Abstract
Purpose
The purpose of this study is to assess the efficacy and safety of everolimus in Korean patients with metastatic renal cell carcinoma (mRCC) for whom initial treatment with a vascular endothelial growth factor receptor-tyrosine kinase inhibitor (VEGFr-TKI) has failed.
Materials and Methods
Eligible patients with mRCC (any histology) who had progressed on or were intolerant of VEGFr-TKI therapy received oral everolimus (10 mg dose once daily). Tumor response was reassessed according to Response Evaluation Criteria in Solid Tumors (RECIST).
Results
This study included 100 patientswith a median follow-up duration of 10.2 months, a median progression-free survival (PFS) of 4.2 months (95% confidence interval [CI], 3.4 to 5.0 months), and an overall survival of 10.1 months (95% CI, 6.9 to 13.3 months). The most common grade 3 or greater adverse events (AEs) overall were anemia (13%), pneumonitis (9%), hyperglycemia (8%), and stomatitis (6%). While the incidence of pneumonitis was similar (26 cases, 26%) to the reported incidence in Western patients, the Korean presentations were more severe: 10 patients permanently discontinued everolimus due to pneumonitis, including two deaths on treatment. Statistically significant relationships were established between biologic toxicities, hyperglycemia and anemia, and PFS (hyperglycemia vs. non-hyperglycemia: hazard ratio [HR], 0.61; p=0.055 and anemia vs. non-anemia: HR, 0.51; p=0.021).
Conclusion
Everolimus was effective in Korean patients with mRCC who had failed initial VEGFr-TKI therapy. While everolimus was well tolerated in general and the AE incidence of this study was similar to those of previous reports, severe pneumonitis was common. Hyperglycemia and anemia showed significant correlation with PFS and thus may be potentially useful as prognostic indicators.
Keywords: Renal cell carcinoma, Everolimus, Treatment outcome, Safety
Introduction
Treatment of metastatic renal cell cancer (mRCC) has changed significantly over the past few years following the introduction of targeted agents that inhibit elements of the vascular endothelial growth factor (VEGF) and mammalian target of rapamycin (mTOR) pathways [1-7]. Based on highest level of evidence, current practice guidelines recommend sunitinib, pazopanib, and bevacizumab plus interferon alpha as first-line therapies for mRCC patients who are of favorable or intermediate risk [2,5,7], and temsirolimus for mRCC patients who are of poor risk [3].
Despite the documented benefits of these newer agents, complete responses (CR) are rare, with most patients eventually becoming resistant to treatment after a median of 6-11 months [1,2,5-9]. Based on positive results from the phase III RECORD-1 and AXIS trials, clinical guidelines have recommended the use of everolimus, an mTOR inhibitor, or axitinib, a new vascular endothelial growth factor receptor-tyrosine kinase inhibitor (VEGFr-TKI), in mRCC cases refractory to VEGFr-TKIs [2,10]. Previously, our team demonstrated a comparable efficacy of sequential VEGFr-TKI versus mTOR inhibitor as a second-line therapy after an initial VEGFr-TKI failure [11]. In the study, the progression-free survival (PFS) was three months for both groups and overall survival (OS) was also similar at 10.6 and 8.2 months, respectively [11]. Similarly, a phase III INTORSECT trial comparing temsirolimus and sorafenib as a second-line treatment for mRCC after sunitinib failure showed that the PFS, the primary endpoint, did not differ significantly between treatment cohorts, while OS showed unexpected improvement in patients treated with sorafenib [12].
Due to drug availability and the reimbursement policies of the National Health Insurance program in Korea, the majority of patients receive everolimus as a second-line systemic therapy following a first-line VEGFr-TKI failure. However, few studies investigating the actual efficacy and safety of everolimus as a second-line targeted therapy for mRCC have been reported, particularly in patients with Asian ethnicity [13,14]. Thus, the aim of the current study is to assess the efficacy and safety of everolimus as a second-line targeted therapy in Korean mRCC patients who failed on VEGFr-TKI. Additional evaluations included an assessment of prognostic factors associated with disease progression and correlation analyses between everolimus-associated adverse events (AEs) and clinical efficacy.
Materials and Methods
1. Patient population
A database of mRCC cases who received VEGFr-TKI therapy at the Asan Medical Center from April 2006 was constructed. Of these, patients with an evaluable lesion who had been treated previously with VEGFr-TKI (sunitinib, sorafenib, or pazopanib) and who received everolimus sequentially due to intolerability or disease progression were enrolled in this study. Patients treated with two or more VEGFr-TKIs or who received immunotherapy (interleukin- 2, interferon) and/or chemotherapy were included. Patients with central nervous system metastases, a Karnofsky performance scale (KPS) score of 50-60, and an uncontrolled medical condition (such as heart failure or diabetes) were also included in the analysis. Patients with a baseline KPS ≤ 40 and prior treatment with another mTOR inhibitor (e.g., temsirolimus) were excluded from analysis. Patients who were lost to follow up during the first two weeks without evidence of disease progression were also excluded.
2. Treatment and data collection
Everolimus at a dose of 10 mg was administered orally once daily. Dose delays and dose modifications were performed in accordance with the everolimus package insert. Comprehensive clinical, laboratory, and radiologic data were collected and reviewed in order to optimize the accuracy and completeness of the patient information in the database following the initiation of everolimus treatment. Patients were primarily followed through outpatient clinic appointments every 2-4 weeks. Physical examination, laboratory testing, and AEs assessment were performed at each visit. The tumor response was assessed every 6-8 weeks during follow-up. Responses were independently reassessed by two investigators (K.P. and L.J.L) using Response Evaluation Criteria in Solid Tumors (RECIST) ver. 1.1 [15]. AE severity was categorized according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) ver. 4.0 [16]. When AEs were present at baseline, the change in AE grade from baseline to follow-up was used to assess the safety of everolimus. This study was approved by the Institutional Review Board of Asan Medical Center, which waived the requirement for patient informed consent due to its retrospective design.
3. Statistical analysis
Summary characteristics and descriptive statistics of the study population are reported as proportions and medians. PFS was defined as the time from everolimus initiation until either first documentation of RECIST-defined disease progression or death due to any cause, whichever came first. OS was defined as the time from everolimus initiation until death from any cause. Kaplan-Meier estimates were used for analysis of the PFS and OS values. Univariate and multivariate analyses using Cox proportional hazards regression were performed for determination of clinical prognostic factors associated with the PFS and/or OS.
A primary multivariate model was developed and analyzed, which first incorporated basic clinical factors. Previous VEGFr-TKI-related factors (i.e., response to and treatment duration of initial VEGFr-TKI, number of previous VEGFr-TKIs) and everolimus-associated AE development were then reanalyzed individually in the primary multivariate model. All statistical analyses were two-sided and were performed using SPSS ver. 19.0 (SPSS Inc., Chicago, IL); p < 0.05 were accepted as statistically significant.
Results
1. Baseline characteristics
Among the pooled database of 376 patients with mRCC who were treated with a targeted agent, 113 consecutive patients received everolimus. Of these 113 patients, 8 cases were excluded from this study because they had received everolimus as a first-line therapy. Other exclusions included two patients with a KPS of ≤ 40, three patients who were lost to follow up within the first two weeks without documented evidence of disease progression, and one patient did not have an evaluable lesion. In total, 100 patients were included in these analyses.
Patient baseline characteristics are shown in Table 1. The median patient age was 58.1 years (range, 29 to 83 years) and 84% of the subjects had undergone a prior nephrectomy. Of the 95 patients who could be assessed using all topics covered under Heng’s criteria, eight patients had a favorable risk, 64 had intermediate risk, and 23 were at high risk [17]. The initial VEGFr-TKI was sunitinib (n=73), sorafenib (n=17), or pazopanib (n=10). Eighty-one patients (81%) had received one prior VEGFr-TKI therapy, while the remaining 19 patients had undergone prior treatment with two different VEGFr-TKIs. Twenty patients (20%) had previously received an immunotherapy for mRCC. The median treatment duration of initial VEGFr-TKI was 9.2 months (range, 0.9 to 61.7 months) and 89 patients progressed during their VEGFr-TKI treatment. The remaining 11 patients discontinued VEGFr-TKI treatment mainly due to drug intolerance and nine of 11 patients experienced disease progression within six months of their last VEGFr-TKI dose.
Table 1.
Characteristics of Korean mRCC patients
| Characteristic | No. (%) |
|---|---|
| Median age (range, yr) | 58.1 (29-83) |
| Gender | |
| Male | 80 (80) |
| Female | 20 (20) |
| Tumor histology | |
| Clear cell type | 86 (86) |
| Non-clear cell type | 14 (14) |
| Any sarcomatoid features (n=84, nephrectomy cases) | |
| Yes | 10 (10) |
| No | 74 (74) |
| Initial VEGFr-TKI therapy | |
| Sunitinib | 73 (73) |
| Sorafenib | 17 (17) |
| Pazopanib | 10 (10) |
| No. of previous VEGFr-TKI therapies | |
| 1 | 81 (81) |
| ≥ 2 | 19 (19) |
| KPS | |
| 100-90 | 22 (2) |
| 80-70 | 74 (74) |
| 60-50 | 4 (4) |
| Previous nephrectomy | |
| Yes | 84 (84) |
| No | 16 (16) |
| Previous immunotherapy | |
| Yes | 20 (20) |
| No | 80 (80) |
| No. of metastatic sites | |
| ≤ 1 | 16 (16) |
| ≥ 2 | 84 (84) |
| Heng’s criteriaa) | |
| Favorable | 8 (8) |
| Intermediate | 64 (64) |
| Poor | 23 (23) |
| Not applicable | 5 (5) |
mRCC, metastatic renal cell carcinoma; VEGFr-TKI, vascular endothelial growth factorreceptor-tyrosine kinase inhibitor; KPS, Karnofsky performance scale.
Heng’s prognostic criteria: low KPS score < 80, low hemoglobin (< lower limit of normal), high corrected serum calcium concentration (> upperlimit of normal), high platelet count (> upper limit of normal), high neutrophil count (> upper limit of normal), time from diagnosis to treatment < 1 year. Categories are defined as favorable=0, intermediate=1-2, or poor=3-6 risk factors.
2. Treatment administration and safety
At the time of analysis (October 2012), 16 patients were being treated with everolimus and the remaining 84 patients had been taken off this treatment, with a median treatment duration of 4.0 months (range, 0.5 to 30.9 months). Reasons for treatment discontinuation included disease progression (n=63), everolimus-associated AEs (n=12), and other reasons (n=9).
The AEs most frequently associated with everolimus were anemia (80%), hypercholesterolemia (62%), hyperglycemia (56%), asthenia (47%), stomatitis (44%), and pneumonitis (26%). The most commonly reported grade 3/4 AEs were anemia (13%), pneumonitis (9%), hyperglycemia (8%), and stomatitis (6%) (Table 2). In total, 11% of everolimus-treated patients required at least one dose reduction. Among the 12 patients who discontinued everolimus due to treatment-related AEs, 10 patients were diagnosed with pneumonitis (two cases were grade 5), one with bacterial pneumonia and one with stomatitis. Of 84 patients who discontinued everolimus, 50 patients (60%) could not receive subsequent alternative therapy after discontinuation.
Table 2.
Adverse events for everolimus in mRCCa)
| AEs | All grades | ≥ Grade 3 |
|---|---|---|
| Non-hematologic | ||
| Diarrhea | 11 (12) | 0 |
| Asthenia | 45 (47) | 0 |
| Nausea | 23 (24) | 0 |
| Vomiting | 6 (6) | 0 |
| Stomatitis | 42 (44) | 6 (6) |
| Rash | 30 (32) | 1 |
| Peripheral edema | 7 (7) | 1 |
| Pain | 23 (24) | 1 |
| Hemorrhage | 13 (14) | 1 |
| Pneumonitis | 26 (27) | 9 (9) |
| Infection (pneumonia) | 7 (7) | 2 (2) |
| Hematologic | ||
| Hemoglobin decreased | 76 (80) | 12 (13) |
| Neutrophil decreased | 15 (16) | 0 |
| Thrombocytopenia | 33 (35) | 3 (3) |
| Lymphocytopenia | 27 (28) | 3 (3) |
| Laboratory values | ||
| Glucose increased | 53 (56) | 8 (8) |
| Cholesterol increased | 58 (61) | 1 (1) |
| Creatinine increased | 17 (18) | 1 (1) |
| AST increased | 39 (41) | 1 (1) |
| ALT increased | 24 (25) | 0 |
| Bilirubin increased | 3 (3) | 0 |
Values are presented as number (%). mRCC, metastatic renal cell carcinoma; AST, aspartate aminotransferase; ALT, alanine aminotransferase.
The safety population (95 cases) included patients who received safety assessment of one or more following the initiation of everolimus therapy.
3. Efficacy
Of the 99 patients with measurable lesions, there were no CR. Three patients (3%) achieved a confirmed response, 68 (68%) had stable disease, and 25 (25%) had progressive disease (PD). The remaining patient with a non-measurable lesion was categorized as non-CR/non-PD. Thus, the calculated disease control rate (DCR) was 72% (Table 3). With a median follow-up duration of 10.2 months (95% confidence interval [CI], 7.9 to 12.7 months), the median PFS on everolimus was 4.2 months (95 % CI, 3.4 to 5.0 months) and the median OS was 10.1 months (95 % CI, 6.9 to 13.3 months) (Fig. 1).
Table 3.
Response to everolimus in Korean mRCC patients for whom VEGFr-TKI treatment failed
| Response | No. (%) |
| Complete response | 0 |
| Partial response | 3 (3) |
| Stable disease | 69 (69) |
| Progressive disease | 25 (25) |
| Not applicable | 3 (3) |
mRCC, metastatic renal cell carcinoma; VEGFr-TKI, vascular endothelial growth factor-tyrosine kinase inhibitor.
Fig. 1.
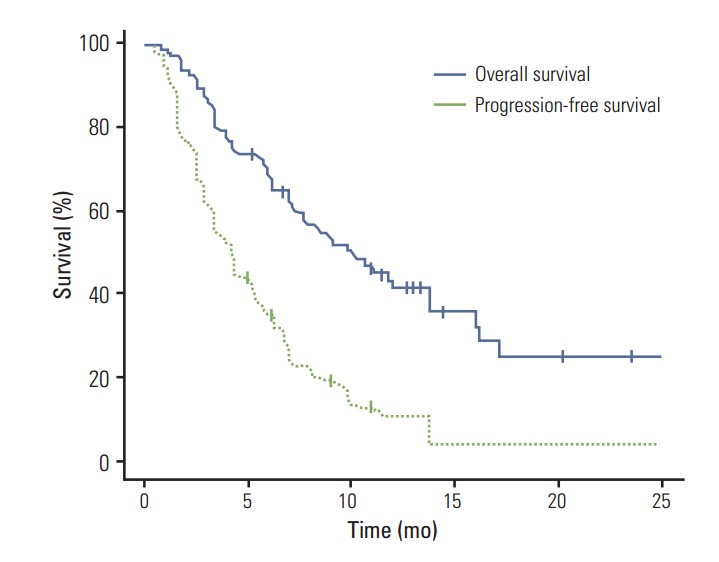
Progression-free survival and overall survival in metastatic renal cell carcinoma patients treated with everolimus.
4. Pretreatment factors showed correlation with everolimus efficacy
Results of univariate analysis showed significant association of young age (< 60 years, p=0.018) and a short time from initial diagnosis to first-line VEGFr-TKI treatment (< 1 year, p=0.003) with a poor PFS. The number of metastatic sites (n ≥ 2, p=0.063), lactate dehydrogenase level (> upper limit of normal, p=0.106), and Heng’s criteria (favorable vs. poor, p=0.080; intermediate vs. poor, p=0.007) were found to show marginal correlation with the PFS. In multivariate analysis, with the exception of patient age, no pretreatment factors showed independent association with the PFS (Table 4). Regarding OS, results of univariate analysis showed significant association of a short time from initial diagnosis to first-line VEGFr-TKI treatment (< 1 year, p=0.015) and an increased number of metastatic lesions (≥ 2, p=0.009) with poor OS. In addition, KPS (≤ 70, p=0.029), serum-corrected calcium level (> upper limit of normal, p=0.003), and Heng’s criteria (favorable vs. poor, p=0.017; intermediate vs. poor, p < 0.001) also showed statistically significant associations. Most of the significant factors from the univariate analysis also remained significant in the multivariate analyses. Heng’s criteria could be used in prediction of the OS in multivariate analysis (favorable vs. poor: HR, 0.09; p=0.014 and intermediate vs. poor: HR, 0.40; p=0.005) (Table 4).
Table 4.
Multivariate analysis of prognostic factors predictive of progression-free survival and overall survival in Korean RCC cases (primary multivariate model)a)
| Variable | Progression-free survival |
Overall survival |
||
| Hazard ratio | p-value | Hazard ratio | p-value | |
| Age (≥ 60 yr vs. < 60 yr) | 0.57 | 0.033 | 0.58 | 0.098 |
| Gender (male vs. female) | 0.83 | 0.534 | 1.01 | 0.972 |
| Histology (non-clear vs. clear) | 1.29 | 0.479 | 0.85 | 0.699 |
| Prior nephrectomy (no vs. yes) | 1.46 | 0.217 | 1.53 | 0.251 |
| No. of metastatic sites (≥ 2 vs. ≤ 1) | 1.79 | 0.134 | 2.81 | 0.037 |
| Type of first VEGFr-TKI | ||||
| (sorafenib vs. non-sorafenib) | 0.76 | 0.473 | 1.86 | 0.162 |
| Heng's criteriab) | ||||
| (favorable vs. poor) | 0.46 | 0.164 | 0.12 | 0.014 |
| (intermediate vs. poor) | 0.55 | 0.023 | 0.39 | 0.003 |
RCC, renal cell carcinoma; VEGFr-TKI, vascular endothelial growth factor-tyrosine kinase inhibitor.
Primary multivariate model analysis was performed for evaluation of basic clinical factors, excluding previous VEGFr-TKI-related factors and development of everolimus-associated adverse event,
Heng’s prognostic criteria: low Karnofsky performance scale (KPS) score < 80, low hemoglobin (< lower limit of normal), high corrected serum calcium concentration (> upper limit of normal), high platelet count (> upperlimit of normal), high neutrophil count (> upperlimit of normal), time from diagnosis to treatment < 1 year. Categories are defined as favorable=0, intermediate=1-2, or poor=3-6 risk factors.
5. Efficacy of everolimus with prior TKI therapy
Clinical outcomes (PFS and OS) for patients treated with everolimus were also analyzed according to response to initial VEGFr-TKI, duration of initial VEGFr-TKI treatment, and number of VEGFr-TKIs used in prior therapy. In univariate analyses, primary refractory disease and a short initial VEGFr-TKI treatment duration (< 6 months, early resistance) showed significant correlation with poor prognosis, which was assessed by both PFS and OS (data not shown). When the univariate analyses excluded patients with primary refractory disease (n=24), short treatment duration was found to show significant correlation with a poor PFS and OS (< 6-month vs. > 6-month PFS: HR, 2.80; p=0.001 and < 6-month vs. > 6-month OS: HR, 2.75; p=0.008). However, number of previously used VEGFr-TKIs did not show correlation with clinical outcomes following everolimus therapy as assessed by both PFS and OS (data not shown).
When the factors related to previous VEGFr-TKI treatment were applied to the aforementioned primary multivariate analysis, a primary refractory response to initial VEGFr-TKI treatment showed significant correlation with poor prognosis as measured by both PFS and OS (PFS in the primary refractory group vs. disease control group: HR, 3.17; p=0.002 and for OS: HR, 4.75; p<0.001). A short duration of initial VEGFr-TKI treatment showed correlation with a poor PFS and OS in the primary multivariate analysis patients if primary refractory disease was excluded from the model (< 6-month vs. > 6-month PFS: HR, 2.62; p=0.028 and for OS: HR, 2.92; p=0.043).
6. Efficacy of everolimus based on AEs
To investigate the relationship between everolimus-associated AEs and antitumor efficacy, we evaluated possible associations between AEs (particularly frequent AEs such as anemia, lymphocytopenia, hyperglycemia, hypercholesterolemia, stomatitis, and pneumonitis) with PFS. Only development of hyperglycemia during everolimus treatment showed significant correlation with a favorable PFS (HR, 0.57; p=0.025), while anemia was only marginally significant (HR, 0.65; p=0.101) (Table 5). When these AEs were applied to the primary multivariate analysis, hyperglycemia showed a marginally significant correlation with a favorable PFS (hyperglycemia group vs. non-hyperglycemia group: HR, 0.58; p=0.056) and anemia showed significant correlation with an improved PFS (anemia group vs. non-anemia group: HR, 0.51; p=0.021).
Table 5.
Univariate analysis of progression-free survival based on adverse event developmenta)
| Variable | Hazard ratio | 95% CI | p-value |
| Anemia (yes vs. none) | 0.63 | 0.38-1.08 | 0.095 |
| Lymphocytopenia (yes vs. none) | 1.01 | 0.62-1.64 | 0.972 |
| Hypercholesterolemia (yes vs. none) | 0.64 | 0.40-1.03 | 0.067 |
| Hyperglycemia (yes vs. none) | 0.60 | 0.37-0.97 | 0.038 |
| Stomatitis (yes vs. none) | 1.08 | 0.68-1.70 | 0.752 |
| Pneumonitis (yes vs. none) | 1.03 | 0.63-1.69 | 0.911 |
CI, confidence interval.
The safety population (95 cases) included patients who received safety assessment of one or more following the initiation of everolimus therapy.
Discussion
The current study findings demonstrate the efficacy and safety of everolimus in Korean mRCC patients for whom a previous VEGFr-TKI therapy had failed. We also evaluated putative prognostic factors associated with favorable treatment outcomes. Our results are generally in line with those reported from the RECORD-1 phase 3 trial and from retrospective studies, however, the OS and safety features from the current data differ from those of previous reports [4,11,14]. Of particular interest, our findings indicate correlation of everolimus-associated anemia and hyperglycemia with improved clinical outcomes in mRCC, with significant benefits in terms of PFS. This result suggests that particular AEs may be potential biomarkers of everolimus treatment efficacy in mRCC.
Our current analyses also show PFS and OS outcomes of everolimus therapy at 4.2 and 9.1 months, respectively, and a DCR of 72%. Our PFS and DCR findings are similar to those reported for the phase 3 RECORD-1 trial, in which the activity of everolimus showed a median PFS of 4.9 months and a DCR of 65%, and of the REACT study, in which the activity of everolimus showed a DCR of 53% [14,18]. However, in our study, the OS outcome was poorer than that in earlier reports. This is likely because we included patients with brain metastases and a poor performance status, whereas previous reports have been of clinical trials with strict inclusion criteria. In addition, 59% of our patients did not receive any subsequent anti-tumor therapy after the discontinuation of everolimus. Some retrospective or subgroup analyses for evaluation of patients who had been previously treated with sunitinib and an mTOR inhibitor have shown that sorafenib or sunitinib retrials as third-line targeted therapies yielded a PFS range of 4-8 months and an OS range of 6-8.4 months [19,20]. Considering the substantial efficacy of third-line targeted therapies, the small proportion of patients who were able to receive such treatments would negatively impact the OS outcome.
Treatment responses and the duration of a previous VEGFr-TKI were found to show correlation with everolimus clinical outcomes. Specifically, patients who showed a poor response (primary refractory or short treatment duration) to initial VEGFr-TKI therapy also showed poor clinical outcomes with everolimus, even though this agent targets a different cellular pathway. This finding is consistent with those of a previous study, which demonstrated generally poor responses to second-line therapy, regardless of whether a VEGFr-TKI or an mTOR inhibitor was administered to patients with primary refractory disease [21]. As a possible explanation for these results, treatment resistance may be driven largely by pathways independent of VEGF and/or mTOR in cases of poor response to initial VEGFr-TKI therapy.
Heng’s criteria, which is the prognostic model of the International Metastatic Renal Cell Carcinoma Database Consortium for patients who receive first-line VEGF pathway inhibitors, has also proven useful for prediction of OS in mRCC patients who received everolimus after VEGFr-TKI failure [17]. However, in the current analyses, the hematologic components of Heng's criteria, including the hemoglobin level, neutrophil count, and platelet count, were found not to be significant predictors of either PFS or OS. In contrast, we found that KPS, time from initial diagnosis to first-line VEGFr-TKI treatment, and the corrected calcium level showed significant correlation with OS. These results differ from those of the phase III RECORD-1 trial, which reported significant association of both the hemoglobin level and neutrophil count with OS [18]. However, our study was conducted in a real practice setting administering second-line everolimus without a substantial time interval and is therefore unlikely to be comparable with other clinical trials that incorporated a time interval between a first-line TKI failure and subsequent treatment. Hence, pretreatment values, particularly hematologic values affected by a previous VEGFr-TKI therapy, may not accurately reflect the efficacy of subsequent treatments.
In the current study, the everolimus AE profile was generally similar to that reported from previous phase III studies [14,18]. Asthenia, stomatitis, anemia, hyperglycemia, and hypercholesterolemia were the most common AEs, whereas severe AEs of grade 3 or 4 were overall rare. Of note, our study included 26 cases of pneumonitis (26%), among whom 10 patients permanently discontinued everolimus therapy and two patients died due to pneumonitis. Our results thus indicate that both the proportion and the severity of pneumonitis was higher than previously reported from large studies such as RECORD-1 and REACT, which reported pneumonitis incidence of 14% and 6%, respectively [14,18]. However, White et al. [22], who reported a 14% incidence of clinical pneumonitis, found a 39% incidence in patients receiving everolimus for mRCC via a careful chest imaging re-analysis of RECORD-1. In another retrospective study, Dabydeen et al. [19] reported a 30% incidence of pneumonitis after mTOR inhibitor therapy as assessed by independent radiologic review. As diagnosis of pneumonitis is based on a constellation of radiologic image findings in conjunction with clinical symptoms, and because differentiation of pneumonitis from infectious pneumonia or progression of a lung metastasis is not easy, its incidence may vary between studies. Some AEs, which were easily detected using standard laboratory evaluations such as those for anemia, hyperglycemia, or hypercholesterolemia, have reported incidences similar to those described in other large clinical trials. Therefore, our current results suggest that Korean patients with mRCC might show an AE incidence similar to that of Western patients, however, pneumonitis can cause more serious consequences for these cases, such as drug interruption or death.
We analyzed everolimus efficacy in relation to associated AEs. With the exception of stomatitis, patients with AEs tended to experience a more favorable PFS compared to subgroups without AEs. Of these AEs, hyperglycemia and anemia showed correlation with a favorable PFS, similar to the identification of hypertension as a biomarker for VEGFr-TKI and rash as a biomarker for EGFR-TKI [21,23,24]. These findings support the hypothesis that everolimus-associated AEs may indicate a favorable prognosis after everolimus treatment in this patient population. Dabydeen et al. [19] reported that patients with pneumonitis show a more favorable prognosis than those without pneumonitis in receiving an mTOR inhibitor. However, according to our current results, development of pneumonitis did not show correlation with a favorable prognosis. Further investigations are thus needed in order to confirm which AEs are specifically associated with everolimus and to perform time dependent analysis to address the possibility that patients treated with everolimus for an extended period of time may experience additional AEs. In consideration of the favorable prognostic trends of these everolimus-associated AEs, early recognition and prompt intervention for severe AEs may help patients to achieve uninterrupted full-dose therapy with everolimus, and therefore further opportunities for improvement of clinical outcomes.
Some limitations of our current study included the use of data from a single center, its retrospective nature with possible selection bias, and some missing data. However, our current data are the first of their kind from a Korean population and are based on a larger sample size than any previous reports from Asia. In addition, unlike the populations in clinical trials, which are stringently selected and are based on effectiveness, our current study included patients who had brain metastases or a poor performance status. Thus, our current study reflects a real-world experience with everolimus and has relevance in daily clinical practice.
Conclusion
Everolimus is an effective treatment in Korean mRCC patients for whom initial VEGFr-TKI therapy has failed. AEs were present in our Korean patients at a frequency comparable to those reported from Western countries, while they tended to be more severe and to lead to permanent discontinuation of the drug, particularly in cases of pneumonitis. AEs such as hyperglycemia and anemia correlate with a favorable prognosis and may represent potential biomarkers of everolimus efficacy in patients with mRCC.
Acknowledgments
This study was supported in part by a grant (no.HI12C17880300) from the Korean Health Technology R&D Project, Ministry of Health and Welfare, and a grant (no. 2013K000281) from the Converging Research Center Program of the Ministry of Science, ICT, and Future Planning, Republic of Korea.
Footnotes
Conflict of interest relevant to this article was not reported.
References
- 1.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–34. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 2.Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370:2103–11. doi: 10.1016/S0140-6736(07)61904-7. [DOI] [PubMed] [Google Scholar]
- 3.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–81. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 4.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–56. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
- 5.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356:115–24. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 6.Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Ou SS, et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol. 2008;26:5422–8. doi: 10.1200/JCO.2008.16.9847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–8. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 8.Patel PH, Senico PL, Curiel RE, Motzer RJ. Phase I study combining treatment with temsirolimus and sunitinib malate in patients with advanced renal cell carcinoma. Clin Genitourin Cancer. 2009;7:24–7. doi: 10.3816/CGC.2009.n.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feldman DR, Baum MS, Ginsberg MS, Hassoun H, Flombaum CD, Velasco S, et al. Phase I trial of bevacizumab plus escalated doses of sunitinib in patients with metastatic renal cell carcinoma. J Clin Oncol. 2009;27:1432–9. doi: 10.1200/JCO.2008.19.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rini BI, Escudier B, Tomczak P, Kaprin A, Szczylik C, Hutson TE, et al. Comparative effectiveness of axitinib versus so-rafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378:1931–9. doi: 10.1016/S0140-6736(11)61613-9. [DOI] [PubMed] [Google Scholar]
- 11.Park K, Lee JL, Park I, Park S, Ahn Y, Ahn JH, et al. Comparative efficacy of vascular endothelial growth factor (VEGF) tyrosine kinase inhibitor (TKI) and mammalian target of rapamycin (mTOR) inhibitor as second-line therapy in patients with metastatic renal cell carcinoma after the failure of first-line VEGF TKI. Med Oncol. 2012;29:3291–7. doi: 10.1007/s12032-012-0227-7. [DOI] [PubMed] [Google Scholar]
- 12.Hutson TE, Escudier B, Esteban E, Bjarnason GA, Lim HY, Pittman K, et al. Temsirolimus versus sorafenib as second line therapy in metastatic renal cell carcinoma: results from the INTORSECT trial. Ann Oncol. 2012;23(Suppl 9):Absr LBA22. doi: 10.1200/JCO.2013.50.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsukamoto T, Shinohara N, Tsuchiya N, Hamamoto Y, Maruoka M, Fujimoto H, et al. Phase III trial of everolimus in metastatic renal cell carcinoma: subgroup analysis of Japanese patients from RECORD-1. Jpn J Clin Oncol. 2011;41:17–24. doi: 10.1093/jjco/hyq166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grunwald V, Karakiewicz PI, Bavbek SE, Miller K, Machiels JP, Lee SH, et al. An international expanded-access programme of everolimus: addressing safety and efficacy in patients with metastatic renal cell carcinoma who progress after initial vascular endothelial growth factor receptor-tyrosine kinase inhibitor therapy. Eur J Cancer. 2012;48:324–32. doi: 10.1016/j.ejca.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 15.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–47. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 16.National Cancer Institute . Common terminology criteria for adverse events (CTCAE). Version 4.0. Bethesda: National Institutes of Health, US Department of Health and Human Services; 2009. [Google Scholar]
- 17.Heng DY, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5794–9. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 18.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma : final results and analysis of prognostic factors. Cancer. 2010;116:4256–65. doi: 10.1002/cncr.25219. [DOI] [PubMed] [Google Scholar]
- 19.Dabydeen DA, Jagannathan JP, Ramaiya N, Krajewski K, Schutz FA, Cho DC, et al. Pneumonitis associated with mTOR inhibitors therapy in patients with metastatic renal cell carcinoma: incidence, radiographic findings and correlation with clinical outcome. Eur J Cancer. 2012;48:1519–24. doi: 10.1016/j.ejca.2012.03.012. [DOI] [PubMed] [Google Scholar]
- 20.Zama IN, Hutson TE, Elson P, Cleary JM, Choueiri TK, Heng DY, et al. Sunitinib rechallenge in metastatic renal cell carcinoma patients. Cancer. 2010;116:5400–6. doi: 10.1002/cncr.25583. [DOI] [PubMed] [Google Scholar]
- 21.George S, Reichardt P, Lechner T, Li S, Cohen DP, Demetri GD. Hypertension as a potential biomarker of efficacy in patients with gastrointestinal stromal tumor treated with suni-tinib. Ann Oncol. 2012;23:3180–7. doi: 10.1093/annonc/mds179. [DOI] [PubMed] [Google Scholar]
- 22.White DA, Camus P, Endo M, Escudier B, Calvo E, Akaza H, et al. Noninfectious pneumonitis after everolimus therapy for advanced renal cell carcinoma. Am J Respir Crit Care Med. 2010;182:396–403. doi: 10.1164/rccm.200911-1720OC. [DOI] [PubMed] [Google Scholar]
- 23.Rini BI, Cohen DP, Lu DR, Chen I, Hariharan S, Gore ME, et al. Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib. J Natl Cancer Inst. 2011;103:763–73. doi: 10.1093/jnci/djr128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perez-Soler R, Chachoua A, Hammond LA, Rowinsky EK, Huberman M, Karp D, et al. Determinants of tumor response and survival with erlotinib in patients with non-small-cell lung cancer. J Clin Oncol. 2004;22:3238–47. doi: 10.1200/JCO.2004.11.057. [DOI] [PubMed] [Google Scholar]


