Abstract
Introduction
The mechanism of the formation of apical cyst has been elusive. Several theories have long been proposed and discussed speculating how an apical cyst is developed and formed in the jaw bone resulting from endododontic infection. Two popular theories are the nutritional deficiency theory and the abscess theory. The nutritional deficiency theory assumes that the over proliferated epithelial cells will form a ball mass such that the cells in the center of the mass will be deprived of nutrition. The abscess theory postulates that when an abscess cavity is formed in connective tissue, epithelial cells proliferate and line the preexisting cavity because of their inherent tendency to cover exposed connective tissue surfaces. Based on the nature of epithelial cells and the epithelium, nutritional theory is a fairy tale, while abscess theory at best just indicates that abscess may be one of the factors that allows the stratified epithelium to form but not to explain a mechanism that makes the cyst to form.
The hypothesis
Apical cyst formation is the result of proliferation of resting epithelial cells, due to inflammation, to a sufficient number such that they are able to form a polarized and stratified epithelial lining against dead tissues or foreign materials. These stratified epithelial lining expands along the dead tissue or foreign materials and eventually wrap around them as a spherical sac, i.e. a cyst. The space in the sac is considered the external environment separating the internal (tissue) environment – the natural function of epithelium.
Evaluation of the hypothesis
This theory may be tested by introducing a biodegradable device able to slowly release epithelial cell mitogens in an in vivo environment implanted with epithelial cells next to a foreign object. This will allow the cells to continuously proliferate which may form a cystic sac wrapping around the foreign object.
Keywords: Apical cyst, Endodontic infection, Epithelium, Embryonic stem cells, Stem cells, Induced pluripotent stem (iPS) cells, Teratoma, Neoplastic, Abscess
Introduction
It has been a mystery as to how the apical cyst is formed resulting from endododontic infection. Several theories have long been proposed. The nutritional deficiency theory and the abscess theory are two popular theories and both lack a strong basis from the perspective of epithelial cell biology.
The nutritional deficiency theory assumes that the over proliferated epithelial cells will form a ball mass such that the cells in the center will be deprived of nutrition. It is unlikely that proliferating epithelial cells will continue to form a ball mass such that the inner cells cannot obtain nutrition. Oral epithelium may reach a thickness of 500 μm [1] and the cells in the outer layers rely on the diffusion of nutrition from the basement membrane. If the epithelial cells in the periodontal ligament begin proliferating in an environment filled with nutrition from all directions, it is likely that cells will always move toward nutritional source while proliferating instead of allowing themselves to be embedded in the center of the cell mass. Unless epithelial cells undergo malignant transformation, they will not form a ball mass.
The abscess theory postulates that when an abscess cavity is formed in connective tissue, epithelial cells proliferate and line the preexisting cavity because of their inherent tendency to cover exposed connective tissue surfaces. Nair et al recently investigated the role of abscess in the formation of apical cysts [2]. Their finding supports the theory that the abscess appears to be a factor causing cyst formation. The effort made by the investigators is admirable and their findings provide a useful piece of information regarding the cause of apical cyst formation. The authors also discussed and summarized the theories of cyst formation. There are three phases of cyst formation; 1) dormant epithelial cell rests proliferate, 2) epithelium-lined cavity established and 3) the cyst grows. The mechanisms underlying the latter two phases are unknown. The authors also showed doubts on the two theories (numbers 1 and 3 below) summarized by an earlier article published by Lin et al [3]. In this article by Lin et al summarized three theories as to how the epithelium-lined apical cyst is formed:
Nutritional deficiency theory: As islands of epithelium expand, more central epithelial cells are distanced from their nutritional supply and undergo necrosis. A cystic cavity results in the center of the cell mass as liquefaction necrosis occurs.
Abscess theory: An abscess cavity is formed in the periapical connective tissues. Subsequently, the abscess is completely surrounded by epithelium because of the natural inclination of stratified squamous epithelium to line exposed connective tissue surfaces.
Merging of epithelial strands theory: As epithelial strands continue to grow, they merge to form a three-dimensional ball mass. When the connective tissue trapped inside the ball mass degenerates, a cyst is formed.
Lin et al considered that the nutritional deficiency theory is flawed as the epithelial strands in apical granulomas are frequently infiltrated by polymorphonuclear leukocytes, but cell necrosis is not often seen in the center of epithelial strands [3]. They also thought that the abscess theory has pitfalls as epithelial proliferation is more prominent in chronic apical periodontitis than apical abscesses.
The authors considered that apical cyst formation is a genetically programmed event [3].
If we understand the nature of epithelial cells and the epithelium, we know that nutritional theory is a fairy tale. While abscess appears to play a role in the formation of apical cysts, the abscess may just be one of the factors that allow the epithelium to form, i.e., the polarization and stratification of the epithelial cells, but is not a theory to explain a mechanism that makes the cyst to form.
The hypothesis
Knowing the nature of epithelial cell biology and the current understanding of epithelial cell proliferation in the apical granulomas, the present author hypothesizes that apical cyst formation is the result of proliferation of resting epithelial cells, due to inflammation, to a sufficient number such that they are able to form a polarized and stratified epithelial lining against dead tissues or foreign materials.
These stratified epithelial lining expands along the dead tissue or foreign materials and eventually wrap around them as a spherical sac, i.e. a cyst.
The space in the sac is considered the external environment separating the internal (tissue) environment – the nature of epithelium.
Evaluation of the hypothesis
Below the author describes the missing link of thoughts in speculating how apical cysts are formed.
1. The nature of cells
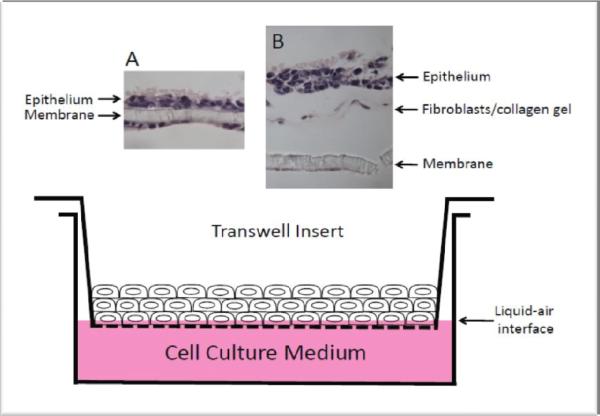
Before we discuss how cyst is formed, we should first review the nature of epithelial cells under normal circumstances. All epithelial cells in the epithelium are polarized. One side of the cells face the basement membrane and the other the luminal surface—the external environment. In fact, this is the main function of epithelium, i.e., separating the internal environment (tissue side) from the external environment. One can artificially induce the formation of stratified epithelial layer in an in vitro setting. When growing primary epithelial cells in the culture dish, the cells maintain a 2-D sheet of monolayer cells. These cells are normally heterogeneous in terms of their levels of differentiation. If growing these cells to confluence in the dish with a set-up that allows the cells to be exposed to the air, these monolayer cells will then become a 3-D stratified layer of epithelium. A common set-up of this type is called transwell system in which there is an insert-well placed into another regular well. The bottom of the insert-well is made of a layer of synthetic membrane with pores. Cells are grown in the insert-well on this membrane and the cell culture medium is added into the regular well that holds the insert-well (Figure 1). If one adds the amount of medium such that the upper surface of the cells is exposed to the air (liquid-air interface), the cells will become stratified. It appears that the exposure to the air provides a signal to the cells to become polarized. The air side is the external environment and the liquid side is the internal. After further proliferation, the cells begin to pile up forming a multilayer.
Figure. 1.
Transwell system. Monolayer epithelial cells are grown to confluence in the insert-well and the cell culture medium level is adjusted such that the monolayer is at the liquid-air interface, allowing the stratification of the cells to occur. (A) Monolayer of oral epithelial cells on insert-well membrane became stratified after grown in liquid-air interface. Some epithelial cells migrated below the membrane and stayed monolayer because being in the liquid phase. (B) Monolayer of oral epithelial cells seeded onto the engineered submucosal tissue (gingival fibroblasts cast in collagen gel on the insert-well membrane) became stratified after grown in liquid-air interface. (A, B) histological analysis with hematoxylin and eosin staining.
2. Cyst cavity formation
The ectoderm-derived epithelial cells form a layer of epithelium -- integumentary system that wraps the entire surface of the body without any breakage, except the erupted teeth which break this integrity. The endoderm-derived epithelial cells also form a layer of epithelium covering the surface of the gastrointestinal system which is mainly a tube or sac shape. Normally, no closed cystic space is formed in the body. Epithelium-lined cyst in the jaw bone is a relatively common form of anomaly of epithelial cell growth.
A type of cultured cell termed embryonic stem (ES) cell is derived from embryos at a stage called blastocyst which is a cystic structure containing an inner cell mass from which the cultured ES cells are derived. The trophoblasts surround the inner cell mass and a fluid-filled blastocyst cavity known as the blastocoele. This is perhaps the only true normal and closed cystic structure (non-glandular) ever occurred during the entire span of a mammalian life. The cavity formation of the blastocoele is part of embryo development and likely to involve cell apoptosis. Nutritional deprivation in the center of the cells has not been considered a cause of the cavity formation.
The human blastocoele is approximately 100 μm in diameter, although there are about 60–100 cells.
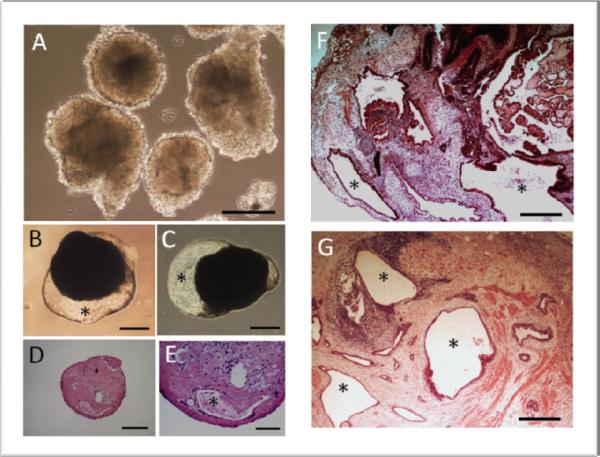
ES cells can form embryoid bodies (EBs) in cell cultures under certain growth conditions. These EBs contain cells differentiating into multiple lineages forming various primitive tissues. Cystic cavities are often formed in EBs. When ES cells are transplanted into immunocompromised mice, they form teratomas containing various immature tissues and are often cystic due to the formation of primitive glandular, epithelial or gastrointestinal tissues. Recently, somatic cells can be reprogrammed to ES cell-like cells termed induced pluripotent stem (iPS) cells. They behave almost like ES cells. Our lab has recently established human iPS cells reprogrammed from dental stem cells [4]. They form EBs and teratomas just like human ES cells forming cystic spaces in the cell mass (Figure 2). The space in the cystic tissues is completely enclosed in the mouse subcutaneous tissues. The developmental programming of the cells, i.e., their ability to proliferate and become polarized, allows the formation of cystic space that represents the external environment.
Figure. 2.
EB and teratoma formation. iPS cells derived from dental stem cells were allowed to form EBs in vitro (A–E) or teratoma in immunocompromised mice (F, G). (A) EB formation for 1week. (B, C) EB of 4.5 weeks. (D, E) Histological analysis of EB of 6 weeks. (F, G) Teratoma formation after 9 weeks in vivo. * indicates cystic space lined by epithelial-like cells. Scale bars: (A, D) 200 μm; (B, C) 500 μm; (E) 100 μm; (F, G) 400 μm. All animal procedures followed a protocol (protocol#AC-AAAB1141) approved by the Institutional Animal Care and Use Committee (IACUC) at the Columbia University (author's previous institute).
3. Neoplastic cyst in the jaw bone
The mechanism of the formation of this type of cyst is still unclear in terms of why the epithelial cell rests reenter the growth phase. Certain gene abnormal activities contributing to the cell proliferation have been under investigation. However, this does not explain how the cystic cavity is formed. As mentioned, epithelial cells of ectodermal origin that form a stratified layer of epithelium are polarized cells.
Only the cell that can self renew (stem cell) is capable of continuous proliferation. One may speculate the following phases of a neoplastic cyst formation: 1) Epithelial cell rests start to proliferate from only a few cells to reaching a spherical-shaped cell mass.
When it reaches certain number of cells, some begin to differentiate. These differentiated cells are likely located in the center of the cell mass and lose proliferating abilities. The outer layer cells of the cell mass are now the proliferating cells (stem cells) and may have established the basement membrane behaving like basal cells that continue to proliferate causing the expansion of the neoplasm. 2) Cavity formation. It is not known when the cavity formation begins during the expansion of the cell mass. Based on the nature of epithelial cells, it is likely at early stage when 4–5 layers (stratifying) of cells are reached. The cells in the center undergo apoptosis leading the cavity formation.
Also, since the epithelium by nature is supposed to cover the internal tissues and stay between these tissues and the external environment, there seems no other way but to form a cystic space which serves as an external environment when the epithelium is completed enclosed in the tissue. 3) The basal cells in contact with the connective tissue continue to proliferate causing the cystic structure to enlarge. The enlargement requires the availability of space. In the jaw bone, this hard tissue must first be resorbed to make space. How would this expansion of epithelial cells cause the bone to resorb? Evidence has shown that these expanding epithelial cells may release bone resorbing factors such as prostaglandins and cytokines [5–7].
4. Apical cyst formation
It may be reasoned that the apical cyst formation may follow a similar principle as the neoplatic cyst formation, except i) the growth of the epithelial cells is not due to neoplastic transformation but cytokine and growth factor stimulation from inflammation; and ii) the cyst cavity is not likely to form from within the ball-shaped epithelial cell mass because it would have been always true cysts in the apical lesions.
The ultimate question is how those strands of epithelial cells commonly seen in granulomas eventually form cysts. Lin et al proposed that merging of these epithelial cell strands may occur to reach the formation of a ball mass. When the connective tissue trapped inside the ball mass degenerates, a cyst is formed (mentioned above).
However, i) formation of a ball mass like the neoplastic cyst formation is unlikely as that will mostly generate a true cyst, and ii) the entrapment of the connective tissue inside the ball mass is unlikely to occur as this is not a natural relationship between connective tissue and epithelium.
Although abscess has been shown to be a cause of apical cyst formation, it is still unclear as to why the stratified epithelium is formed. Abscess is likely an indirect stimulus to the epithelial cells to proliferate. The presence of abscess co-exists with inflammatory cytokines and growth factors for epithelial cell growth.
Abscess is also considered as a foreign material that body is programmed to encapsulate or expel it. Fibrous tissue can do the work but when epithelial cells are around to form epithelium, it is the best tissue for this job.
Abscess may also facilitate the polarization and differentiation of epithelial cells to become epithelium.
Most of the apical cysts are so called “bay cysts” because they are not closed cysts but with an opening connected to the root canal space. With this understanding, one may reason that the epithelial cells are just trying to behave what they are programmed to do – forming a layer of epithelium separating the external environment from the internal tissues. Infected root canal space is part of the continuum of the external environment and therefore it should be excluded by forming the epithelium against it.
5. Tooth -- the only organ protruding out from internal tissue into external space
Tooth is the only organ that protrudes from the internal environment of the host (jaw bone) into the external environment (oral cavity) causing the discontinuity of the oral epithelium. The connection between the tooth surface and the gingival epithelium is established by only hemi-desmosomes than desmosomes between epithelial cells. This contact is weak and can be easily separated by trauma and microbial invasion. In response to microbial invasion, the body retreats by resorbing bone and allowing the epithelium to grow apically. As a result, the affected tooth is further exposed to the external environment. Ultimately, if untreated, the bone continues to retreat allowing the epithelium to reach the apex and reunite again. At this point, the entire tooth is being “pushed” into the external environment and “disposed”. Apical cyst appears to perform the same function - excluding the external environment which is the infected root canal space. From the developmental perspective, epithelial cell rests are the remnant of the Hertwig's root sheath which originates from the gingival epithelium.
Therefore, it seems logical that forming a layer of “internal gingival epithelium-like” cystic structure at the apex is the best way to protect internal tissues.
Given the right condition, the merging of epithelial cell strands may become polarized forming a sac of epithelium-lined cyst wrapping up the abscess or foreign debris.
Conclusion
In summary, we may speculate the phases of apical cyst formation as the following:
Epithelial cell rests re-enter the growth phase due the cytokine and growth factor stimulation in the inflammatory environment.
Each epithelial cell rest proliferates to form epithelial cell strands.
The cell strands are polarized due to encountering of abscess or foreign debris.
With time and the right condition, the epithelial cells strands merge wrapping the abscess or foreign debris and become cysts.
The cysts continue to expand as the growth stimulus persists allowing the internal epithelial cell layer (basal cell) to grow and expand.
Testing the hypothesis
Having understood of the nature of epithelial cell and epithelium biology, animal experimental designs can be established to test the above hypothesis. The experimental model should allow the epithelial cells to proliferate into sufficient cell number and the foreign materials should be present in the in vivo location to allow epithelial cells to form epithelium and wrap the foreign materials separating from the internal tissues.
Use the same animal model- Spraque Dawley rats, employed by Nair et al [2].
Insert various foreign objects or materials including abscess, cement, dead tissue, and air into the subcutaneous tissue of rats.
Introduce a biodegradable device able to slowly release epithelial cell mitogens (e.g., EGF, epithelial growth factor) around the foreign objects or materials.
Implant epithelial cells next to a foreign object. This will allow the cells to continuously proliferate may form a cystic sac wrapping around the foreign object(s).
All animal procedures should follow a protocol approved by the Institutional Animal Care and Use Committee (IACUC). The protocol should include details of the study design (control groups, steps taken to minimize subjective bias); experimental procedures (drug formulation and dose, anesthetic and surgical procedures, equipment); experimental animals (species, strain, sex, developmental stage); housing and husbandry; sample size (total animals used, including sample size calculation used); and statistical methods used.
Reporting the scientific findings will follow the “Animal Research: Reporting In Vivo Experiments” (ARRIVE) guidelines, developed by NC3Rs.
This journal utilizes the LOCKSS system to create a distributed archiving system among participating libraries and permits those libraries to create permanent archives of the journal for purposes of preservation and restoration.
Acknowledgments
The author wishes to thank Dr. Xing Yang for the iPS cell work presented in Figure 2. This work was supported in part by grants from the National Institutes of Health (NIH) R21 DE14585 (G.T.-J.H.) and R01 DE019156-01 (G.T.-J.H.).
List of abbreviations
- 2D or 3D
Two or three dimensional.
- ES
Embryonic stem cells.
- EB
Embryoid body.
- iPS cells
Induced pluripotent stem cells.
- EGF
Epithelial growth factor.
Footnotes
Conflicts of interests The author has no financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
- Main idea: by GT.
- Literature search: by GT.
- Data collection: by GT.
- Data interpretation: by GT.
- Manuscript preparation: by GT.
- Funds Collection: GT.
References
- 1.Markiewicz MR, Margarone JE, Barbagli G, Scannapieco FA. Oral mucosa harvest: an overview of anatomic and biologic considerations. EAU-EBU Update series. 2007;5:179–87. [Google Scholar]
- 2.Nair PN, Sundqvist G, Sjögren U. Experimental evidence supports the abscess theory of development of radicular cysts. Oral surg oral med oral pathol oral radiol endodo. 2008;106:294–303. doi: 10.1016/j.tripleo.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Lin LM, Huang GT, Rosenberg PA. Proliferation of Epithelial Cell Rests, Formation of Apical Cysts, and Regression of Apical Cysts after Periapical Wound Healing. J Endod. 2007;33:908–16. doi: 10.1016/j.joen.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 4.Yan X, Qin H, Qu C, Tuan RS, Shi S, Huang GT. iPS Cells Reprogrammed From Human Mesenchymal-Like Stem/Progenitor Cells of Dental Tissue Origin. Stem Cell Dev. 2010;19:469–80. doi: 10.1089/scd.2009.0314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris H. odontogenic cyst growth and pros-taglandin-induced bone resorption. Ann Royal Coll Surg Engl. 1978;60:85–91. [PMC free article] [PubMed] [Google Scholar]
- 6.Meghji S, Qureshi W, Henderson B, Harris M. The role of endotoxin and cytokines in the pathogenesis of odontogenic cysts. Arch Oral Biol. 1996;41:523–31. doi: 10.1016/0003-9969(96)00032-5. [DOI] [PubMed] [Google Scholar]
- 7.Birek C, Heersche JN, Jez D, Brunette DM. Secretion of a bone resorbing factor by epithelial cells cultured from porcine rests of Malassez. J Periodontal Res. 1983;18:75–81. doi: 10.1111/j.1600-0765.1983.tb00337.x. [DOI] [PubMed] [Google Scholar]