feedback changed to having impaired learning from negative feedback but restored learning from positive feedback after 12 weeks of DA therapy.[29] In a previous study we demonstrated that PD patients without ICBs but on a DA were performing similarly to non-PD patients with pathological gambling.[16] This suggests that DA medication might trigger cognitive impulsivity in all PD patients, but intact cortical inhibition may prevent some patients from developing an ICB. It is however unclear whether a proportion of these patients will develop an ICB in the future.
It has been suggested that the STN acts as a “brake” on the cortico-striatal loop in high conflict situations to “buy more time” before making a decision.[9] In PD patients treated with DBS, impulsive choices have been reported to be increased.[30] We did not find an increase in impulsive choices in patients with DBS on the beads test. However in previous studies most PD patients were taking a DA in addition to DBS and acute effects of DBS were tested. Furthermore the main outcome measure in previous studies was reaction time[9–30], whereas in our study the focus was number of draws.
To our best knowledge no behavioural test in STN-DBS patients with an active ICB has been carried out so far. We excluded patients with ICBs from this study as reflection impulsivity may persist long after the behavioural addiction has stopped and therefore might have confounded our results.[31] Thus we cannot exclude an interaction between STN-DBS and DA in patients with ICBs and our results should therefore be interpreted with care.
The STN may also be more involved with decisions made under time pressure. Activation of the anterior cingulate and the ventral striatum but not STN occurs in normal volunteers on the beads task.[12] DA medication may cause a reduction in “top down” cortical control of the basal ganglia. An imaging study in volunteers showed that pramipexole increased activity of the mesolimbic dopaminergic neurons during anticipation of monetary rewards and simultaneously reduced prefrontal cortex activity.[32]
We acknowledge that the sample size is small in our study. However our results are robust and therefore we do not think that the data would significantly change if a larger cohort would have been tested.
In summary our results indicate that neither STN-DBS nor L-dopa monotherapy increases impulsive choices in the context of information sampling in a cohort of PD patients who have never had an ICB. Our data suggests that STN-DBS in combination with L-dopa therapy may be considered as a potential treatment for PD patients with ICBs. Future studies comparing patients who had pallidal DBS versus STN-DBS on decision making tasks would be of considerable interest.
Acknowledgments
This study was funded by the Reta Lila Weston Institute for Neurological Studies
Glossary
- DA
dopamine agonists
- DBS
deep brain stimulation
- ICBs
impulsive compulsive behaviours
- LEU
L-dopa equivalent units
- MMSE
mini mental state examination
- PD
Parkinson’s disease
- STN
subthalamic nucleus
Footnotes
Atbin Djamshidian: Concept, design, statistical analysis and interpretation of data, drafting manuscript
Sean S. O’Sullivan: Concept, design, interpretation of data, revising manuscript
Thomas Foltynie: Acquisition of data, interpretation of data, revising manuscript
Iciar Aviles-Olmos: Acquisition of data, interpretation of data, revising manuscript
Patricia Limousin: Acquisition of data, interpretation of data, revising manuscript
Alastair Noyce: Acquisition of data, interpretation of data, revising manuscript
Ludvic Zrinzo: Acquisition of data, interpretation of data, revising manuscript
Andrew J. Lees: Study supervision, concept, design, interpretation of data, revising manuscript
Bruno B. Averbeck: Study supervision, concept, design, statistical analysis and interpretation of data, revising manuscript
Disclosure Statement
Atbin Djamshidian received funding from TEVA Pharmaceuticals to attend the Movement disorders congress in Dublin 2012.
Sean O’Sullivan received honoraria from Britannia Pharmaceuticals, Teva, Lundbeck.
Thomas Foltynie received honoraria from Abbott, St Jude Medical, Data Monitoring Committee for Oxford Biomedical and grants from Parkinson’s UK, Cure Parkinson’s Trust and European Union FP7.
Iciar Aviles-Olmos reports no disclosures.
Patricia Limousin has received honoraria from industry (Medtronic and St Jude Medical) and for invited talks.
Alastair Noyce received funding and travel support from the National Institute of Health Research and grants from the Parkinson’s UK, National Institute of Health Research; consultancy: Elan Pharmaceuticals.
Ludvic Zrinzo has received honoraria from industry (Medtronic and St Jude Medical) and for invited talks.
Andrew Lees is a consultant for Genus, received honoraria from Novartis, Teva, Meda, Boehringer Ingelheim, GSK, Ipsen, Lundbeck, Allergan, Orion, grants from the PSP Association, Weston Trust – The Reta Lila Howard Foundation.
Bruno Averbeck received grants from Wellcome, and the Intramural research program of the NIH.
References
- 1.Vlaar A, Hovestadt A, van Laar T, Bloem BR. The treatment of early Parkinson’s disease: levodopa rehabilitated. Pract Neurol. 2011;11(3):145–52. doi: 10.1136/practneurol-2011-000011. practneurol-2011-000011 [pii] [DOI] [PubMed] [Google Scholar]
- 2.Foltynie T, Zrinzo L, Martinez-Torres I, et al. MRI-guided STN DBS in Parkinson’s disease without microelectrode recording: efficacy and safety. J Neurol Neurosurg Psychiatry. 2010;82(4):358–63. doi: 10.1136/jnnp.2010.205542. jnnp.2010.205542 [pii] [DOI] [PubMed] [Google Scholar]
- 3.Ardouin C, Voon V, Worbe Y, et al. Pathological gambling in Parkinson’s disease improves on chronic subthalamic nucleus stimulation. Mov Disord. 2006;21(11):1941–6. doi: 10.1002/mds.21098. [DOI] [PubMed] [Google Scholar]
- 4.Bandini F, Primavera A, Pizzorno M, Cocito L. Using STN DBS and medication reduction as a strategy to treat pathological gambling in Parkinson’s disease. Parkinsonism Relat Disord. 2007;13(6):369–71. doi: 10.1016/j.parkreldis.2006.07.011. S1353-8020(06)00162-3 [pii] [DOI] [PubMed] [Google Scholar]
- 5.Lhommee E, Klinger H, Thobois S, et al. Subthalamic stimulation in Parkinson’s disease: restoring the balance of motivated behaviours. Brain. 2012;135(Pt 5):1463–77. doi: 10.1093/brain/aws078. aws078 [pii] [DOI] [PubMed] [Google Scholar]
- 6.Zahodne LB, Susatia F, Bowers D, et al. Binge eating in Parkinson’s disease: prevalence, correlates and the contribution of deep brain stimulation. J Neuropsychiatry Clin Neurosci. 2011;23(1):56–62. doi: 10.1176/appi.neuropsych.23.1.56. 23/1/56 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halbig TD, Tse W, Frisina PG, et al. Subthalamic deep brain stimulation and impulse control in Parkinson’s disease. Eur J Neurol. 2009;16(4):493–7. doi: 10.1111/j.1468-1331.2008.02509.x. ENE2509 [pii] [DOI] [PubMed] [Google Scholar]
- 8.Moum SJ, Price CC, Limotai N, et al. Effects of STN and GPi deep brain stimulation on impulse control disorders and dopamine dysregulation syndrome. PLoS One. 2012;7(1):e29768. doi: 10.1371/journal.pone.0029768. PONE-D-11-15508 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frank MJ, Samanta J, Moustafa AA, Sherman SJ. Hold your horses: impulsivity, deep brain stimulation, and medication in parkinsonism. Science. 2007;318(5854):1309–12. doi: 10.1126/science.1146157. 1146157 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Rogers RD, Wielenberg B, Wojtecki L, Elben S, Campbell-Meiklejohn D, Schnitzler A. Deep brain stimulation of the subthalamic nucleus transiently enhances loss-chasing behaviour in patients with Parkinson’s disease. Exp Neurol. 2011;231(1):181–9. doi: 10.1016/j.expneurol.2011.06.007. S0014–4886(11)00229-9 [pii] [DOI] [PubMed] [Google Scholar]
- 11.van Wouwe NC, Ridderinkhof KR, van den Wildenberg WP, et al. Deep brain stimulation of the subthalamic nucleus improves reward-based decision-learning in Parkinson’s disease. Front Hum Neurosci. 2011;5:30. doi: 10.3389/fnhum.2011.00030. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Furl N, Averbeck BB. Parietal cortex and insula relate to evidence seeking relevant to reward–related decisions. J Neurosci. 2011;31(48):17572–82. doi: 10.1523/JNEUROSCI.4236-11.2011. 31/48/17572 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69(4):680–94. doi: 10.1016/j.neuron.2011.01.020. S0896-6273(11)00068-7 [pii] [DOI] [PubMed] [Google Scholar]
- 14.Averbeck BB, Evans S, Chouhan V, Bristow E, Shergill SS. Probabilistic learning and inference in schizophrenia. Schizophr Res. 2011;127(1–3):115–22. doi: 10.1016/j.schres.2010.08.009. S0920-9964(10)01455-6 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garety PA, Freeman D. Cognitive approaches to delusions: a critical review of theories and evidence. Br J Clin Psychol. 1999;38 (Pt 2):113–54. doi: 10.1348/014466599162700. [DOI] [PubMed] [Google Scholar]
- 16.Djamshidian A, O’Sullivan SS, Sanotsky Y, et al. Decision making, impulsivity, and addictions: do Parkinson’s disease patients jump to conclusions? Mov Disord. 2012;27(9):1137–45. doi: 10.1002/mds.25105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1988;51(6):745–52. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans AH, Katzenschlager R, Paviour D, et al. Punding in Parkinson’s disease: its relation to the dopamine dysregulation syndrome. Mov Disord. 2004;19(4):397–405. doi: 10.1002/mds.20045. [DOI] [PubMed] [Google Scholar]
- 19.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4. Washington, DC: 2000. text rev. [Google Scholar]
- 20.McElroy SL, Keck PE, Jr, Pope HG, Jr, Smith JM, Strakowski SM. Compulsive buying: a report of 20 cases. J Clin Psychiatry. 1994;55(6):242–8. [PubMed] [Google Scholar]
- 21.Voon V, Hassan K, Zurowski M, et al. Prevalence of repetitive and reward-seeking behaviors in Parkinson disease. Neurology. 2006;67(7):1254–7. doi: 10.1212/01.wnl.0000238503.20816.13. 01.wnl.0000238503.20816.13 [pii] [DOI] [PubMed] [Google Scholar]
- 22.Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999;146(4):348–61. doi: 10.1007/pl00005481. 91460348.213 [pii] [DOI] [PubMed] [Google Scholar]
- 23.Weintraub D, Siderowf AD, Potenza MN, et al. Association of dopamine agonist use with impulse control disorders in Parkinson disease. Arch Neurol. 2006;63(7):969–73. doi: 10.1001/archneur.63.7.969. 63/7/969 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lule D, Heimrath J, Pinkhardt EH, Ludolph AC, Uttner I, Kassubek J. Deep brain stimulation and behavioural changes: is comedication the most important factor? Neurodegener Dis. 2012;9(1):18–24. doi: 10.1159/000328817. 000328817 [pii] [DOI] [PubMed] [Google Scholar]
- 25.Demetriades P, Rickards H, Cavanna AE. Impulse control disorders following deep brain stimulation of the subthalamic nucleus in Parkinson’s disease: clinical aspects. Parkinsons Dis. 2011;2011:658415. doi: 10.4061/2011/658415. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Milenkova M, Mohammadi B, Kollewe K, et al. Intertemporal choice in Parkinson’s disease. Mov Disord. 2011;26(11):2004–10. doi: 10.1002/mds.23756. [DOI] [PubMed] [Google Scholar]
- 27.Poletti M, Frosini D, Lucetti C, Del Dotto P, Ceravolo R, Bonuccelli U. Decision making in de novo Parkinson’s disease. Mov Disord. 2010;25(10):1432–6. doi: 10.1002/mds.23098. [DOI] [PubMed] [Google Scholar]
- 28.Djamshidian A, Cardoso F, Grosset D, Bowden-Jones H, Lees AJ. Pathological gambling in Parkinson’s disease--a review of the literature. Mov Disord. 2011;26(11):1976–84. doi: 10.1002/mds.23821. [DOI] [PubMed] [Google Scholar]
- 29.Bodi N, Keri S, Nagy H, et al. Reward-learning and the novelty-seeking personality: a between- and within-subjects study of the effects of dopamine agonists on young Parkinson’s patients. Brain. 2009;132(Pt 9):2385–95. doi: 10.1093/brain/awp094. awp094 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cavanagh JF, Wiecki TV, Cohen MX, et al. Subthalamic nucleus stimulation reverses mediofrontal influence over decision threshold. Nat Neurosci. 2011;14(11):1462–7. doi: 10.1038/nn.2925. nn.2925 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clark L, Robbins TW, Ersche KD, Sahakian BJ. Reflection impulsivity in current and former substance users. Biol Psychiatry. 2006;60(5):515–22. doi: 10.1016/j.biopsych.2005.11.007. S0006-3223(05)01397-1 [pii] [DOI] [PubMed] [Google Scholar]
- 32.Ye Z, Hammer A, Camara E, Munte TF. Pramipexole modulates the neural network of reward anticipation. Hum Brain Mapp. 2010 doi: 10.1002/hbm.21067. published Online First: Epub Date. [DOI] [PMC free article] [PubMed] [Google Scholar]


