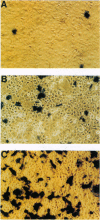
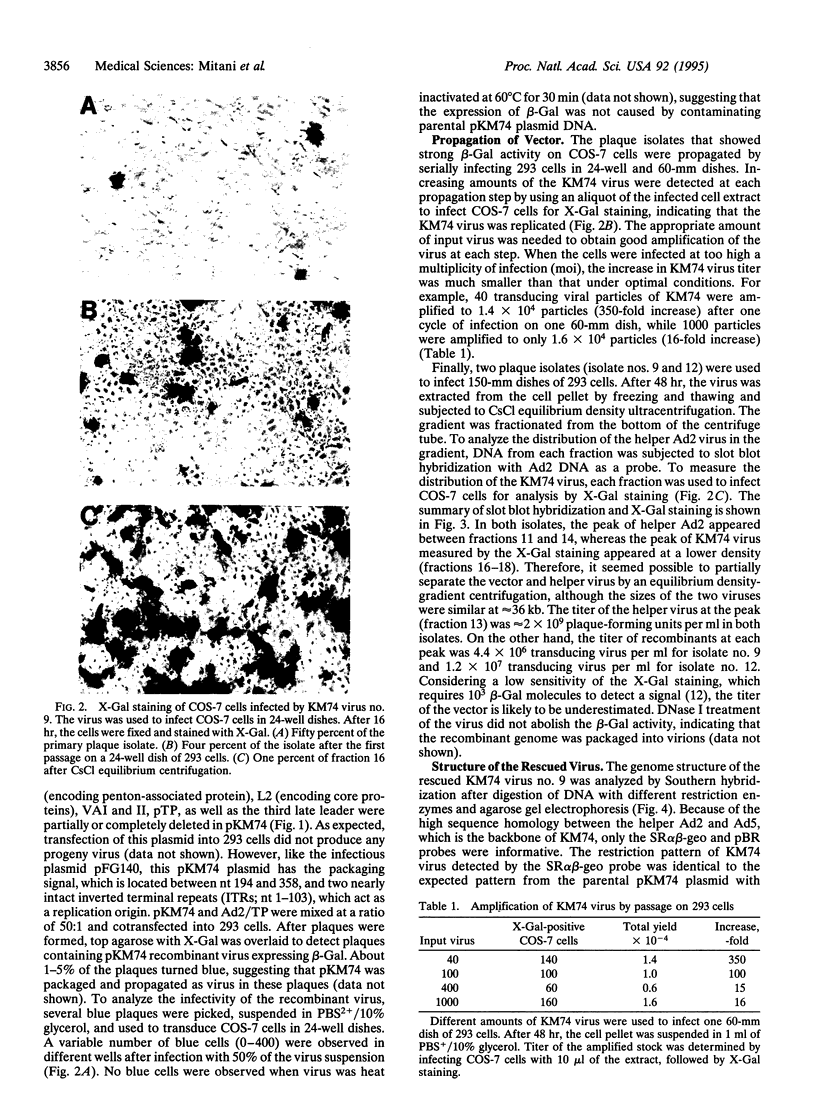
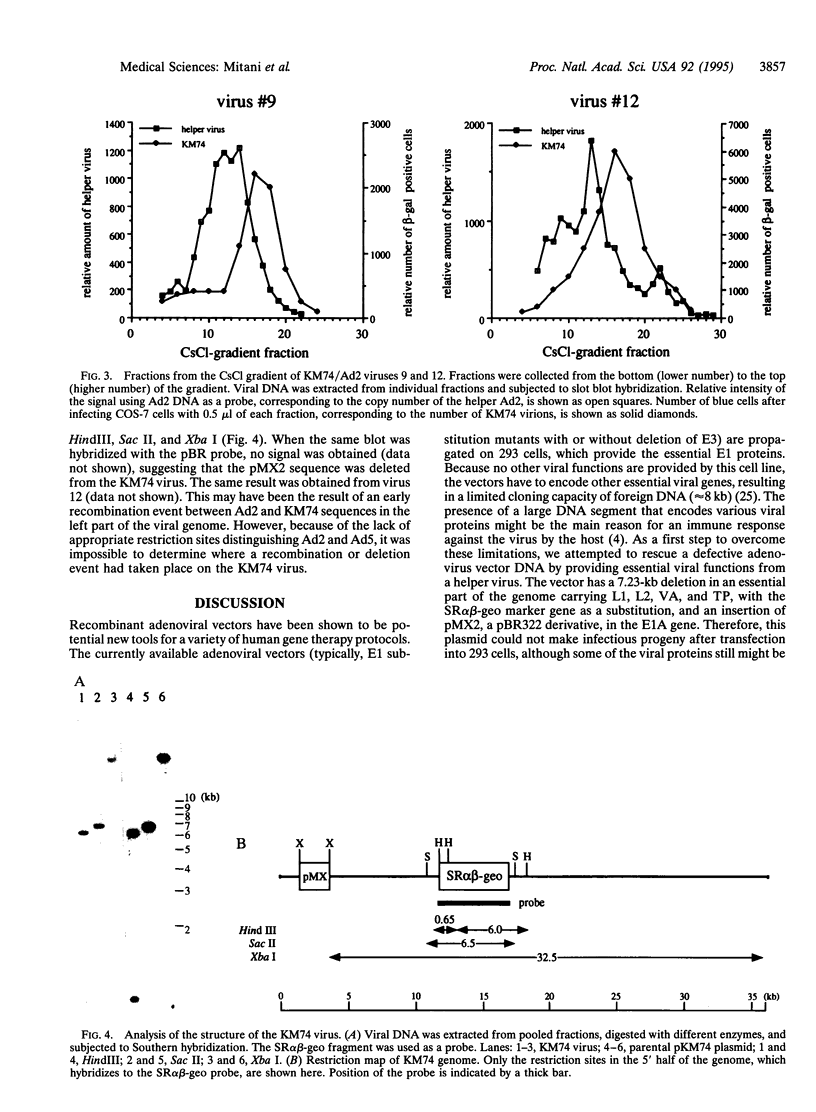
Abstract
Adenoviral vectors are widely used as highly efficient gene transfer vehicles in a variety of biological research strategies including human gene therapy. One of the limitations of the currently available adenoviral vector system is the presence of the majority of the viral genome in the vector, resulting in leaky expression of viral genes particularly at high multiplicity of infection and limited cloning capacity of exogenous sequences. As a first step to overcome this problem, we attempted to rescue a defective human adenovirus serotype 5 DNA, which had an essential region of the viral genome (L1, L2, VAI + II, pTP) deleted and replaced with an indicator gene. In the presence of wild-type adenovirus as a helper, this DNA was packaged and propagated as transducing viral particles. After several rounds of amplification, the titer of the recombinant virus reached at least 4 x 10(6) transducing particles per ml. The recombinant virus could be partially purified from the helper virus by CsCl equilibrium density-gradient centrifugation. The structure of the recombinant virus around the marker gene remained intact after serial propagation, while the pBR sequence inserted in the E1 region was deleted from the recombinant virus. Our results suggest that it should be possible to develop a helper-dependent adenoviral vector, which does not encode any viral proteins, as an alternative to the currently available adenoviral vector systems.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berkner K. L. Expression of heterologous sequences in adenoviral vectors. Curr Top Microbiol Immunol. 1992;158:39–66. doi: 10.1007/978-3-642-75608-5_3. [DOI] [PubMed] [Google Scholar]
- Bett A. J., Haddara W., Prevec L., Graham F. L. An efficient and flexible system for construction of adenovirus vectors with insertions or deletions in early regions 1 and 3. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8802–8806. doi: 10.1073/pnas.91.19.8802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bett A. J., Prevec L., Graham F. L. Packaging capacity and stability of human adenovirus type 5 vectors. J Virol. 1993 Oct;67(10):5911–5921. doi: 10.1128/jvi.67.10.5911-5921.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breakefield X. O., DeLuca N. A. Herpes simplex virus for gene delivery to neurons. New Biol. 1991 Mar;3(3):203–218. [PubMed] [Google Scholar]
- Chinnadurai G., Chinnadurai S., Green M. Enhanced infectivity of adenovirus type 2 DNA and a DNA-protein complex. J Virol. 1978 Apr;26(1):195–199. doi: 10.1128/jvi.26.1.195-199.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deuring R., Doerfler W. Proof of recombination between viral and cellular genomes in human KB cells productively infected by adenovirus type 12: structure of the junction site in a symmetric recombinant (SYREC). Gene. 1983 Dec;26(2-3):283–289. doi: 10.1016/0378-1119(83)90198-1. [DOI] [PubMed] [Google Scholar]
- Deuring R., Klotz G., Doerfler W. An unusual symmetric recombinant between adenovirus type 12 DNA and human cell DNA. Proc Natl Acad Sci U S A. 1981 May;78(5):3142–3146. doi: 10.1073/pnas.78.5.3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerfler W. Uptake, fixation, and expression of foreign DNA in mammalian cells: the organization of integrated adenovirus DNA sequences. Curr Top Microbiol Immunol. 1982;101:127–194. doi: 10.1007/978-3-642-68654-2_6. [DOI] [PubMed] [Google Scholar]
- Friedrich G., Soriano P. Promoter traps in embryonic stem cells: a genetic screen to identify and mutate developmental genes in mice. Genes Dev. 1991 Sep;5(9):1513–1523. doi: 10.1101/gad.5.9.1513. [DOI] [PubMed] [Google Scholar]
- Gluzman Y., Van Doren K. Palindromic adenovirus type 5-simian virus 40 hybrid. J Virol. 1983 Jan;45(1):91–103. doi: 10.1128/jvi.45.1.91-103.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L. Covalently closed circles of human adenovirus DNA are infectious. EMBO J. 1984 Dec 1;3(12):2917–2922. doi: 10.1002/j.1460-2075.1984.tb02232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Gräble M., Hearing P. cis and trans requirements for the selective packaging of adenovirus type 5 DNA. J Virol. 1992 Feb;66(2):723–731. doi: 10.1128/jvi.66.2.723-731.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarskjöld M. L., Winberg G. Encapsidation of adenovirus 16 DNA is directed by a small DNA sequence at the left end of the genome. Cell. 1980 Jul;20(3):787–795. doi: 10.1016/0092-8674(80)90325-6. [DOI] [PubMed] [Google Scholar]
- Hassell J. A., Lukanidin E., Fey G., Sambrook J. The structure and expression of two defective adenovirus 2/simian virus 40 hybrids. J Mol Biol. 1978 Apr 5;120(2):209–247. doi: 10.1016/0022-2836(78)90065-7. [DOI] [PubMed] [Google Scholar]
- Hay R. T., Stow N. D., McDougall I. M. Replication of adenovirus mini-chromosomes. J Mol Biol. 1984 Jun 5;175(4):493–510. doi: 10.1016/0022-2836(84)90181-5. [DOI] [PubMed] [Google Scholar]
- Mansour S. L., Grodzicker T., Tjian R. An adenovirus vector system used to express polyoma virus tumor antigens. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1359–1363. doi: 10.1073/pnas.82.5.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D. Retroviral vectors. Curr Top Microbiol Immunol. 1992;158:1–24. doi: 10.1007/978-3-642-75608-5_1. [DOI] [PubMed] [Google Scholar]
- Takebe Y., Seiki M., Fujisawa J., Hoy P., Yokota K., Arai K., Yoshida M., Arai N. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988 Jan;8(1):466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Lewis J. A., Grodzicker T. Overproduction of the protein product of a nonselected foreign gene carried by an adenovirus vector. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3567–3571. doi: 10.1073/pnas.82.11.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Nunes F. A., Berencsi K., Furth E. E., Gönczöl E., Wilson J. M. Cellular immunity to viral antigens limits E1-deleted adenoviruses for gene therapy. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4407–4411. doi: 10.1073/pnas.91.10.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]