Abstract
Objectives
This study aimed to 1) investigate the relationship between the acoustic change complex (ACC) and perceptual measures of frequency and intensity discrimination, and gap detection; and 2) examine the effects of acoustic change on the amplitudes and latencies of the ACC.
Design
Psychophysical thresholds for frequency and intensity discrimination and gap detection, as well as ACCs elicited by stimuli containing increments in frequency, or intensity or gaps, were recorded from the same group of subjects. The magnitude of the acoustic change was systematically varied for the ACC recording.
Study Sample
Twenty-six adults with normal hearing ranging in age between 19 and 39 years.
Results
Electrophysiological and psychophysical measures for frequency and intensity discrimination were significantly correlated. Electrophysiological thresholds were comparable to psychophysical thresholds for intensity discrimination but were higher than psychophysical thresholds for gap detection and frequency discrimination. Increasing magnitude of acoustic change increased the ACC amplitude but did not show consistent effects across acoustic dimensions for ACC latency.
Conclusions
The ACC can be used as an objective index of auditory discrimination in frequency and intensity. The ACC amplitude is a better indicator for auditory processing than the ACC latency.
Keywords: auditory evoked potential, auditory event-related response, auditory discrimination
INTRODUCTION
Understanding daily conversation depends on the ability of the auditory system to detect ongoing changes in the spectral and temporal patterns of incoming signals. Typically, auditory discrimination abilities in adult listeners are assessed using behavioral measures. Such psychophysical measures can provide useful information about a listener's auditory perception of a dynamic sound. However, performing these measures requires a significant amount of linguistic experience and cognitive ability. This presents a challenge when trying to obtain similar, and reliable, behavioral responses from infants and young children. Compared with behavioral tasks, many electrophysiological measures do not require active participation from listeners and can be reliably recorded from infants and very young children. In addition, unlike psychophysical measures, electrophysiological assessment can be more objective and less affected by factors such as memory, motivation, task, and response criteria. Therefore, such measures can provide a non-behavioral means of investigating the auditory processing of sound, and may provide information about underlying physiological mechanisms.
The P1-N1-P2 complex is an auditory evoked potential that can be recorded from surface electrodes placed on the scalp and is thought to reflect primarily cortical generators. When recorded from adult listeners using a brief acoustic stimulus, the P1-N1-P2 complex consists of three peaks (two vertex positive, one vertex negative) that occur during the time window between 50 to 250 msec after stimulus onset. The P1-N1-P2 complex is typically recorded in response to brief acoustic stimuli, such as clicks, tones, and short-duration speech tokens. However, studies have shown that it can also be elicited by changes in a continuous stimulus or a stimulus with long duration. Ostroff et al. (1998) recorded cortical potentials in response to three naturally produced speech tokens: /s/, /ei/, and /sei/ in eight normal hearing listeners. They found that the response evoked by the speech token /sei/ consisted of two overlapping onset responses – one to the fricative /s/ and one to the vowel /ei/. Ostroff et al. (1998) referred to the response elicited by the vowel /ei/ when presented in the context of an ongoing syllable /sei/ as the “N1-P2 acoustic change complex (ACC).” They suggested that the ACC response might indicate auditory discrimination capacity. It has been suggested that the ACC response represents a change detection response rather than a simple onset response even though the P1-N1-P2 complex elicited by a brief acoustic stimulus and the ACC response show similar general characteristics (Martin et al., 2008). The ACC response can only be evoked by long-duration, time-varying stimuli.
In a series of studies, Martin and Boothroyd (1999, 2000) investigated the ACC responses evoked by changes in periodicity, intensity, and spectrum of long-duration, ongoing stimuli. They found that the ACC response could be reliably elicited by changes along these acoustic dimensions. Jones and colleagues (Jones et al., 1998; Jones and Perez, 2001, 2002) reported that the ACC response could also be recorded in response to changes in pitch and/or timbre of synthesized music. In addition, several studies have shown that the ACC response can be recorded using ongoing stimuli that contain silent gaps of various durations (Michalewski et al., 2005; Pratt et al., 2005, 2007; Lister et al., 2007; Atcherson et al., 2009). Results of these studies showed that the ACC amplitude increased with increasing magnitude of acoustic changes in intensity (Martin and Boothroyd, 2000; Harris et al., 2007; Dimitrijevic et al., 2009, 2011), spectrum (Harris et al., 2008; Dimitrijevic et al., 2009, 2011), and gap duration (Michalewski et al., 2005). Therefore, the ACC response might function as an electrophysiological measure of the neural processes that underlie detection of changes in an ongoing acoustic signal. Consequently, it has been suggested that the ACC response can serve as an index of auditory discrimination ability (Martin et al., 2008). Studies have shown that the ACC response can be recorded not only from normal-hearing listeners but also from listeners with sensorineural hearing loss (Martin et al., 2008), cochlear implant users (Friesen and Tremblay, 2006; Brown et al., 2008), and patients with auditory neuropathy spectrum disorder (ANSD) (Michalewski et al., 2005; Dimitrijevic et al., 2011; He et al., 2010). Moreover, the ACC response can be reliably recorded from children as young as 6 years of age (Martin et al., 2010). Therefore, the ACC is a very promising tool for the objective evaluation of auditory discrimination abilities. Such a tool would be particularly useful in populations where the reliability of voluntary (behavioral) responses is questionable, such as in infants and very young children.
Despite the significance of such a clinical application, research on the relationship between the ACC response and auditory discrimination abilities is relatively sparse. The relationship between behavioral and electrophysiological measures of gap detection was investigated in normal-hearing subjects (Atcherson et al., 2009; Pratt et al., 2005) as well as in ANSD patients (Michalewski et al., 2005). It was found that both methodologies provided similar threshold estimates in normal-hearing listeners (Atcherson et al., 2009; Pratt et al., 2005). However, the behavioral gap detection thresholds were measured using either a modified Békésy-type tracking paradigm (Atcherson et al., 2009) or by recording the percentage of correct identifications along with associated reaction times (Pratt et al., 2005). It has been shown that results of behavioral measures can be affected by testing procedures (e.g., Wier et al., 1977; Freyman and Nelson, 1987). Therefore, it is of an interest to determine how well these gap detection thresholds compare with those reported in the psychophysical literature measured using a multiple-alternative forced-choice procedure. Although Michalewski et al. (2005) used such a procedure to obtain gap detection thresholds and showed a good agreement between psychophysical and electrophysiological measures, this conclusion was based on results obtained from ANSD patients. It is known that ANSD patients have poor temporal processing abilities (e.g., Zeng et al., 1999). Therefore, it is unclear how well the conclusion is generalizable to other listeners. In terms of other acoustic dimensions, it has been shown that ACC responses can be reliably evoked by intensity increments as small as 2 dB in young (Martin and Boothroyd, 2000; Dimitrijevic et al., 2009) and older listeners (Harris et al., 2007). Harris et al. (2008) showed that the ACC response to a frequency change of 4 Hz could be recorded from young adults with normal hearing. However, psychophysical measures of intensity and frequency discrimination were not recorded for these study participants. Therefore, the relationship between electrophysiological and psychophysical measures of frequency and intensity discrimination has not yet been systematically investigated.
Although it is apparent that the amplitude of the ACC complex increases as a function of the magnitude of the acoustic change (Martin and Boothroyd, 2000; Harris et al., 2007; Dimitrijevic et al., 2008, 2009, 2011; Michalewski et al., 2005), the effect of acoustic change on ACC latency has not been consistently reported across different acoustic dimensions. It has been shown that the ACC latency decreases as the magnitude of frequency change increases (Dimitrijevic et al., 2008; Harris et al., 2008). In terms of the effect of gap duration on ACC latency, the findings have not been consistent. Whereas some researchers found a significant effect (Pratt et al., 2005; Lister et al., 2007), other researchers reported no effect at all (Michalewski et al., 2005). In addition, the effect of intensity change on ACC latency is still unknown. Such information would be important for the clinical application of the ACC; i.e., ACC latency may be a clinically uninformative metric if it varies idiosyncratically across different dimensions of acoustic change.
The primary aim of this study was to systematically investigate the relationship between the ACC and perceptual measures of auditory discrimination along the acoustic dimensions of temporal continuity, frequency, and intensity in normal-hearing adults. It was hypothesized that the psychophysical and electrophysiological discrimination thresholds would show a robust correlation. In addition, this study investigated the effect of acoustic change magnitude on the amplitudes and latencies of the ACC response in order to gauge the predictive value of these response parameters in terms of potential clinical applications. This underscores our long-term goal of developing a time-efficient tool for examining auditory discrimination capacity that is suitable for use in the clinic, especially for pediatric patients.
GENERAL METHODS
Subjects
A total of 26 normal-hearing adults ranging in age from 19 to 39 years were recruited (13 females). These subjects were divided into three groups of twelve adults, with each group being tested separately on one of the three test dimensions of auditory discrimination: temporal continuity, frequency and intensity. Five subjects completed experiments for all three acoustic dimensions. All listeners had normal hearing sensitivity as defined by pure-tone detection thresholds of 20 dB HL or better at octave frequencies from 250 to 8000 Hz (ANSI, 2004). None of the listeners had a history of chronic ear disease or neurological disorder. All listeners signed informed consents following local IRB guidelines for testing human subjects. Each listener was paid for participation in the study.
Stimuli
For assessing auditory discrimination of frequency and intensity, the standard stimulus was a 500-Hz pure tone. For gap detection, the standard stimulus was a broad-band Gaussian noise. All tonal stimuli were gated on and off using 5-msec linear ramps and were presented at 70 dB SPL except for the stimuli that contained an intensity increment. The Gaussian noise was gated on and off using 1-msec linear ramps. Psychophysical measures were implemented using custom MATLAB (Mathworks) script that controlled a digital signal processor (RP2, Tucker-Davis Technologies). This platform controlled all signal generation, presentation gating/timing, and response collection. Stimuli were generated in the frequency domain based on 218 points and a 24.4-kHz sampling rate. From the output of the real-time digital signal processor, stimuli were routed through a headphone buffer and then presented monaurally to the left earphone of a Sennheiser HD 265 linear headset. For electrophysiological recordings, the same stimuli were loaded onto an Intelligent Hearing System SmartEP system and presented to the left ear through an Etymotic EAR3-A insert earphone. All stimuli were calibrated to 70 dB SPL using a Larson-Davis sound level meter.
Psychophysical Measures
Gap detection thresholds and auditory discrimination thresholds for frequency and intensity were obtained using a three alternative forced choice (3AFC), two-down one-up adaptive strategy estimating 70.7% correct detection (Levitt, 1971). Two of the listening intervals contained standard sounds whereas the third interval, chosen at random, contained a signal that differed in either frequency, intensity, or temporal continuity (i.e., it contained a brief interruption, or gap). Durations for listening intervals and inter-stimulus intervals were 500 msec and 400 msec, respectively. The initial step size of the change was 20 msec for gap detection and 20 Hz for frequency discrimination, respectively. This step changed by a factor of 1.414 () until the second reversal point and thereafter by a factor of 1.189 (). For intensity discrimination, the initial step size was 2 dB and this changed to 1 dB after two reversals. A threshold track stopped after eight reversals, and the signal level at the final six reversals was averaged (geometrically for gaps and frequency, arithmetically for intensity). At least three such estimates were obtained for each condition. Threshold was defined as the average of all estimates obtained for each condition. The behavioral threshold measure for each acoustic dimension took approximately 10-15 minutes.
Although only adults were tested in this study, the psychophysical procedures used were designed for children because of other ongoing studies that include a wide age range. Listening intervals were marked visually using animation on a computer screen. Over the course of a track, a cartoon picture was revealed, in the style of a jigsaw puzzle, with one piece revealed following each correct response. No visual feedback was provided for any incorrect response. A progress bar at the top of the screen indicated the number of track reversals obtained up to that point. At the end of the track the puzzle was completed and the underlying image performed a two-second animation. Listeners were tested in a double-walled sound-attenuating booth.
Electrophysiological Recordings
The electrophysiological measure was obtained during the same testing session as the psychophysical measure. The ACC responses were recorded using the Intelligent Hearing System SmartEP Continuous Acquisition Module. In keeping with our long-term goal of developing a clinically-applicable test suitable for pediatric patients, our recording methodology represented a compromise between an ideal approach and a practical, yet informative, approach. Specifically, we implemented a single-channel recording using a convenient electrode montage, as motivated by the consideration of clinical feasibility with child participants. A single channel recording cannot provide information about differences in EEG pattern across the hemispheres (Pratt et al., 2005; Tremblay et al., 2009) but avoids the significant patient preparation time required for multi-channel recordings that can be prohibitively challenging in pediatric patients in a clinical setting. In terms of electrode montage, responses were recorded differentially between a non-inverting electrode placed at high forehead (hairline) and an inverting electrode placed on the mastoid contralateral to the ear of sound presentation. A ground electrode was placed on the low forehead. Response amplitude is likely to be somewhat compromised by this montage but morphology is nevertheless typically robust. In addition to the factor of readily accessible electrode sites, the contralateral mastoid is often used as the reference site for measuring auditory evoked potentials in cochlear implant users in order to minimize effects of stimulus artifacts. Therefore, this montage can potentially be applied to this group of patients. As a result, the recording methodology implemented here is very time efficient and can potentially be used in different patient populations, which is crucial for any clinical procedures used in pediatric populations. Electrode impedances were maintained below 5000 Ohms with an inter-electrode impedance difference of less than 2000 Ohms. The EEG signal was recorded in epochs and averaged online. It was sampled at a rate of 1000 Hz. Responses were amplified (X10,000 gain) and filtered between 1 and 30 Hz (12 dB/octave rolloff) prior to averaging. Ocular movements were monitored from electrodes located above and below the orbit of the eye contralateral to the stimulated ear. Responses with large amplitude voltages (>70 μV) indicated contamination with electromyogenic activity and were rejected from averaging. The recording window consisted of a 100-msec prestimulus period and a 2100-msec poststimulus period. The averaged response was baseline corrected by subtracting the average value recorded between 1700-2000 msec after stimulus onset from all values in the epoch. This long-latency region was selected for the estimate of baseline because it occurred after the cessation of any stimulus-evoked cortical activity. During all recording sessions, subjects were seated in a recliner in a sound-attenuated booth and allowed to watch captioned videos to maintain alertness while remaining quiet. Subjects were instructed to ignore the auditory stimuli. They were also instructed to relax but not to sleep. Recordings were suspended and the subject re-instructed if she/he made excessive movements as monitored by a high-sensitivity microphone installed in the sound booth and/or amplitude of the raw EEG input. Breaks were provided as needed.
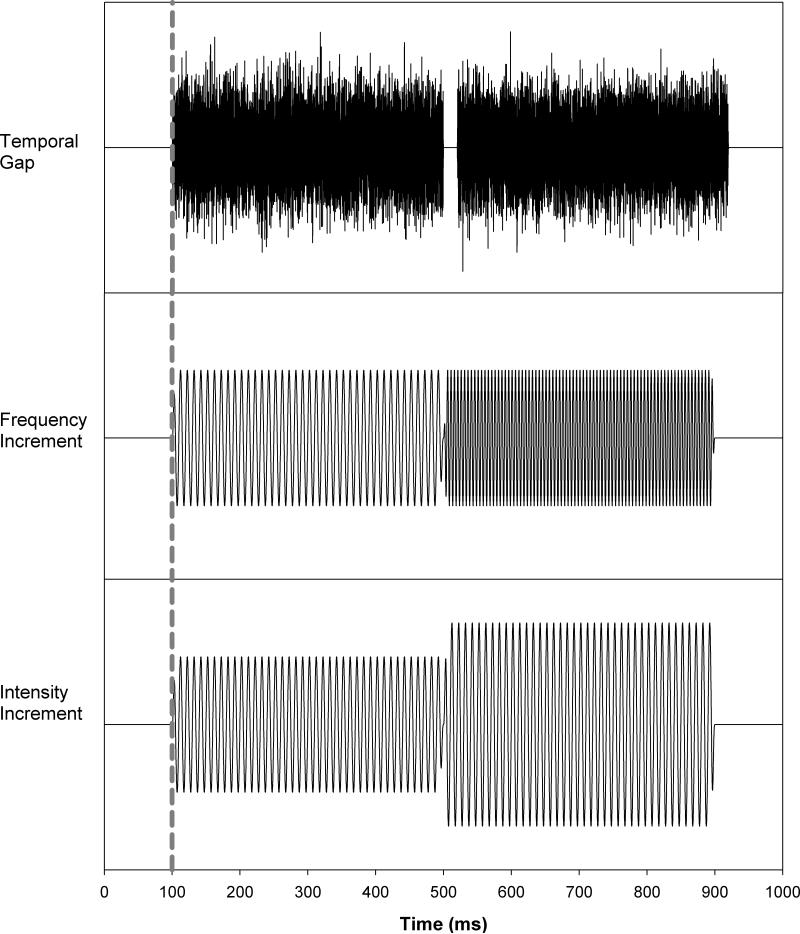
Two basic stimulus configurations were used in the ACC paradigm. In the “standard configuration”, the stimulus was either an 800-msec, 500-Hz pure tone (for spectral and intensity changes) or a Gaussian noise (for temporal changes). In the “change configuration”, the stimulus consisted of two sequential segments, each 400 msec in duration. In the pure tone conditions, the leading segment was 500-Hz and the trailing segment contained an increment in either the frequency or intensity. In the noise condition, the two segments were separated by a silent gap. Figure 1 shows a schematic of the stimuli used in this study. The upper, middle, and lower panels display the “change configuration” for temporal continuity, frequency increment, and intensity increment, respectively. The dotted line indicates stimulus onset. For ACC responses elicited by frequency changes, the frequency increments were 5, 8, 10, 20, 50, and 100 Hz. For four subjects whose behavioral frequency discrimination thresholds were lower than 3 Hz, an additional frequency increment of 3 Hz was also collected. For ACC responses elicited by intensity changes, increments of 2, 3, 4, 6, and 8 dB were used. An increment of 1 dB was also used in seven subjects whose behavioral thresholds were less than 2 dB. For ACC responses elicited by silent gaps, gap durations of 5, 8, 10, 20, 50, and 100 msec were employed. In addition, a gap duration of 3 msec was used in a subgroup of eight subjects whose gap detection thresholds were less than 5 msec. All stimuli were presented at 70 dB SPL with an interstimulus interval of 3030 msec. In all conditions, the ACC response was recorded in blocks of 50 artifact-free sweeps except for two subjects (S25 and S26) whose responses were recorded in blocks of 100 artifact-free sweeps in order to improve the signal to noise ratio of recorded responses. At least two blocks of responses were recorded for each stimulation condition for each subject, with a third block for the “standard configuration” collected from 4 subjects. The order of conditions was randomly interleaved to guard against order effects. Measuring the ACC for each acoustic dimension took approximately 90 minutes.
Figure 1.
Stimulus schematic for changes in temporal continuity (upper panel), frequency increment (middle panel), and intensity increment (lower panel).
Averaged cortical responses based on 50 sweeps (10 subjects) or 100 sweeps (2 subjects: S25 & S26) were examined offline. Replicate responses were averaged together and then smoothed using a 40-msec wide boxcar filter before determination of amplitude and latency values. The group average waveforms were used to determine the latency windows for identification of N1 and P2 components. N1 was defined as the largest negativity occurring between 80 and 180 msec for the onset response and between 450 and 600 msec for the ACC responses elicited by changes in frequency and intensity. The latency range of the N1 component for the ACC responses elicited by gap stimuli was 450-700 msec. P2 was defined as the largest positivity occurring within an 80-100 msec window after the N1 component. Both onset and ACC response peaks were labeled using the standard nomenclature of P1, N1 and P2. Response peaks were identified visually and independently by three experienced researchers (not all at the same institution), and were in agreement for 90% of the identified peaks. In cases where the three judges initially differed in peak identification (10%), the differences were mutually resolved following consultation. In some cases there was agreement that particular peaks were not identifiable.
Amplitude and latency measurements were carried out separately for the onset and the ACC responses. Peak-to-peak amplitudes reflect the voltage difference between N1 and P2. Latencies were measured from the onset of the stimulus or the onset of the acoustic change to the respective negative and positive peaks for the onset P1-N1-P2 complex and the ACC response. The resulting measures consisted of the N1-P2 amplitude and the latencies of N1 and P2 for both the onset and the ACC responses. Peaks were not labeled in conditions where there was no consensus among the three independent judges or where it was agreed that the components were not discernable from background EEG noise. The ACC threshold was defined as the smallest acoustic change that could reliably elicit the ACC response.
The test-retest reliability between every pair of onset response traces (averaged recordings of 100 or 200 sweeps) within and across recording sessions was evaluated for the five subjects who completed all test sessions using an intraclass correlation test with a two-way mixed model assessing the absolute agreement. In this model, responses measured from these subjects are considered as fixed effects. In other words, there was no generalization to responses recorded from other subjects due to individual variability in levels of background EEG noise. Recordings obtained using the same stimuli (i.e. 500-Hz pure tone or broad-band Guassian noise) are considered as random effects. For each dimension of acoustic change (intensity, frequency and temporal continuity), repeated measures analyses of variance (ANOVAs) were used to evaluate ACC amplitude and latency data for the group of listeners within that dimension. Data were included in the analyses only for conditions where responses were obtained from every subject tested in each group. Specifically, the analyses were performed for frequency increments of 10, 20, 50, and 100 Hz; for gap durations of 10, 20, 50, and 100 msec; and for level increments of 4, 6, and 8 dB. The ACC and the behavioral discrimination thresholds were compared using Paired Sample T tests. Correlations between the ACC threshold and the corresponding behavioral discrimination threshold were assessed using Pearson Product Moment Correlation tests.
RESULTS
Psychophysical Results
Behavioral gap detection thresholds ranged from 4.1 to 6.6 msec with a mean of 4.89 msec (SEM= 0.19 msec). Frequency discrimination thresholds ranged from 1.9 to 5.7 Hz with a mean of 3.55 Hz (SEM=0.36 Hz). Intensity discrimination thresholds ranged from 1 to 2.44 dB with a mean of 1.77dB (SEM=0.14 dB). Individual and group mean data are listed in Table 1. Note that the behavioral frequency discrimination threshold for subject S25 was 18.4 Hz, which is more than 12 standard deviations higher than the mean threshold measured for all other subjects. This threshold was designated as an outlier and excluded from analysis. All other data from this subject, including gap detection and intensity discrimination thresholds – as well as cortical auditory evoked potentials – were within normal limits and included.
Table 1.
Psychophysical thresholds of gap detection frequency discrimination, and intensity discrimination recorded from individual subjects, as well as group mean and standard error of the mean (SEM).
| Gap Detection (ms) | Frequency Discrimination (Hz) | Intensity Discrimination (dB) | ||||||
|---|---|---|---|---|---|---|---|---|
| Subject Number | Thresholds |
Subject Number | Thresholds |
Subject Number | Thresholds |
|||
| Behavioral Threshold | ACC Threshold | Behavioral Threshold | ACC Threshold | Behavioral Threshold | ACC Threshold | |||
| S1 | 5.64 | 5 | S1 | 5.68 | 8 | S1 | 1.96 | 2 |
| S2 | 5.06 | 5 | S2 | 3.07 | 5 | S2 | 2.48 | 2 |
| S3 | 4.81 | 5 | S4 | 4.83 | 10 | S5 | 2.11 | 2 |
| S6 | 4.82 | 5 | S6 | 4.65 | 5 | S6 | 2.44 | 3 |
| S8 | 6.56 | 8 | S9 | 4.01 | 5 | S7 | 2.07 | 2 |
| S10 | 4.43 | 5 | S13 | 2.49 | 3 | S11 | 1.73 | 2 |
| S12 | 4.74 | 5 | S17 | 1.92 | 5 | S14 | 1.78 | 2 |
| S16 | 4.53 | 5 | S18 | 2.23 | 5 | S15 | 1.00 | 1 |
| S20 | 4.66 | 5 | S19 | 3.19 | 5 | S22 | 1.37 | 2 |
| S23 | 5.04 | 5 | S21 | 4.08 | 8 | S24 | 1.11 | 1 |
| S25 | 4.30 | 5 | S25 | 18.4* | 5 | S25 | 2.00 | 3 |
| S26 | 4.10 | 5 | S26 | 2.9 | 5 | S26 | 1.30 | 2 |
| Mean (SEM) | 4.89 (0.19) | 5.25 (0.25) | Mean (SEM) | 3.55 (0.36) | 5.81 (0.60) | Mean (SEM) | 1.77 (0.14) | 2.00 (0.17) |
Outlier.
Electrophysiological Results
Responses from Individual Subjects
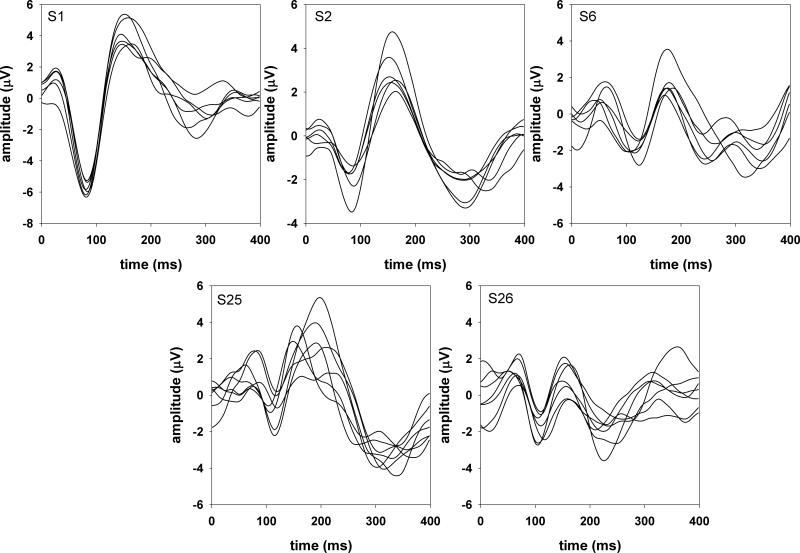
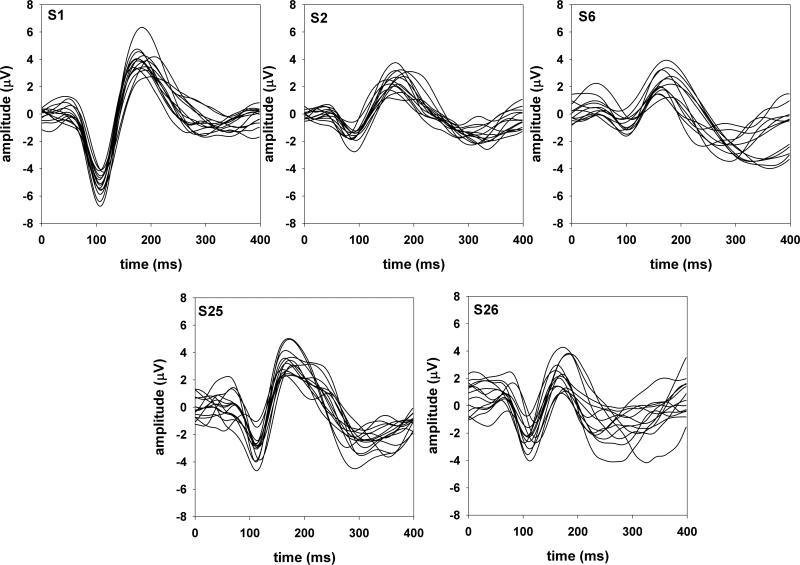
The onset P1-N1-P2 complex showed considerable variability across subjects for all three acoustic dimensions. However, responses recorded from each individual subject were relatively stable both within and across recording sessions. This is illustrated in Figures 2 and 3 that show the onset P1-N1-P2 complex recorded from the five subjects who were tested in all three acoustic dimensions. Figure 2 shows responses evoked by Gaussian noise (gap detection conditions) and figure 3 shows responses evoked by 500-Hz tones (frequency and intensity discrimination conditions). Each trace represents an averaged response of 100 sweeps for subjects S1, S2, and S6 and 200 sweeps for subjects S25 and S26. For each of these five subjects, the responses were recorded across three different sessions separated by intervals ranging from 2 days to 1 month.
Figure 2.
Onset responses evoked by Gaussian noise in five subjects.
Figure 3.
Onset responses evoked by 500-Hz tones in five subjects.
The test-retest reliability of the onset P1-N1-P2 complex between every pair of traces was examined using intraclass correlation tests with a two-way fixed effect model assessing the absolute agreement. In general, the mean intraclass correlation coefficients for these five subjects ranged from 0.62 to 0.95 and 0.63 to 0.94 for responses evoked by Gaussian noise and 500-Hz pure tones, respectively. The mean correlation coefficients across stimulus type (responses evoked by Gaussian noise vs. 500-Hz pure tones) ranged from 0.53 to 0.85. These cross-stimuli coefficients were significantly lower than the within-stimulus coefficients (t=3.97, p<0.05).
For all subjects, the most identifiable component of the response was the N1 peak. For the onset responses elicited by Gaussian noise, the N1 latencies ranged from 70 to 124 msec with a mean of 94.2 msec. The N1-P2 amplitude ranged from 0.8 to 11.7 μV with a mean of 4.27 μV. For the onset responses evoked by a 500-Hz pure tone, the N1 latencies ranged from 70 to 137 msec with a mean of 101.3 msec. The N1-P2 amplitude ranged from 1.2 to 12.5 μV with a mean of 5.2 μV. Results of student T tests showed that the N1-P2 amplitudes of the onset responses evoked by pure tones were significantly larger than those of the onset responses evoked by Gaussian noise (t=2.57, p<0.05). However, there was no significant difference in N1 latency between the onset responses evoked by these two stimuli (t=1.37, p=0.17).
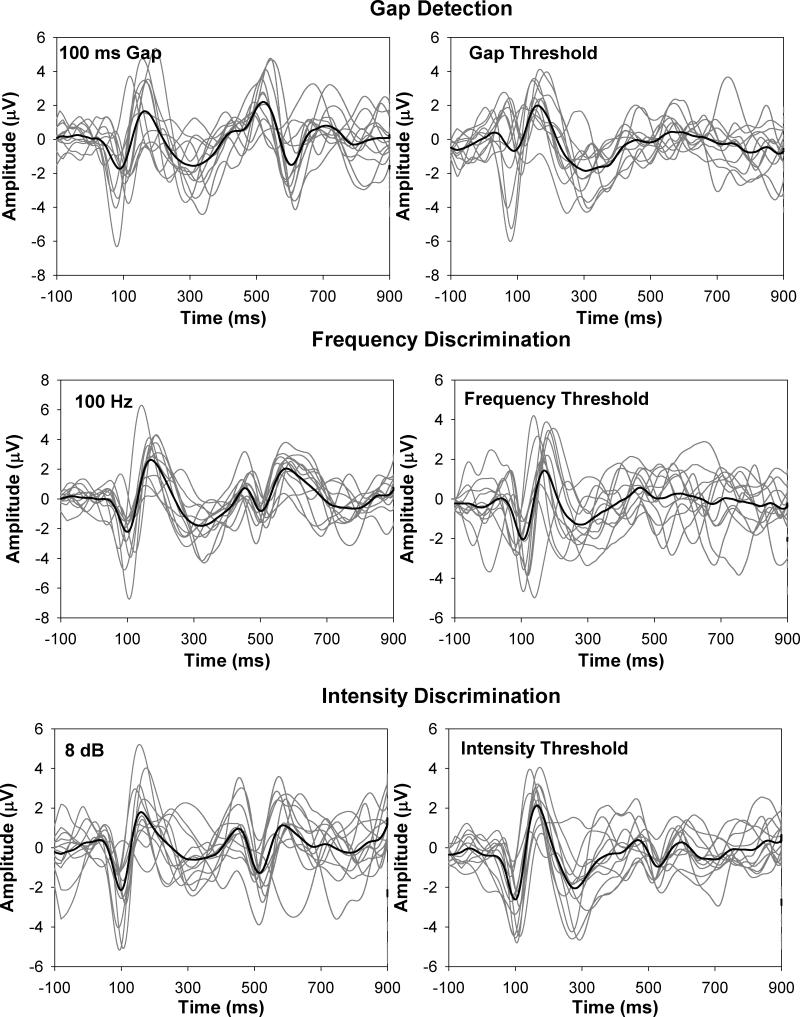
Figure 4 shows responses evoked by two magnitudes of acoustic change (suprathreshold and threshold) in each acoustic dimension. The upper, middle, and lower panels show, respectively, results for the temporal continuity (gap), frequency, and intensity measures. Each panel shows responses from each of the 12 subjects (gray lines) as well as the group-average waveform (black line). The waveforms in the left column show results for the largest acoustic change that was tested for that particular acoustic dimension (i.e., a 100-ms gap, a 100-Hz increment, and an 8-dB increment). The waveforms in the right column show results for the smallest acoustic change that could elicit the ACC response (i.e. the ACC threshold).
Figure 4.
Onset and ACC responses recorded from individual subjects and group-mean waveforms. The left graph in each panel shows responses recorded to the largest acoustic change in each acoustic dimension, and the right graph shows responses recorded at ACC threshold.
For the majority of subjects, the general morphology of the ACC response was similar to their onset P1-N1-P2 complex. Amplitude and latency measures for ACC responses shown in Figure 4 are summarized in Table 2. Results of paired sample T tests showed that the N1-P2 amplitudes evoked by a frequency increment of 100 Hz and by an intensity increment of 8 dB were significantly smaller than the onset responses recorded in the same testing conditions (frequency increment: t=3.42, p<0.05; intensity increment: t=2.58, p<0.05). There was no significant difference in amplitude between the ACC recorded for a 100-ms gap and the onset response for this stimulus (t=1.56, p=0.15). As expected, the N1 latency of the ACC response for the gap stimuli was significantly longer than the N1 latency of the onset responses (t=-12.81, p<0.001). However, there was no difference in latency of the N1 component between the ACC and the onset response for either frequency or intensity increments (p>0.05).
Table 2.
Latencies and amplitudes of the ACC responses elicited by two levels of change in each acoustic dimension for individual subjects.
| Gap Duration (msec) |
Frequency Increment (Hz) |
Intensity Increment (dB) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 100 | Threshold | 100 | Threshold | 8 | Threshold | |||||||||
| Subject | N1 latency (ms) | N1-P2 amplitude (μV) | N1 latency (ms) | N1-P2 amplitude (μV) | Subject | N1 latency (ms) | N1-P2 amplitude (μV) | N1 latency (ms) | N1-P2 amplitude (μV) | Subject | N1 latency (ms) | N1-P2 amplitude (μV) | N1 latency (ms) | N1-P2 amplitude (μV) |
| S1 | 165.1 | 1.57 | 83.1 | 1.67 | S1 | 110 | 3.47 | 97 | 1.86 | S1 | 105.3 | 4.02 | 124.5 | 1.08 |
| S2 | 193.1 | 6.23 | 90.5 | 1.46 | S2 | 94 | 4.84 | 122 | 1.62 | S2 | 115.11 | 1.79 | 136.5 | 0.9 |
| S3 | 204.9 | 4.31 | 110.7 | 0.34 | S4 | 116 | 1.51 | 185 | 0.66 | S5 | 103.12 | 3 | 122.1 | 0.77 |
| S8 | 172.9 | 2.60 | 102.3 | 2.35 | S8 | 106.5 | 1.85 | 133.5 | 0.42 | S7 | 137.1 | 3.10 | 142.3 | 1.18 |
| S10 | 191 | 2.74 | 151 | 1.26 | S9 | 97.9 | 3.53 | 92.9 | 0.27 | S6 | 102.9 | 1.97 | 158.9 | 1.06 |
| S6 | 217.1 | 4.19 | 91.7 | 0.93 | S13 | 135 | 3.75 | 148 | 1.37 | S11 | 125.53 | 4.63 | 134.9 | 3.50 |
| S12 | 225 | 2.59 | 172 | 1.62 | S17 | 92.3 | 3.41 | 109.1 | 0.57 | S14 | 138 | 3.56 | 149 | 2.21 |
| S16 | 201 | 3.93 | 82 | 2.71 | S18 | 103 | 3.94 | 130 | 2.21 | S15 | 120 | 6.17 | 139 | 1.81 |
| S20 | 196 | 5.49 | 112 | 1.24 | S19 | 90 | 6.85 | 152 | 1.27 | S22 | 117 | 2.5 | 142 | 1.49 |
| S23 | 224.5 | 2.30 | 108.9 | 0.75 | S21 | 109 | 1.84 | 122 | 0.55 | S24 | 98 | 4.99 | 117 | 2.67 |
| S25 | 117.1 | 5.37 | 87.7 | 3.6 | S25 | 120.9 | 4.77 | 133.9 | 1.82 | S25 | 126.1 | 5.36 | 133.9 | 3.05 |
| S26 | 112.3 | 3.89 | 150.1 | 0.68 | S26 | 138.1 | 2.40 | 143.5 | 1.43 | S26 | 120.5 | 3.18 | 141.5 | 0.85 |
| Mean (SEM) | 202.42 (5.72) | 3.77 (0.42) | 108.98 (8.61) | 1.62 (0.26) | Mean (SEM) | 109.39 (4.56) | 3.51 (0.44) | 131.16 (7.41) | 1.18 (0.19) | Mean (SEM) | 98.73 (0.37) | 3.68 (0.40) | 136.8 (3.67) | 1.71 (0.17) |
Inspection of Figure 4 also indicates that the general morphology of the ACC response did not change as the magnitude of acoustic change decreased. However, results of paired sample T tests showed that N1-P2 amplitude of the ACC response elicited by the largest acoustic change (i.e., 100-msec gap, 100-Hz increment or 8-dB increment) was significantly larger than that of the ACC response at threshold (gap stimuli: t=4.47, p<0.05; frequency increment: t=6.13, p<0.001; intensity increment: t=6.72, p<0.001). In terms of latency, the ACC response elicited by a 100-msec gap was significantly delayed relative to the response elicited by a gap at threshold (t=9.87, p<0.01), whereas the N1 latency was significantly shorter for the ACC response elicited by a frequency increment of 100 Hz or an intensity increment of 8 dB relative to the response at threshold (t=3.52, p<0.05; t=13.61, p<0.01, respectively).
Grand Mean Waveforms
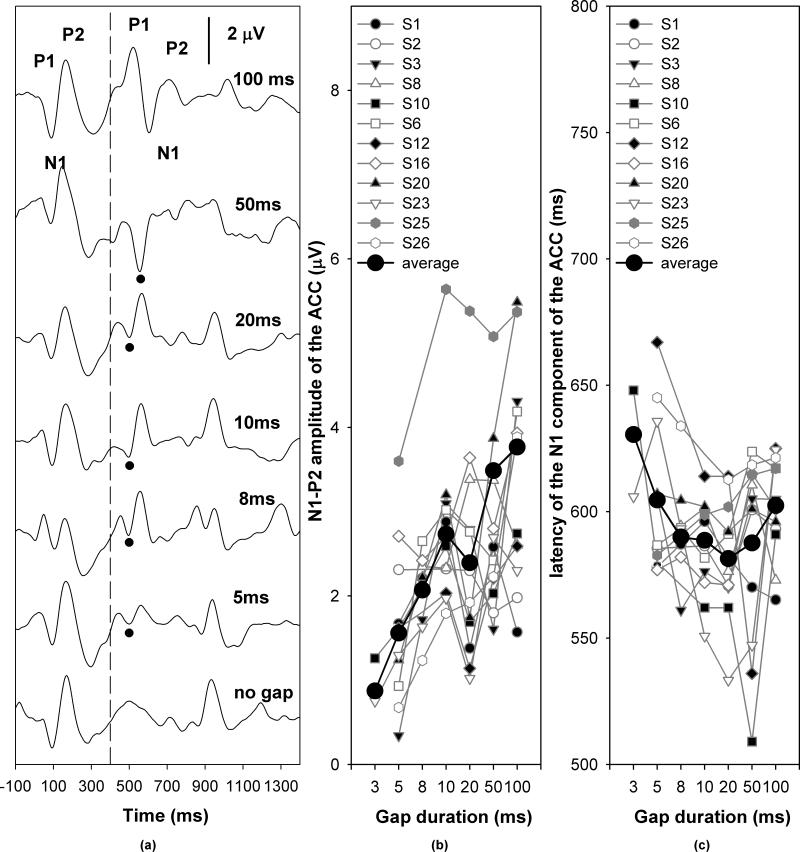
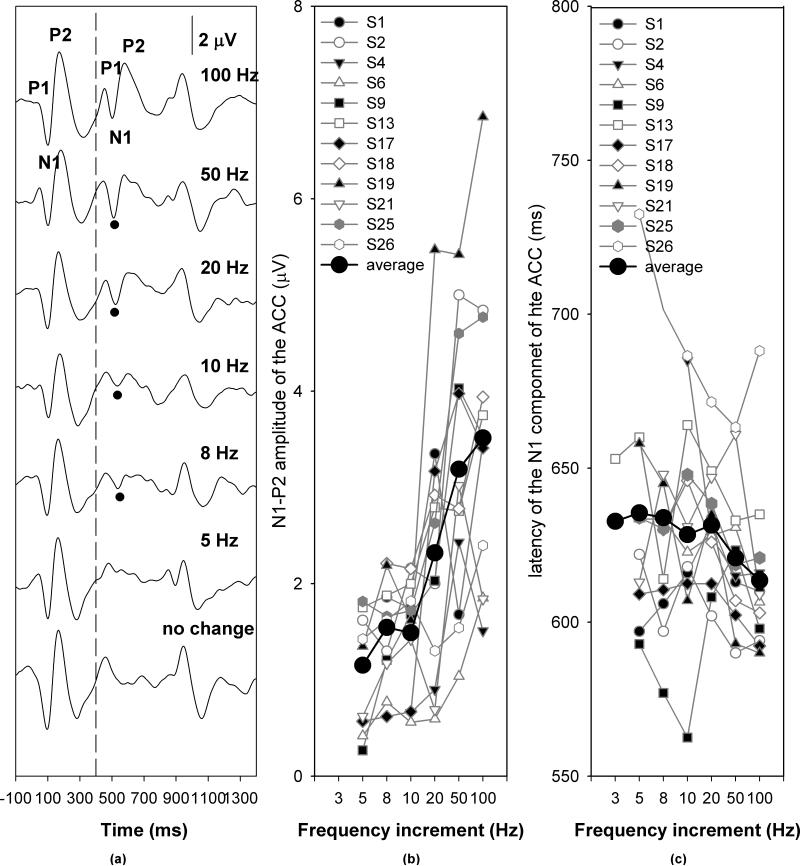
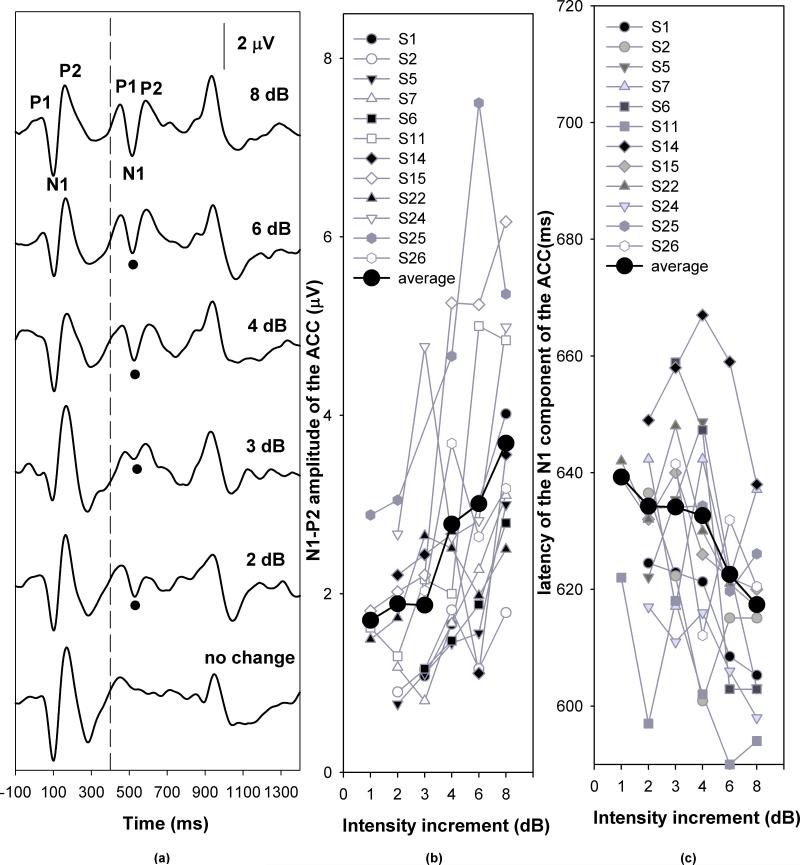
Figures 5, 6 and 7 show the grand mean average ACC responses for temporal continuity (gap), frequency and intensity measures, respectively. In each graph, Panel (a) shows the grand mean waveforms averaged across listeners for each step of acoustic change magnitude. The vertical dashed line indicates the time when the first 400-msec segment of stimulus ended. The black dots indicate the N1 peaks of the ACC responses. The specific gap durations, frequency increments, and intensity increments are labeled for each trace. Panels (b) and (c) plot the respective response amplitudes and latencies as a function of the magnitude of stimulus change. Symbols connected by grey lines represent data obtained from individual subjects, as noted in the legend. The black dots connected by a solid line represent the group mean.
Figure 5.
ACC responses recorded in response to changes in temporal continuity. Panel (a) shows the grand mean averaged waveforms. Panel (b) and (c) shows the effect of gap durations on ACC amplitudes and latencies, respectively.
Figure 6.
ACC responses recorded in response to frequency increments. Panel (a) shows the grand mean averaged waveforms. Panel (b) and (c) shows the effect of frequency increments on ACC amplitudes and latencies, respectively.
Figure 7.
ACC responses recorded in response to intensity increments. Panel (a) shows the grand mean averaged waveforms. Panel (b) and (c) shows the effect of changes in intensity on ACC amplitudes and latencies, respectively.
Inspection of Figure 5 indicates that the ACC amplitude increased as the gap duration increased, whereas the effect of gap duration on the ACC latency was nonmonotonic. The ACC latency decreased as the gap duration increased up to 20 ms and then began to increase beyond this. Results of a repeated measures ANOVA for conditions where responses were obtained from every subject tested for gap discrimination (i.e., gap durations of 10, 20, 50, and 100 msec) showed a main effect of gap duration on ACC amplitude (F[3,33]=5.73, p<0.05) and latency (F[3,33]=54.39, p<0.05). Post-hoc analysis of the amplitude effect showed that the amplitudes of ACC responses elicited by the 100-ms gap were larger than responses for the 20-ms gap (p<0.05). There were no significant differences between any of the other conditions. Post-hoc analysis of the latency effect showed that the ACC responses for the 100-ms gap had significantly longer latencies than for gaps of any other duration (p<0.05). There was no difference in ACC latency between any other gap conditions.
Inspection of Figure 6 indicates that the ACC amplitude increased as the frequency increment increased, whereas the effect of frequency increments on the ACC latency was nonmonotonic. The ACC latency was not affected by frequency increments between 3 and 20 Hz and decreased as the frequency increment increased from 20 to 100 Hz. Analysis of the effects of frequency increment for conditions where responses were obtained from every subject tested (i.e., 10, 20, 50, and 100 Hz) on ACC amplitude and latency showed that the magnitude of frequency increment (10-100 Hz) had a main effect on ACC amplitudes (F[3, 33]=13.51, p<0.01). Post-hoc analysis revealed that the ACC amplitude was larger for a 100-Hz increment than for both 20- and 10-Hz increments (p<0.05). The ACC response recorded for a 50-Hz increment was larger than that obtained for 10-Hz increment (p<0.05). There was no difference in ACC amplitude between any the other conditions. There was also a main effect of frequency increment on ACC latency (F[3,33]=3.96, p<0.05). Post-hoc analysis indicated that the latency of ACC responses for a 100-Hz and 50 Hz increment was shorter than for 20- and 10-Hz increments (p<0.05). There was no significant difference between latencies measured for the other conditions.
Inspection of Figure 7 suggests that the ACC amplitude increased as the magnitude of intensity change increased and that the ACC latency decreased as the magnitude of intensity change increased from 4 to 8 dB. For an intensity change less than 4 dB, the effect of intensity increment was not robust. Results of a repeated measures ANOVA for conditions where responses were obtained from every subject tested (i.e., 4, 6, and 8 dB) showed a significant effect of level increment (4-8 dB) on ACC amplitude (F[2, 22]=4.36, p<0.05) and latency (F[2,22]=5.99, p<0.05) (Fig. 6). Post hoc analysis showed that the ACC amplitude was significantly larger for 8-dB than for 4- or 6-dB increments (p<0.05). There was no difference in ACC amplitude between 4- and 6-dB increments. The ACC response showed a longer latency for 4-dB than for 6- and 8-dB increments (p<0.05). There was no difference between latencies measured for 6- and 8-dB increments.
Comparison of Electrophysiological and Psychophysical Measures
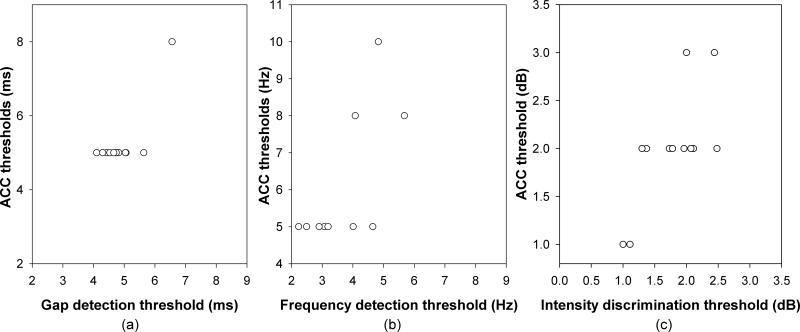
The ACC thresholds for gap detection ranged from 5.0 to 8.0 msec with a mean of 5.25 msec (SEM= 0.25 msec). Thresholds for frequency discrimination ranged from 5.0 to 10.0 Hz with a mean of 5.81 Hz (SEM=0.6 Hz). Finally, thresholds for intensity discrimination, ranged from 1.0 to 3.0 dB with a mean of 2 dB (SEM=0.17 dB). Figure 8 compares the auditory discrimination thresholds determined by electrophysiological and psychophysical measures. Panel a - c show thresholds of gap detection, frequency discrimination, and intensity discrimination, respectively. Paired Sample T tests showed that gap detection and frequency discrimination thresholds determined by the electrophysiological measure were significantly larger than the behavioral thresholds (for gap detection: t=2.37, p<0.05; for frequency discrimination: t=5.2, p<0.05 ). However, there was no significant difference between thresholds measured by the two procedures for intensity discrimination (t=-1.80, p=0.1). One-way Pearson Product Moment Correlation tests showed a significant correlation between the thresholds measured using the two methodologies for frequency discrimination (r=0.70, p<0.05), and intensity discrimination (r=0.72, p<0.05). However, a test of correlation is not valid for gap detection because the result is largely dependent on one point (see panel (a)).
Figure 8.
The correlation between the ACC threshold and the psychophysical threshold obtained for the same group of listeners. Panel (a), (b), and (c) illustrate the results for gap stimuli, frequency and intensity increments, respectively.
DISCUSSION
In this study, behavioral thresholds for gap detection, frequency discrimination and intensity discrimination were measured for normal-hearing adults. Our results showed that the averaged thresholds were 4.9 msec and 1.8 dB for gap detection and intensity discrimination, respectively. These results are generally consistent with results reported by other researchers using similar stimuli (e.g. Buss et al., 2009; Harris et al., 2010). The averaged threshold for frequency discrimination was 3.6 Hz, which is generally consistent with results of Wier et al. (1977). Our results also show that the ACC thresholds are more variable than behavioral thresholds as indicated by large standard errors of the means for all three acoustic dimensions, which could be due to the larger step sizes that were used in the electrophysiological measures than those used for behavioral measures. In general, the electrophysiological ACC thresholds recorded in the present study are consistent with results reported by others for all three acoustic dimensions (Martin and Boothroyd, 2000; Michalewski et al., 2005; Harris et al., 2007; 2008).
Variations in the onset P1-N1-P2 complex are observed. Some subjects show well-defined peaks with large amplitudes, whereas other subjects show less robust peaks, especially for P1 components. Test-retest reliability of the onset P1-N1-P2 complex was assessed within and across recording sessions for five subjects using intraclass correlation tests. The mean correlation coefficients across traces range from 0.62 to 0.95, which is consistent with published literature (e.g. Hensch et al., 2008; Friesen and Tremblay, 2006). These results indicate that: 1) the onset P1-N1-P2 complex is stable within and across recording sessions; 2) differences in the P1-N1-P2 complex across subjects are due to individual variability rather than noisy recordings.
The primary goal of the study was to investigate the relationship between the ACC response and auditory discrimination ability across the acoustic dimensions of temporal continuity, frequency, and intensity. For frequency and intensity increments, our results showed a significant correlation between the ACC responses and the psychophysical measures of these auditory discrimination abilities. Listeners who had higher ACC thresholds also showed poorer auditory discrimination abilities as indicated by higher behavioral thresholds. However, these correlations must be considered cautiously since the range of thresholds for both behavioral and electrophysiological tests was relatively restricted. For gap detection, the lack of variation of the electrophysiological threshold undermined the meaningfulness of its correlation with the behavioral threshold. It is likely that the association between ACC thresholds and behavioral thresholds could be further clarified by including subjects representing a greater range of performance. For example, (Michalewski et al., 2005) tested gap detection in ANSD patients and found substantially larger thresholds in this population for both ACC and behavioral measures. Inclusion of patient populations such as children with ANSD is a future direction for this line of research. Whereas the relative lack of variation in the thresholds for our normal-hearing adult population undermines tests of association between behavioral and electrophysiological measures, it is encouraging that the thresholds across the two test procedures fall within similar ranges. For intensity discrimination, the thresholds were comparable across electrophysiological and psychophysical measures. The behavioral thresholds of frequency discrimination and gap detection were significantly lower than the electrophysiological ACC thresholds but still within the same general range of performance. Overall, these results suggest that the ACC response can be used as an objective indicator of behavioral sensitivity to changes in an ongoing acoustic signal. It can accurately predict behavioral thresholds of intensity discrimination.
The second goal of the study was to investigate the effect of acoustic changes on the amplitude and latency of the ACC responses. This focus was expected to clarify the parameter of choice for applications in clinical settings. Results of the present study showed that the ACC response increased in amplitude as the magnitude of change increased in all three acoustic dimensions, which is consistent with results reported in the literature (Atcherson et al., 2009; Martin and Boothroyd, 1999, 2000; Martin, 2010; Michalewski et al., 2005). However, the effect of acoustic change on ACC latency was not consistent across conditions. On the one hand, our results showed that the ACC latency decreased as the magnitude of frequency increment increased for acoustic changes greater than 20 Hz, consistent with the results of Dimitrijevic et al. (2008). On the other hand, changes in intensity and temporal continuity had nonmonotonic effects on ACC latency. The ACC latency increased as the intensity increment decreased up to 4 dB and reached a plateau afterwards. The effect of gap duration on the ACC latency showed a more complicated pattern. While the ACC latency decreased with gap duration up to 20 ms, it started to increase for gaps with longer durations. Overall, our results suggest that the ACC amplitude is a better indicator for auditory processing since it is more consistent across acoustic dimensions.
It is should be noted that there was only a minimum of 100-200 artifact-free sweeps recorded for each stimulating condition in this study. Therefore, it is possible that the ACC response recorded in this study might contain high levels of background EEG noise. However, results of intra-class correlation tests obtained from five subjects who participated in recording sessions across all three acoustic dimensions showed that the mean correlation coefficients across traces evoked by the same type of stimulus range from 0.62 to 0.95, which is consistent with published literature (e.g. Hensch et al., 2008; Friesen and Tremblay, 2006). Therefore, it is unlikely that our results were affected by the background EEG noise.
CONCLUSIONS
Results of electrophysiological and psychophysical measures of acoustic discrimination showed a significant correlation for frequency and intensity discrimination. The intensity discrimination thresholds measured using these two paradigms are comparable. However, the electrophysiological measures of gap detection and frequency discrimination are less sensitive than the behavioral measures. Our results suggest that the ACC amplitude might be a better indicator for auditory processing than the ACC latency since it shows a relatively consistent pattern across stimulus dimensions.
ACKNOWLEDGEMENTS
Portions of this paper were presented at the 33rd MidWinter meeting of the Association for Research in Otolaryngology in February of 2010, San Diego CA. We acknowledge funding provided by the Deafness Research Foundation and the NIH/NIDCD (1R21DC011383). The authors thank all of the subjects who participated in this study. The authors also wish to acknowledge Sara Mamo who assisted in pilot data collection and Emily Buss for help with programming. We also gratefully acknowledge Eun Kyung Jeon and Likuei Chiou from the University of Iowa for assistance with data analysis.
Acronyms and Abbreviations
- ACC
Acoustic Change Complex
- ANOVA
Analysis of Variance
- ANSD
Auditory Neuropathy Spectrum Disorder
- ANSI
American National Standards Institute
- dB
Decibel
- EEG
Electroencephalogram
- Hz
Hertz
- IRB
Institutional Review Board
- msec
Millisecond
- RP2
Real-time Processor 2
- HD
High Definition
- SEM
Standard Error of the Mean
- sec
Second
- SPL
Sound Pressure Level
- 3AFC
three alternative forced choice
Footnotes
DECLARATION OF INTEREST
Authors have no conflict of interests to claim for this work.
REFERENCES
- ANSI . Specification for audiometers. American National Standards Institute; New York: 2004. ANSI S3.6-2004. [Google Scholar]
- Atcherson SR, Gould HJ, Mendel MI, Ethington CA. Auditory N1 component to gaps in continuous narrowband noises. Ear Hear. 2009;30:687–695. doi: 10.1097/AUD.0b013e3181b1354f. [DOI] [PubMed] [Google Scholar]
- Brown CJ, Etler C, He S, O'Brien S, Erenberg S, et al. The electrically evoked auditory change complex: preliminary results from Nucleus cochlear implant users. Ear Hear. 2008;29:704–717. doi: 10.1097/AUD.0b013e31817a98af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss E, Hall JW, 3rd, Grose JH. Psychometric functions for pure tone intensity discrimination: slope differences in school-aged children and adults. J. Acoust. Soc. Am. 2009;125:1050–1058. doi: 10.1121/1.3050273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrijevic A, Michalewski HJ, Zeng FG, Pratt H, et al. Frequency changes in a continuous tone: auditory cortical potentials. Clin. Neurophysiol. 2008;119:2111–2124. doi: 10.1016/j.clinph.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrijevic A, Lolli B, Michalewski HJ, Pratt H, Zeng FG, et al. Intensity changes in a continuous tone: auditory cortical potentials comparison with frequency changes. Clin. Neurophysiol. 2009;120:374–383. doi: 10.1016/j.clinph.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic A, Starr A, Bhatt S, Michalewski HJ, Zeng FG, et al. Auditory cortical N100 in pre- and post-synaptic auditory neuropathy to frequency or intensity changes of continuous tones. Clin Neurophysiol. 2011;122:594–604. doi: 10.1016/j.clinph.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freyman RL, Nelson DA. Frequency discrimination of short-versus long-duration tones by normal and hearing-impaired listeners. J. Speech Hear.Res. 1987;30:28–36. doi: 10.1044/jshr.3001.28. [DOI] [PubMed] [Google Scholar]
- Friesen LM, Tremblay KL. Acoustic change complexes recorded in adult cochlear implant users. Ear Hear. 2006;27:678–685. doi: 10.1097/01.aud.0000240620.63453.c3. [DOI] [PubMed] [Google Scholar]
- Harris KC, Mills JH, Dubno JH. Electrophysiologic correlates of intensity discrimination in cortical evoked potentials of younger and older adults. Hear. Res. 2007;228:58–68. doi: 10.1016/j.heares.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KC, Mills JH, He NJ, Dubno JR. Age-related differences in sensitivity to small changes in frequency assessed with cortical evoked potentials. Hear. Res. 2008;243:47–56. doi: 10.1016/j.heares.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KC, Eckert MA, Ahlstrom JB, Dubno JR. Age-related differences in gap detection: effects of task difficulty and cognitive ability. Hear. Res. 2010;264:21–29. doi: 10.1016/j.heares.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, Grose JH, Buchman CA. Electrically-evoked cortical potentials and speech perception in children with auditory neuropathy.. Objective Measures in Auditory Implants-6th International Symposium; St. Louis, MO, USA.. September 22-25.2010. [Google Scholar]
- Hensch T, Herold U, Diers K, Armbruster D, Brocke B. Reliability of intensity dependence of auditory-evoked potentials. Clin. Neurophysiol. 2008;119:224–236. doi: 10.1016/j.clinph.2007.09.127. [DOI] [PubMed] [Google Scholar]
- Jones SJ, Longe O, Vaz Pato M. Auditory evoked potentials to abrupt pitch and timbre change of complex tone: electrophysiological evidence of ‘streaming’? Electroencephalogr Clin. Neurophysiol. 1998;108:131–142. doi: 10.1016/s0168-5597(97)00077-4. [DOI] [PubMed] [Google Scholar]
- Jones SJ, Perez N. The auditory ‘C-process’: analyzing the spectral envelope of complex sounds. Clin. Neurophysiol. 2001;112:965–975. doi: 10.1016/s1388-2457(01)00515-6. [DOI] [PubMed] [Google Scholar]
- Jones SJ, Perez N. The auditory ‘C-process’ of spectral profile analysis. Clin. Neurophysiol. 2002;113:1558–1565. doi: 10.1016/s1388-2457(02)00219-5. [DOI] [PubMed] [Google Scholar]
- Levitt H. Transformed up-down methods in psychoacoustics. J. Acoust. Soc. Am. 1971;49:467–477. [PubMed] [Google Scholar]
- Lister JJ, Maxfield ND, Pitt GJ. Cortical evoked response to gaps in noise: within- channel and across channel conditions. Ear Hear. 2007;28:862–878. doi: 10.1097/AUD.0b013e3181576cba. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BA, Boothroyd A. Cortical, auditory, event-related potentials in response to periodic and aperiodic stimuli with the same spectral envelope. Ear Hear. 1999;20:33–44. doi: 10.1097/00003446-199902000-00004. [DOI] [PubMed] [Google Scholar]
- Martin BA, Boothroyd A. Cortical, auditory, evoked potentials in response to changes of spectrum and amplitude. J. Acoust. Soc. Am. 2000;107:2155–2161. doi: 10.1121/1.428556. [DOI] [PubMed] [Google Scholar]
- Martin BA, Tremblay KL, Korczak P. Speech evoked potentials: from the laboratory to the clinic. Ear Hear. 2008;29:285–313. doi: 10.1097/AUD.0b013e3181662c0e. [DOI] [PubMed] [Google Scholar]
- Martin BA, Boothroyd A, Ali D, Leach-Berth T. Stimulus presentation strategies for eliciting the acoustic change complex: increasing efficiency. Ear Hear. 2010;31:356–366. doi: 10.1097/AUD.0b013e3181ce6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalewski HJ, Starr A, Nguyen TT, Kong YY, Zeng FG. Auditory temporal processes in normal-hearing individuals and in patients with auditory neuropathy. Clin. Neurophysiol. 2005;116:669–680. doi: 10.1016/j.clinph.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Ostroff JM, Martin BA, Boothroyd A. Cortical evoked response to acoustic change within a syllable. Ear Hear. 1998;19:290–297. doi: 10.1097/00003446-199808000-00004. [DOI] [PubMed] [Google Scholar]
- Pratt H, Bleich N, Mittelman N. The composite N1 component to gaps in noise. Clin. Neurophysiol. 2005;116:2648–2623. doi: 10.1016/j.clinph.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Pratt H, Starr A, Michalewski HJ, Dimitrijevic A, Bleich N, et al. The N1 complex to gaps in noise: effects of preceding noise duration and intensity. Clin. Neurophysiol. 2007;118:1078–1087. doi: 10.1016/j.clinph.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Tremblay KL, Shahin AJ, Pitcon T, Ross B. Auditory training alters the physiological detection of stimulus-specific cues in humans. Clin. Neurophysiol. 2009;120:128–135. doi: 10.1016/j.clinph.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wier CC, Jesteadt W, Green DM. Frequency discrimination as a function of frequency and sensation level. J. Acoust. Soc. Am. 1977;61:178–184. doi: 10.1121/1.381251. [DOI] [PubMed] [Google Scholar]
- Zeng FG, Oba S, Garde S, Sininger Y, Starr A. Temporal and speech processing deficits in auditory neuropathy. Neuroreport. 1999;10:3429–3435. doi: 10.1097/00001756-199911080-00031. [DOI] [PubMed] [Google Scholar]