Abstract
The incidence of cancer increases with advanced age. Unfortunately, there is a significant lack of evidence regarding the safety and efficacy of treatments. The oncology community also lacks information regarding which older patients are most likely to benefit from treatment without undue toxicities. Interventions to lower symptoms and reduce long-term complications from cancer and cancer treatment in older patients are urgently needed. Establishing research priorities in geriatric oncology could help guide researchers and focus efforts on interventions that have the highest likelihood of improving outcomes. The Cancer and Aging Research Group, in partnership with the National Institute on Aging and National Cancer Institute, held linked conferences as part of a U13 grant in September of 2010 and November of 2012, summarising the gaps in knowledge in geriatric oncology and recommending ways to close these gaps. The overall purpose of this review is to highlight the important research priorities in geriatric oncology from the literature and from the previous U13 meetings. More evidence regarding the treatment of older cancer patients is urgently needed given the rapid aging of the population.
Approximately 60% of all cancers and 70% of cancer mortality occur in people aged 65 years and over.1 It is anticipated that 70% of all cancer diagnoses will occur in adults aged ≥ 65 by the year 2030.2 Because clinical trials include only a small proportion of older patients,3 we lack important and necessary information on efficacy and safety of therapeutic oncology treatments in patients who are older, with health status issues besides their cancer.
Older cancer patients often have other health status concerns. For example, in a group of older patients with newly diagnosed cancer from a population-based database, 15% had three or more significant comorbidities, disability and geriatric syndromes (figure 1). The Comprehensive Geriatric Assessment (CGA) is an evaluation tool utilised by geriatricians to assess an older patient’s health status. CGA includes an evaluation of functional status, comorbidity burden, cognition, social support system, nutrition and medication review. Studies of geriatric oncology patients reveal that measures within CGA can predict postoperative morbidity, toxicity of chemotherapy and mortality.4 Because of the perceived importance of the CGA in the geriatric oncology community, research has focused on defining the reliability and predictive value of the CGA in cancer patients. Although the oncology community has come a long way in recognising aging issues, there is much more that could be done to improve the outcomes of older cancer patients. Below, we summarise research priorities in geriatric oncology for 2013 and beyond.
Figure 1.
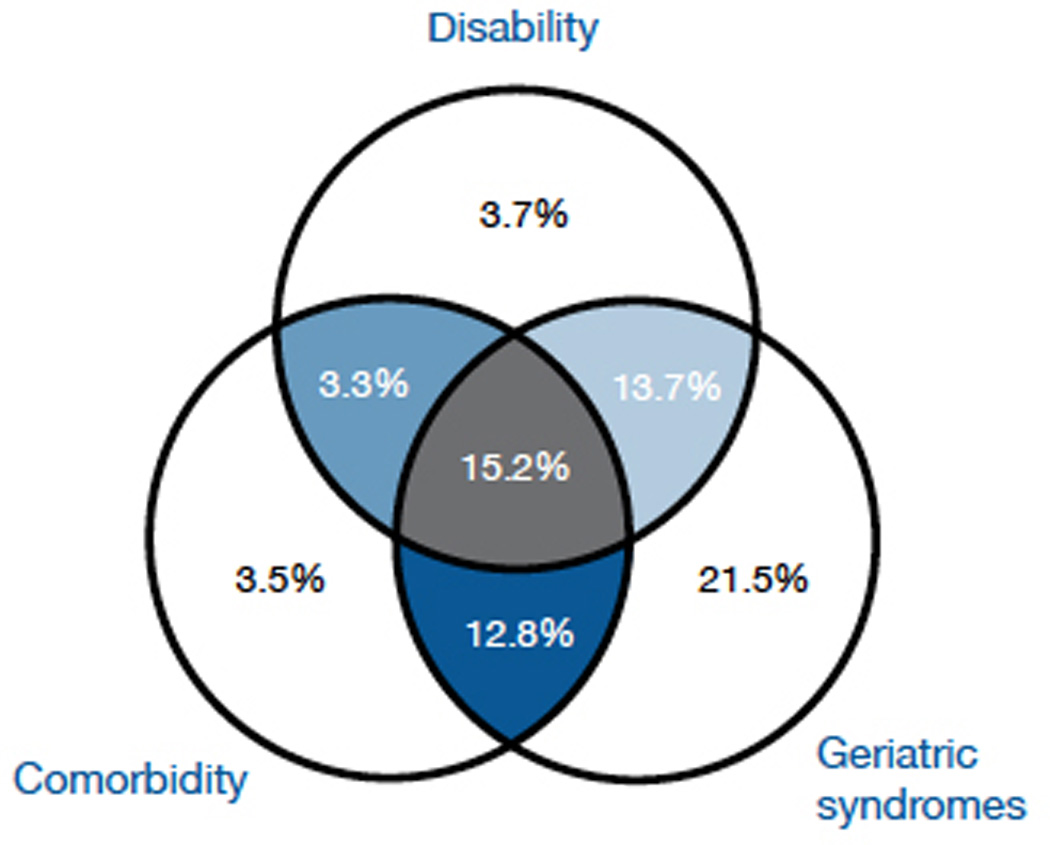
Disability, comorbidity, and geriatric syndromes among older, newly-diagnosed cancer patients.
Incorporate geriatric assessment tools into clinical trials that predict adverse outcomes for older adults with cancer
Currently, oncologists assess functional status by assigning a Karnofsky performance status (KPS) or Eastern Cooperative Oncology Group (ECOG) performance status score,5,6 generic scales which are a one-item numeric global assessment of functional status rated by the healthcare provider. They are applied to all adult cancer patients, regardless of age, and are used to estimate functional status in order to determine a treatment course, assess eligibility for clinical trials and predict treatment toxicity and survival.7,8 Although KPS and ECOG performance measures are commonly used and do correlate with treatment toxicity, these tools do not predict survival or outcomes as well as CGA in the elderly.9–11 For this reason, according to the National Cancer Comprehensive Network guidelines, CGA should be a key part of the treatment approach for older cancer patients.12
Key domains within the CGA are assessments of functional status and physical performance. Commonly utilised tools for evaluation of functional status in the geriatric population are evaluation of ADLs (Activities of Daily Living) and Instrumental (I)ADLs. ADLs are skills required for basic self-care,13 and IADLs include the ability to perform daily tasks required for independent living.14 The need for ADL and IADL assistance has been associated with poorer overall survival in geriatric oncology patients.15 Older cancer patients have a higher prevalence of ADL and IADL deficiencies when compared to age-matched controls.16 Physical performance measures objectively evaluate mobility and fall risk.17,18 Objective measures of physical performance include the Short Physical Performance Battery, gait speed, six-minute walk test, chair stands, isometric grip strength and the ‘Timed Get Up and Go’ test. These directly observed measures can supplement self-report measures of functional status.
Other domains in the CGA, such as an assessment of comorbidity, nutritional status and cognition, can also identify older cancer patients at risk for adverse outcomes. Among cancer patients, comorbidity is associated with poorer treatment tolerance and overall survival.19–23 Furthermore, patients with comorbid conditions often take several medications which may predispose patients to the risks of polypharmacy and drug-drug interactions.24 Poor nutritional status is associated with an increased need for functional assistance and poorer overall survival in the geriatric population.25 Unintentional weight loss during the six months prior to chemotherapy is associated with lower chemotherapy response rates and lower overall survival.26 High-risk patients can be identified through self-reported weight loss of >10% of body weight, calculation of Body Mass Index (BMI) with BMI < 20 associated with adverse outcomes, and/or the Mini-Nutritional Assessment (MNA). The MNA has been shown to be a sensitive and specific tool for identifying malnutrition in the elderly population.27 A cognitive assessment is needed to determine if the patient has the capacity to consent to treatment, to adhere to supportive care medication instructions and to understand the indications to seek attention. In the presence of cognitive impairment, the involvement of the patient’s family or caregiver is required to maintain safety.28–31 In both the geriatric and oncology literature, social isolation has been linked to an increased risk of mortality.32–34
Two studies have rigorously evaluated the role of CGA for predicting toxicity from chemotherapy. In a study led by Hurria and performed by the Cancer and Ageing Research Group (CARG) (n = 500), CGA variables were associated with grade 3–5 toxicity.35 A risk-stratification schema which scored patients from 0–23 was developed. Factors that were associated with risk included age ≥72, GI or GU cancer type, receiving full standard dose or more than one chemotherapy agent, the presence of anemia [<11G/dL (male), <10G/dL (female)] or renal insufficiency (creatinine clearance <34 ml/min) a recent fall, having hearing impairment, needing assistance with IADLs, limited in walking one block and having decreased social activity. A second study, led by Extermann, developed the Chemotherapy Risk Assessment Scale for High-Age Patients Score in over 500 patients.36 The best model for hematologic toxicity included IADL score, LDH level, diastolic blood pressure and chemotherapy intensity. The best predictive model for non-hematologic toxicity included performance status, Mini-Mental State Examination score, MNA score and chemotherapy intensity. Information from two-thirds of the patients was used to develop the risk stratification scheme, and the tool was validated in the remaining one-third of patients.
CGA can also help predict overall survival. One study performed by Kanesvaran et al. evaluated the impact of CGA domains on overall survival and developed a prognostic scoring system, including these elements for use by clinicians. This study included 249 patients of any cancer type, stage and functional status. The majority of patients had GI, GU or lung malignancies, and 84.7% had advanced-stage disease. Factors that were independently associated with overall survival included low albumin, EGOG PS ≥ 2, abnormal geriatric depression screen, advanced stage disease, malnutrition and advanced age. A nomogram to predict one-year, two-year and three-year overall survival for individual patients, that weights each of these independent variables, was created for use by clinicians.37 More recently, two studies evaluated the predictive value of geriatric assessment tools for survival. Giantin et al examined the value of the Multidimensional Prognostic Index (MPI) in predicting mortality in 160 patients with inoperable or metastatic solid tumour malignancy.38 The MPI was used to stratify individuals into three grades of mortality risk. By six months and 12 months, 34.4% and 46.9% of patients had died, respectively. In multivariable models, the MPI was able to predict six-month and 12-month mortality. Van der Geest et al. examined factors that predict mortality in patients undergoing chemotherapy for colorectal cancer.39 Patients aged 70 and over were enrolled (n=143), with the sample including those receiving adjuvant (38%) or palliative (62%) chemotherapy in a single comprehensive cancer centre in The Netherlands.39 Nutritional status (measured by the MNA) and frailty (Groningen Frailty Indicator) predicted mortality, but only in patients treated with palliative intent.
This research has shown that pre-treatment CGA variables can help identify older adults at increased risk of chemotherapy toxicity and help predict survival. However, we still need validation studies of several of these models for use for specific cancers and treatments. Incorporation of validated tools into clinical research, and potentially clinical care, can help identify which older patients are the most likely to tolerate and benefit from treatment. These tools can be utilised in future research to identify and test interventions to reduce chemotherapy toxicity and improve outcomes in vulnerable older populations.
Test the ability of a geriatric assessment model of care for improving outcomes of older cancer patients
There is a critical gap in knowledge regarding how to improve outcomes in older adults with cancer.40–42 Despite the fact that the majority of cancer patients are in older age groups, most oncologists have received little specific training in the care of older patients.43 As a result, common problems facing an aging population of cancer patients may go unrecognised and produce serious consequences.40,44 Although CGA may help predict risk from chemotherapy toxicity and survival in older cancer patients, there is no evidence-based approach regarding the use of specific interventions to reduce risk from cancer treatment. CGA-driven interventions were identified as an important area of research by geriatric oncology experts during the first U13 conference, and examples of interventions used within the University of Rochester and University of Chicago Specialised Oncology Care and Research in the Elderly clinics to address vulnerabilities in selected geriatric domains are listed in table 1.45
Table 1.
SOCARE Pilot Data on GA and GA-driven interventions
| GA domains in relationship to cancer and chemotherapy in older adults |
Examples of patient/ caregiver concern (from pilot work) |
Rating of importance 0–10 with 10=very important median range |
GA-driven interventions | Proportion | |
|---|---|---|---|---|---|
| Cancer treatment recommendations |
|
Patient | caregiver |
|
|
| 7 (4–10) | 8 (7–10) | ||||
| Functional abilities, physical performance and/or falls |
|
10 (8–10) | 8 (6–10) |
|
|
| Comorbidity polypharmacy |
|
7 (0–10) | 6 (0–10) |
|
|
| Cognition |
|
9 (1–10) | 8 (4–10) |
|
|
| Psychological status |
|
8 (0–10) | 8 (4–10) |
|
|
| Nutrition |
|
9 (6–10) | 9 (6–10) |
|
|
| Social support |
|
8 (1–10) | 7 (6–10) |
|
|
In community dwelling older adults, interventions guided by CGA improve health outcomes – including prevention of disability, reduction in the risk of falls, reduction in unplanned hospitalisations and decreased nursing home admissions – providing evidence supporting the use of a multidimensional approach in older patients.46–48 Several studies have shown that the implementation of CGA and CGA-driven interventions into the clinical care of older cancer patients is feasible.49–52 The Comprehensive Geriatric Assessment in the decision-making process in elderly patients with cancer: ELCAPA study illustrated that providing CGA information and geriatric assessment-driven interventions to oncology teams can influence treatment decisions, although outcomes from these changes were not measured.50 Another pilot study showed that CGA affected the oncology treatment plan.53 Unfortunately, there are few published randomised studies evaluating outcomes from CGA and CGA-driven interventions in older cancer patients. In a study by McCorkle et al,54 geriatric nurse practitioners conducted CGA with cancer patients, and this led to a survival advantage of 67% in the intervention group compared with 40% in the control group. In a study by Goodwin et al, breast cancer patients in the CGA-driven interventions group were significantly more likely to return to normal functioning than the controls.55
A conceptual model (figure 2) demonstrates how information from CGA can guide interventions and decision-making. CGA-driven interventions and/or changes in chemotherapy treatment decisions (eg. selection of regimen, dosing of chemotherapy, use of supportive care medications) could improve outcomes.
Figure 2.
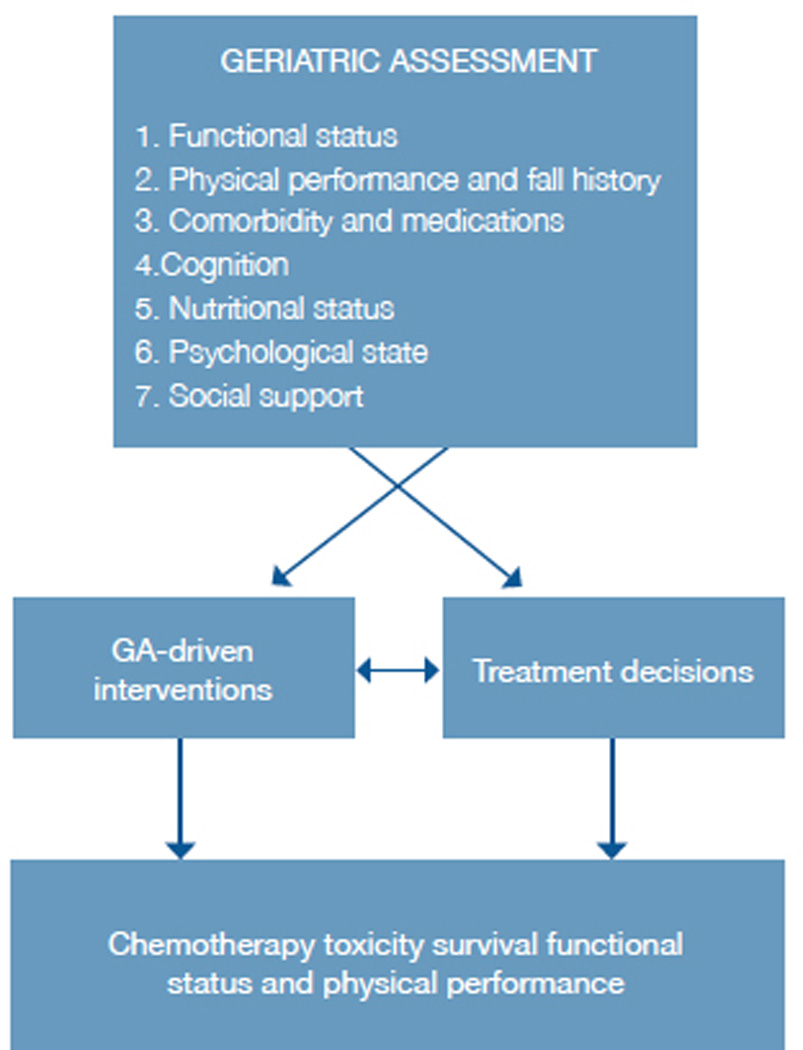
Conceptual model of geriatric assessment use in chemotherapy choices.
There is a great need for randomised studies to evaluate CGA and CGA-driven interventions for improving decisions for cancer treatment and for improving outcomes. At this stage, there is no consensus on how to best incorporate CGA-driven interventions into oncology care. Two studies are ongoing which will utilise expert opinion to develop a consensus of geriatric assessment and geriatric assessment-driven interventions in oncology. The next necessary step would be to test whether these approaches improve outcomes in randomised studies.
Understand the impact of oncology therapeutics in the general population of older cancer patients
Because the average age of patients enrolled on cancer clinical trials is lower than the average age of patients with the disease, and since older patients enrolled in clinical trials are generally healthier than most patients seen in practice, it is difficult to apply the results of clinical trials to patients in the general population.56–58 More data on the safety and efficacy of treatments in older patients are needed.
There are several possible reasons why older patients are under-represented in clinical trials. First, these trials often have stringent inclusion and exclusion criteria which would preclude their ability enrol, such as excluding patients with certain comorbidities, mild organ dysfunction, or a history of a past cancer, even though these issues are unlikely to affect outcomes. Second, the infrastructure - time or resources, required to safely enrol older patients in studies - is not usually built into the study protocols. Therefore, it is often very difficult for older patients to travel to a tertiary care centre frequently for repeated study visits and procedures. As a research community, different structures and novel approaches to data collection, such as telemedicine, should be considered to allow for the inclusion of an appropriate proportion of older patients. Third, the majority of older adults are treated in the community, not at academic medical centres. Therefore, enrolment in clinical trials should also be more widely available in community oncology practices, where the majority of older adults with cancer are treated. Community oncology practices need to be reimbursed for the extra time and resources required to enrol and retain older patients in trials. Fourth, there is often a concern for higher toxicity in older patients, which speaks to the need for trials specifically for older patients with safety parameters and endpoints of relevance. Because of difficulties with recruitment and enrolment of older adults, only 9% of patients enrolled in registration trials were 75 years or older in FDA-registration trials.44 This contrasts with the fact that approximately 30% of cancer patients are in this age range.
The oncology community needs to focus on developing trials where the results can be generalised to the population with the disease. The gap may only be able to be closed if multidisciplinary teams work together to design elderly-specific trials to include patients who are older and/or have other health status issues. All trials, especially trials studying therapeutics for cancers that occur commonly in older populations, should have a specific target accrual for patients aged 65 and over. These studies would provide data that are necessary for clinicians to utilise in daily practice.
Identify and test interventions to improve symptoms and maintain quality of life of older cancer patients
In addition to including these measures as part of the baseline evaluation, longitudinal inclusion of a CGA would further our understanding of the impact of both the cancer and its treatment on geriatric outcomes such as functional status and cognition. One large population database of mostly cancer survivors showed that cancer survivors were more likely to be vulnerable, have a disability, or to have geriatric syndromes than people without a history of cancer.59 This data suggests that cancer and/or cancer treatment could have long-term consequences on the quality of survivorship in an older patient. Endpoints should be included in clinical trials that evaluate impact of therapies on geriatric domains. This is especially important in curative intent trials, or trials for cancers with a long clinical history.
Another routine part of clinical trials is to evaluate the toxicity of the cancer therapy. Toxicity of chemotherapy is generally graded by the National Cancer Institute Common Terminology Criteria for Adverse Events.60 Grade 3 (severe or medically significant), 4 (life-threatening) or 5 (treatment-related mortality) toxicities are typically captured and reported in clinical trials and are considered to be ‘dose limiting.’ Grade 2 toxicities, such as diarrhoea or neuropathy, could also significantly affect quality of life in older patients and may also be ‘dose-limiting’ particularly in the geriatric population. Therefore, grade 2 toxicities should be captured. Trials should also report consequences of toxicities such as health care utilisation and changes in care. Hospitalisations, rehabilitation and transitions to a higher level of care, such as assisted living or nursing home, are important outcomes to capture so that these risks can be discussed with the patient during treatment decision-making.
There are some under-studied, but important long-term symptoms of cancer and cancer treatment that can affect quality of life and should be studied. Sarcopenia is the progressive generalised loss of skeletal muscle mass, strength and function. Cachexia has no uniform definition, and is a complex metabolic syndrome associated with cancer that is characterised by weight loss >10%, reduced food intake (<1500 kcal/d) and systemic inflammation (CRP >10mg/L).61 It is estimated that 50% of people older than 80 years have sarcopenia. Half of all cancer patients lose some body weight; one third lose > 5% body weight and up to 20% of all cancer deaths are directly linked to cachexia.61 To date, no clinically applied regimen has been completely successful in reversing cancer-associated muscle or weight loss. Interventions for these issues including cachexia and sarcopenia are needed to improve the quality of survivorship for the older patient with cancer. The third conference of the CARG-NIH U13 Grant, ‘Geriatric oncology research to improve clinical care,’ will address this research need by bringing a multidisciplinary group of researchers together to develop a research agenda focusing on interventions for improving the quality of survivorship of older and/or frail adults with cancer.
Conclusions
New priorities in geriatric oncology research focusing on the needs of older cancer patients are necessary to meet the needs of a rapidly aging population. Older patients, caregivers and health care providers would ultimately benefit from research that improves the evidence base for oncology care in older adults. Significant current gaps in knowledge ultimately lead to wide variation in patterns of care in the treatment of older adults with cancer, potentially increasing health care burdens and costs due to both over and under-treatment of older adults with cancer. Focusing efforts on geriatric oncology research would provide a better evidence base to inform decision-making, with the ultimate goal of improving the quality of care of older adults with cancer.
Acknowledgement
We would like to acknowledge the funding mechanism, U13 AG038151 (Geriatric Oncology Research to Improve Clinical Care).
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Pal SK, Hurria A. Impact of age, sex, and comorbidity on cancer therapy and disease progression. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2010;28:4086–4093. doi: 10.1200/JCO.2009.27.0579. [DOI] [PubMed] [Google Scholar]
- 3.Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Jr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341:2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 4.Extermann M, Hurria A. Comprehensive geriatric assessment for older patients with cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2007;25:1824–1831. doi: 10.1200/JCO.2007.10.6559. [DOI] [PubMed] [Google Scholar]
- 5.Karnofsky DA, Ellison RR, Golbey RB. Selection of patients for evaluation of chemotherapeutic procedures in advanced cancer. J Chronic Dis. 1962;15:243–249. doi: 10.1016/0021-9681(62)90006-1. [DOI] [PubMed] [Google Scholar]
- 6.Zubrod C, Schniderman M, Frei E. Appraisal of methods for the study of chemotherapy of cancer in man. Journal of Chronic Diseases. 1960:7–33. [Google Scholar]
- 7.Albain KS, Crowley JJ, Hutchins L, Gandara D, O'Bryan RM, Von Hoff DD, et al. Predictors of survival following relapse or progression of small cell lung cancer. Southwest Oncology Group Study 8605 report and analysis of recurrent disease data base. Cancer. 1993;72:1184–1191. doi: 10.1002/1097-0142(19930815)72:4<1184::aid-cncr2820720409>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 8.Albain KS, Crowley JJ, LeBlanc M, Livingston RB. Survival determinants in extensive-stage non-small-cell lung cancer: the Southwest Oncology Group experience. J Clin Oncol. 1991;9:1618–1626. doi: 10.1200/JCO.1991.9.9.1618. [DOI] [PubMed] [Google Scholar]
- 9.Extermann M, Overcash J, Lyman GH, Parr J, Balducci L. Comorbidity and functional status are independent in older cancer patients. J Clin Oncol. 1998;16:1582–1587. doi: 10.1200/JCO.1998.16.4.1582. [DOI] [PubMed] [Google Scholar]
- 10.Rockwood K, Stadnyk K, MacKnight C, McDowell I, Hebert R, Hogan DB. A brief clinical instrument to classify frailty in elderly people. Lancet. 1999;353:205–206. doi: 10.1016/S0140-6736(98)04402-X. [DOI] [PubMed] [Google Scholar]
- 11.Reuben DB, Rubenstein LV, Hirsch SH, Hays RD. Value of functional status as a predictor of mortality: results of a prospective study. Am J Med. 1992;93:663–669. doi: 10.1016/0002-9343(92)90200-u. [DOI] [PubMed] [Google Scholar]
- 12.Hurria A, Browner IS, Cohen HJ, Denlinger CS, deShazo M, Extermann M, et al. Senior adult oncology. Journal of the National Comprehensive Cancer Network : JNCCN. 2012;10:162–209. doi: 10.6004/jnccn.2012.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Katz S, Ford AB, Moskowitz RW, Jackson BA, Jaffe MW. Studies of Illness in the Aged. The Index of Adl: A Standardized Measure of Biological and Psychosocial Function. JAMA : the journal of the American Medical Association. 1963;185:914–919. doi: 10.1001/jama.1963.03060120024016. [DOI] [PubMed] [Google Scholar]
- 14.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. The Gerontologist. 1969;9:179–186. [PubMed] [Google Scholar]
- 15.Wedding U, Rohrig B, Klippstein A, Pientka L, Hoffken K. Age, severe comorbidity and functional impairment independently contribute to poor survival in cancer patients. J Cancer Res Clin Oncol. 2007;133:945–950. doi: 10.1007/s00432-007-0233-x. [DOI] [PubMed] [Google Scholar]
- 16.Patel KV, Peek MK, Wong R, Markides KS. Comorbidity and disability in elderly Mexican and Mexican American adults: findings from Mexico and the southwestern United States. Journal of aging and health. 2006;18:315–329. doi: 10.1177/0898264305285653. [DOI] [PubMed] [Google Scholar]
- 17.Guralnik JM, Simonsick EM, Ferrucci L, Glynn RJ, Berkman LF, Blazer DG, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. Journal of gerontology. 1994;49:M85–M94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 18.Bylow K, Hemmerich J, Mohile SG, Stadler WM, Sajid S, Dale W. Obese frailty, physical performance deficits, and falls in older men with biochemical recurrence of prostate cancer on androgen deprivation therapy: a case-control study. Urology. 2011;77:934–940. doi: 10.1016/j.urology.2010.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Extermann M, Balducci L, Lyman GH. What threshold for adjuvant therapy in older breast cancer patients? J Clin Oncol. 2000;18:1709–1717. doi: 10.1200/JCO.2000.18.8.1709. [DOI] [PubMed] [Google Scholar]
- 20.Frasci G, Lorusso V, Panza N, Comella P, Nicolella G, Bianco A, et al. Gemcitabine plus vinorelbine versus vinorelbine alone in elderly patients with advanced non-small-cell lung cancer. J Clin Oncol. 2000;18:2529–2536. doi: 10.1200/JCO.2000.18.13.2529. [DOI] [PubMed] [Google Scholar]
- 21.Firat S, Byhardt RW, Gore E. Comorbidity and Karnofksy performance score are independent prognostic factors in stage III non-small-cell lung cancer: an institutional analysis of patients treated on four RTOG studies. Radiation Therapy Oncology Group. Int J Radiat Oncol Biol Phys. 2002;54:357–364. doi: 10.1016/s0360-3016(02)02939-5. [DOI] [PubMed] [Google Scholar]
- 22.Piccirillo JF, Tierney RM, Costas I, Grove L, Spitznagel EL., Jr Prognostic importance of comorbidity in a hospital-based cancer registry. JAMA. 2004;291:2441–2447. doi: 10.1001/jama.291.20.2441. [DOI] [PubMed] [Google Scholar]
- 23.Satariano WA, Ragland DR. The effect of comorbidity on 3-year survival of women with primary breast cancer. Ann Intern Med. 1994;120:104–110. doi: 10.7326/0003-4819-120-2-199401150-00002. [DOI] [PubMed] [Google Scholar]
- 24.Maggiore RJ, Gross CP, Hurria A. Polypharmacy in older adults with cancer. The oncologist. 2010;15:507–522. doi: 10.1634/theoncologist.2009-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Landi F, Zuccala G, Gambassi G, Incalzi RA, Manigrasso L, Pagano F, et al. Body mass index and mortality among older people living in the community. J Am Geriatr Soc. 1999;47:1072–1076. doi: 10.1111/j.1532-5415.1999.tb05229.x. [DOI] [PubMed] [Google Scholar]
- 26.Dewys WD, Begg C, Lavin PT, Band PR, Bennett JM, Bertino JR, et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern Cooperative Oncology Group. Am J Med. 1980;69:491–497. doi: 10.1016/s0149-2918(05)80001-3. [DOI] [PubMed] [Google Scholar]
- 27.Vellas B, Guigoz Y, Garry PJ, Nourhashemi F, Bennahum D, Lauque S, et al. The Mini Nutritional Assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition. 1999;15:116–122. doi: 10.1016/s0899-9007(98)00171-3. [DOI] [PubMed] [Google Scholar]
- 28.Wadley VG, Okonkwo O, Crowe M, Ross-Meadows LA. Mild cognitive impairment and everyday function: evidence of reduced speed in performing instrumental activities of daily living. Am J Geriatr Psychiatry. 2008;16:416–424. doi: 10.1097/JGP.0b013e31816b7303. [DOI] [PubMed] [Google Scholar]
- 29.Sauvaget C, Yamada M, Fujiwara S, Sasaki H, Mimori Y. Dementia as a predictor of functional disability: a four-year follow-up study. Gerontology. 2002;48:226–233. doi: 10.1159/000058355. [DOI] [PubMed] [Google Scholar]
- 30.Dodge HH, Kadowaki T, Hayakawa T, Yamakawa M, Sekikawa A, Ueshima H. Cognitive impairment as a strong predictor of incident disability in specific ADL-IADL tasks among community-dwelling elders: the Azuchi Study. The Gerontologist. 2005;45:222–230. doi: 10.1093/geront/45.2.222. [DOI] [PubMed] [Google Scholar]
- 31.Hurria A, Li D, Hansen K, Patil S, Gupta R, Nelson C, et al. Distress in older patients with cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2009;27:4346–4351. doi: 10.1200/JCO.2008.19.9463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seeman TE, Berkman LF, Kohout F, Lacroix A, Glynn R, Blazer D. Intercommunity variations in the association between social ties and mortality in the elderly. A comparative analysis of three communities. Ann Epidemiol. 1993;3:325–335. doi: 10.1016/1047-2797(93)90058-c. [DOI] [PubMed] [Google Scholar]
- 33.Kroenke CH, Kubzansky LD, Schernhammer ES, Holmes MD, Kawachi I. Social networks, social support, and survival after breast cancer diagnosis. J Clin Oncol. 2006;24:1105–1111. doi: 10.1200/JCO.2005.04.2846. [DOI] [PubMed] [Google Scholar]
- 34.Kornblith AB, Herndon JE, 2nd, Zuckerman E, Viscoli CM, Horwitz RI, Cooper MR, et al. Social support as a buffer to the psychological impact of stressful life events in women with breast cancer. Cancer. 2001;91:443–454. doi: 10.1002/1097-0142(20010115)91:2<443::aid-cncr1020>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 35.Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, Gross CP, et al. Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol. 2011;29:3457–3465. doi: 10.1200/JCO.2011.34.7625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Extermann M, Boler I, Reich RR, Lyman GH, Brown RH, DeFelice J, et al. Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer. 2011 doi: 10.1002/cncr.26646. [DOI] [PubMed] [Google Scholar]
- 37.Kanesvaran R, Li H, Koo KN, Poon D. Analysis of prognostic factors of comprehensive geriatric assessment and development of a clinical scoring system in elderly Asian patients with cancer. J Clin Oncol. 2011;29:3620–3627. doi: 10.1200/JCO.2010.32.0796. [DOI] [PubMed] [Google Scholar]
- 38.Giantin V, Valentini E, Iasevoli M, Falci C, Sivero P, De Luca E, et al. Does the Multidimensional Prognostic Index (MPI), based on a Comprehensive Geriatric Assessment (CGA), predict mortality in cancer patients? Results of a prospective observational trial. Journal of Geriatric Oncology. 2013;4:208–217. doi: 10.1016/j.jgo.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 39.Aaldriks Ab A, van der Geest L, Giltay E, Cessie S, Johanneke EA. Frailty and malnutrition predictive of mortality risk in older patients with advanced colorectal cancer receiving chemotherapy. Journal of Geriatric Oncology. 2013;4:218–226. doi: 10.1016/j.jgo.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 40.Hurria A, Cohen HJ, Extermann M. Geriatric Oncology Research in the Cooperative Groups: A Report of a SIOG Special Meeting. Journal of geriatric oncology. 2010;1:40–44. doi: 10.1016/j.jgo.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hurria A, Cirrincione CT, Muss HB, Kornblith AB, Barry W, Artz AS, et al. Implementing a geriatric assessment in cooperative group clinical cancer trials: CALGB 360401. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29:1290–1296. doi: 10.1200/JCO.2010.30.6985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pal SK, Katheria V, Hurria A. Evaluating the older patient with cancer: understanding frailty and the geriatric assessment. CA: a cancer journal for clinicians. 2010;60:120–132. doi: 10.3322/caac.20059. [DOI] [PubMed] [Google Scholar]
- 43.Hurria A, Balducci L, Naeim A, Gross C, Mohile S, Klepin H, et al. Mentoring junior faculty in geriatric oncology: report from the Cancer and Aging Research Group. J Clin Oncol. 2008;26:3125–3127. doi: 10.1200/JCO.2008.16.9771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hurria A, Mohile SG, Dale W. Research priorities in geriatric oncology: addressing the needs of an aging population. J Natl Compr Canc Netw. 2012;10:286–288. doi: 10.6004/jnccn.2012.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dale W, Mohile SG, Eldadah BA, Trimble EL, Schilsky RL, Cohen HJ, et al. Biological, clinical, and psychosocial correlates at the interface of cancer and aging research. J Natl Cancer Inst. 2012;104:581–589. doi: 10.1093/jnci/djs145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cohen HJ, Feussner JR, Weinberger M, Carnes M, Hamdy RC, Hsieh F, et al. A controlled trial of inpatient and outpatient geriatric evaluation and management. N Engl J Med. 2002;346:905–912. doi: 10.1056/NEJMsa010285. [DOI] [PubMed] [Google Scholar]
- 47.Stuck AE, Siu AL, Wieland GD, Adams J, Rubenstein LZ. Comprehensive geriatric assessment: a meta-analysis of controlled trials. Lancet. 1993;342:1032–1036. doi: 10.1016/0140-6736(93)92884-v. [DOI] [PubMed] [Google Scholar]
- 48.Stuck AE, Aronow HU, Steiner A, Alessi CA, Büla CJ, Gold MN, et al. A trial of annual in-home comprehensive geriatric assessments for elderly people living in the community. N Engl J Med. 1995;333:1184–1189. doi: 10.1056/NEJM199511023331805. [DOI] [PubMed] [Google Scholar]
- 49.Rao AV, Hsieh F, Feussner JR, Cohen HJ. Geriatric evaluation and management units in the care of the frail elderly cancer patient. J Gerontol A Biol Sci Med Sci. 2005;60:798–803. doi: 10.1093/gerona/60.6.798. [DOI] [PubMed] [Google Scholar]
- 50.Caillet P, Canoui-Poitrine F, Vouriot J, Berle M, Reinald N, Krypciak S, et al. Comprehensive geriatric assessment in the decision-making process in elderly patients with cancer: ELCAPA study. J Clin Oncol. 2011;29:3636–3642. doi: 10.1200/JCO.2010.31.0664. [DOI] [PubMed] [Google Scholar]
- 51.Ingram SS, Seo PH, Martell RE, Clipp EC, Doyle ME, Montana GS, et al. Comprehensive assessment of the elderly cancer patient: the feasibility of self-report methodology. J Clin Oncol. 2002;20:770–775. doi: 10.1200/JCO.2002.20.3.770. [DOI] [PubMed] [Google Scholar]
- 52.Hurria A, Gupta S, Zauderer M, Zuckerman EL, Cohen HJ, Muss H, et al. Developing a cancer-specific geriatric assessment: a feasibility study. Cancer. 2005;104:1998–2005. doi: 10.1002/cncr.21422. [DOI] [PubMed] [Google Scholar]
- 53.Horgan AM, Leighl NB, Coate L, Liu G, Palepu P, Knox JJ, et al. Impact and Feasibility of a Comprehensive Geriatric Assessment in the Oncology Setting: A Pilot Study. Am J Clin Oncol. 2011 doi: 10.1097/COC.0b013e318210f9ce. [DOI] [PubMed] [Google Scholar]
- 54.McCorkle R, Strumpf NE, Nuamah IF, Adler DC, Cooley ME, Jepson C, et al. A specialized home care intervention improves survival among older post-surgical cancer patients. J Am Geriatr Soc. 2000;48:1707–1713. doi: 10.1111/j.1532-5415.2000.tb03886.x. [DOI] [PubMed] [Google Scholar]
- 55.Goodwin JS, Satish S, Anderson ET, Nattinger AB, Freeman JL. Effect of nurse case management on the treatment of older women with breast cancer. J Am Geriatr Soc. 2003;51:1252–1259. doi: 10.1046/j.1532-5415.2003.51409.x. [DOI] [PubMed] [Google Scholar]
- 56.Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Jr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer- treatment trials. The New England journal of medicine. 1999;341:2061–2067. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 57.Yee KW, Pater JL, Pho L, Zee B, Siu LL. Enrollment of older patients in cancer treatment trials in Canada: why is age a barrier? J Clin Oncol. 2003;21:1618–1623. doi: 10.1200/JCO.2003.12.044. [DOI] [PubMed] [Google Scholar]
- 58.Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. J Clin Oncol. 2004;22:4626–4631. doi: 10.1200/JCO.2004.02.175. [DOI] [PubMed] [Google Scholar]
- 59.Mohile SG, Xian Y, Dale W, Fisher SG, Rodin M, Morrow GR, et al. Association of a cancer diagnosis with vulnerability and frailty in older Medicare beneficiaries. Journal of the National Cancer Institute. 2009;101:1206–1215. doi: 10.1093/jnci/djp239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yesavage JA. Geriatric Depression Scale. Psychopharmacology bulletin. 1988;24:709–711. [PubMed] [Google Scholar]
- 61.Fearon K, Strasser F, Anker SD, Bosaeus I, Bruera E, Fainsinger RL, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]


