Abstract
OBJECTIVE
To estimate the frequency of and to characterize the adverse drug events at a terciary care hospital.
METHODS
A retrospective review was carried out of 128 medical records from a hospital in Rio de Janeiro in 2007, representing 2,092 patients. The instrument used was a list of triggers, such as antidotes, abnormal laboratory analysis results and sudden suspension of treatment, among others. A simple random sample of patients aged 15 and over was extracted. Oncologic and obstetric patients were excluded as were those hospitalized for less than 48 hours or in the emergency room. Social and demographic characteristics and those of the disease of patients who underwent adverse events were compared with those of patients who did not in order to test for differences between the groups.
RESULTS
Around 70.0% of the medical records assessed showed at least one trigger. Adverse drug events triggers had an overall positive predictive value of 14.4%. The incidence of adverse drug events was 26.6 per 100 patients and 15.6% patients suffered one or more event. The median length of stay for patients suffering an adverse drug event was 35.2 days as against 10.7 days for those who did not (p < 0.01). The pharmacological classes most commonly associated with an adverse drug event were related to the cardiovascular system, nervous system and alimentary tract and metabolism. The most common active substances associated with an adverse drug event were tramadol, dypirone, glibenclamide and furosemide. Over 80.0% of events provoked or contributed to temporary harm to the patient and required intervention and 6.0% may have contributed to the death of the patient. It was estimated that in the hospital, 131 events involving drowsiness or fainting 33 involving falls, and 33 episodes of hemorrhage related to adverse drug effects occur annually.
CONCLUSIONS
Almost one-sixth of in-patients (16,0%) suffered an adverse drug event. The instrument used may prove useful as a technique for monitoring and evaluating patient care results. Psycothropic therapy should be critically appraised given the frequency of associated events, such as excessive sedation, lethargy, and hypotension.
Keywords: Pharmaceutical Preparations, adverse effects; Drug Toxicity, therapeutic use; Withholding Treatment; Mass Screening; Predictive Value of Tests; Adverse Drug Reaction Reporting Systems; Tertiary Healthcare; Patient Safety
INTRODUCTION
The interest in adverse drug events (ADE) in hospitalized adults is growing.
The frequencies found for the occurrence of ADE in hospitalized adults are disparate. A systematic review of 29 studies found between 1.7 and 51.8 events per 100 hospitalizations. The variability in the estimates could be put down to differences in the definitions and the ways of recording ADE, to population characteristics and to prescribing habits. 2
There are several approaches used to capture ADE. In those which are implicit, the technique for identifying events is less systematic, is subjective and there is poor reliability between the judgments of the evaluators, even when they are experienced. 1 Approaches using automated procedures, active searches or monitoring identify a higher number of events than those that depend on spontaneous notifications. 9,16 Using automated surveillance 16 or computerized systems supporting clinical decisions 17 means more objective evaluations. Investigations based on nationwide administrative databases, 23 together with the International Classification of Diseases, 3,15 contributes to decreasing the time spent on analysis and improves the cost-benefit ratio of identifying events.
Using signs, or triggers, is a recent approach, and they act as clues in identifying adverse events and are used within hospitals, both prospectively 5 and retrospectively, 10 in intervention studies 6 or in the emergency services. 22
ADE have been shown to be promising in a variety of environments and using different approaches. The aim of this study was to estimate the frequency of ADE and to characterize them in tertiary care hospitals
METHODS
ADE are the occurrence of any harm to the patient related to medical intervention with the use of medication, resulting in a temporary or permanent physical or psychological disturbance in the body or in its structure. The definition includes prescribing, dispensing and administrating errors and adverse reactions. 21 Events existing upon hospital admission were excluded.
A retrospective review was carried out of medical records in a hospital in Rio de Janeiro, RJ, Southeastern Brazil, containing around 450 tertiary care beds distributed between medical and surgical clinics, which treated Brazilian Unified Health System (SUS) patients. A team of researchers from the Fundação Oswaldo Cruz /Ministry of Health was in charge of coordinating the study. The medical record evaluation was conducted together with doctors, pharmacists and nurses involved in risk management in the hospital.
Four simple random samples were extracted from hospital discharge sheets for January, April, July and October 2007, based on databases recording hospitalizations. Data were collected at different periods in order to prevent the study being affected by seasonal variation in the frequency and type of events. Medical records were selected for the first month of each three-month period, considering variations throughout the year. Each hospital patient had equal chance of being selected to participate in the study. The following parameters were considered when calculating the sample size: error of 10.0%; statistical significance of 95%; frequency of the event studied, 10.0%; and losses of 10.0%. Patients aged under 15 years were excluded, as were cancer patients, those who spent fewer than 48 hours in hospital and those in the emergency and obstetrics departments. Every day of hospitalization was examined, excluding those that were spent in intensive care units. There were no readmissions. A total of 2,092 medical records were deemed to be eligible, giving a sample of 128 medical records corresponding to 1,862 days of hospitalization. There were no irreplaceable losses.
The sources of the data were: hospital discharge summaries, prescriptions, progress notes and SUS hospital admission authorization forms. The data collected covered: demographic data and data on existing comorbidities on admission; the dose, frequency and administration method of prescribed pharmaceutics; results of laboratory tests; patients progress, recorded by the medical, nursing and pharmacy team; description and dates of triggers and of adverse events recorded as such or as interactions which could be associated with medication.
A data entry program for laptops was developed in order to avoid errors and facilitate recording the data extracted.
The triggers used, after adjustments to the list proposed by Rozich, 21 were grouped into three types. The first included: use of antihistamines; coagulants; flumazenil; anti-emetics; naloxone; anti-diarrheal; sodium polystyrene. And also digoxin, when accompanied by signs and symptoms of intoxication (arrhythmia, bradycardia, nausea, vomiting, anorexia, visual changes). The second group included: blood sugar below 50 mg/dL, partial thromboplastin time (PTT) above 100 seconds; International Normalized Ratio (INR) above 6; white count below 3,000 x 106/μl blood cells, platelet counts below 50,000; elevated serum creatinine. The third group included: excessive sedation, lethargy, hypotension, falls, skin rash, sudden interruption of the medication; transfer to care of a higher level complexity.
Each medical record selected was independently evaluated by two health care professionals, looking for triggers and adverse events. The association between the adverse event and the suspected medication is established considering the temporal relationship between them and the patient's clinical evolution, as well as descriptions in the literature of similar events. The medical records in which ADE were identified by at least one evaluator were examined in detail in meetings with all members of the evaluation team. Thus, doubts were settled and consensus reached on the occurrence and the severity of each event and its link to medication.
Further details on triggers can be found published elsewhere. 11,20
The subgroups of patients with and without ADE were compared and the figures presented as absolute, proportions, means and standard deviation. The differences between the subgroups according to the variables selected, were tested for statistical significance (Chi-square, Fisher's exact and Student's t tests).
The overall Positive Predictive Value (PPV) was calculated for the sets of triggers and for each trigger. PPV was defined as: number of patients for whom a trigger was indicating an ADE found divided by the number of patients for whom a trigger was indicating an ADE found plus the number of patients for whom a trigger did not indicate and AME. 12 The rate of "triggers per 100 records" and the rate of "AME per 100 records" for each trigger.
The total incidence of ADE was calculated, as was its distribution by type of event, each corresponding to an ADE-implicated medication partnership, classified according to the severity of the harm caused to the patient, based on the National Coordinating Council for Medication Error Reporting and Prevention Index for Categorizing Errors. 21
The medications implied were classified according to the Anatomical Therapeutic Chemical (ATC) 24,a and distribution was presented as absolute and relative frequency.
The Charlson Index 4,8 was used to analyze comorbidities and enabled the severity of the patient's illness based on 19 clinical conditions. The index was used in principal and secondary diagnosis.
The project was approved by the ethics committee of the hospital studied (Process no. CEP 000.283/2007).
RESULTS
Mean age was 53.2 years (SD = 17.4 years), females predominated (60.2%) and the mean length of hospitalization was 14.6 days (SD = 19.9 days). In 73.0% of cases, the medical record contained information on occupation of which 47.9% were retired and 18.1% were homemakers (Table 1). The most commonly found principal diagnoses were neoplasms (25.8%), diseases of the digestive system (20.3%), diseases of the genitourinary system (15.6%) and diseases of the circulatory system (14.8%).
Table 1.
Characteristics of the hospitalized patients according to occurrence of adverse drug events, identified using triggers in a federal hospital. Rio de Janeiro, RJ, Southeastern Brazil, 2007.
| Variable | No ADE | ADE | Total | Pa | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| Mean ageb | 53.3(17.3) | – | 52.8(18.2) | – | 53.2(17.4) | – | 0.908 | |
| Sex | 0.988 | |||||||
| Female | 65 | 84.4 | 12 | 15.6 | 77 | 60.2 | ||
| Male | 43 | 84.3 | 8 | 15.7 | 51 | 39.8 | ||
| Race/Skin color | 0.919 | |||||||
| White | 48 | 82.8 | 10 | 17.2 | 58 | 48.7 | ||
| Black | 19 | 86.4 | 3 | 13.6 | 22 | 18.5 | ||
| Mixed race | 33 | 84.6 | 6 | 15.4 | 39 | 32.8 | ||
| Schooling | 0.57 | |||||||
| Did not finish primary education | 54 | 80.6 | 13 | 19.4 | 67 | 73.6 | ||
| Did not finish secondary education | 18 | 90.0 | 2 | 10.0 | 20 | 22.0 | ||
| Further education | 3 | 75.0 | 1 | 25.0 | 4 | 4.4 | ||
| Occupation | 0.037 | |||||||
| Retired | 37 | 82.2 | 8 | 17.8 | 45 | 47.9 | ||
| Homemaker | 12 | 70.6 | 5 | 29.4 | 17 | 18.1 | ||
| Other | 31 | 96.9 | 1 | 3.1 | 32 | 34.0 | ||
| CCI | 0.312 | |||||||
| 0 | 67 | 87.0 | 10 | 13.0 | 77 | 60.2 | ||
| ≥ 1 | 41 | 80.4 | 10 | 19.6 | 51 | 39.8 | ||
| Mean length of hospitalization (days)b | 10.7(15.3) | – | 35.2(28.0) | – | 14.6(19.9) | – | < 0.01 | |
CCI: Charlson Comorbidity Index; ADE: adverse drug event
Chi-square or Fisher’s exact tests for categorical variables and Student’s t test for continual variables.
The values shown are the means and, in brackets, the respective standard deviation.
There was a significant difference (p < 0.01) in the length of hospitalizations between patients who experienced ADE and those who did not, with shorter stays for the latter group (10.7 days versus 35.2 days). The characteristics of the patient related to the severity of the case were not statistically significant in the comparison between the groups, according to the Charlson Index. The probability of having an ADE was higher in those with more, and more severe, comorbidities. Those who stated that they were "housewives" had a higher chance of having an ADE compared with those who said they were "retired" (p = 0.037) (Table 1).
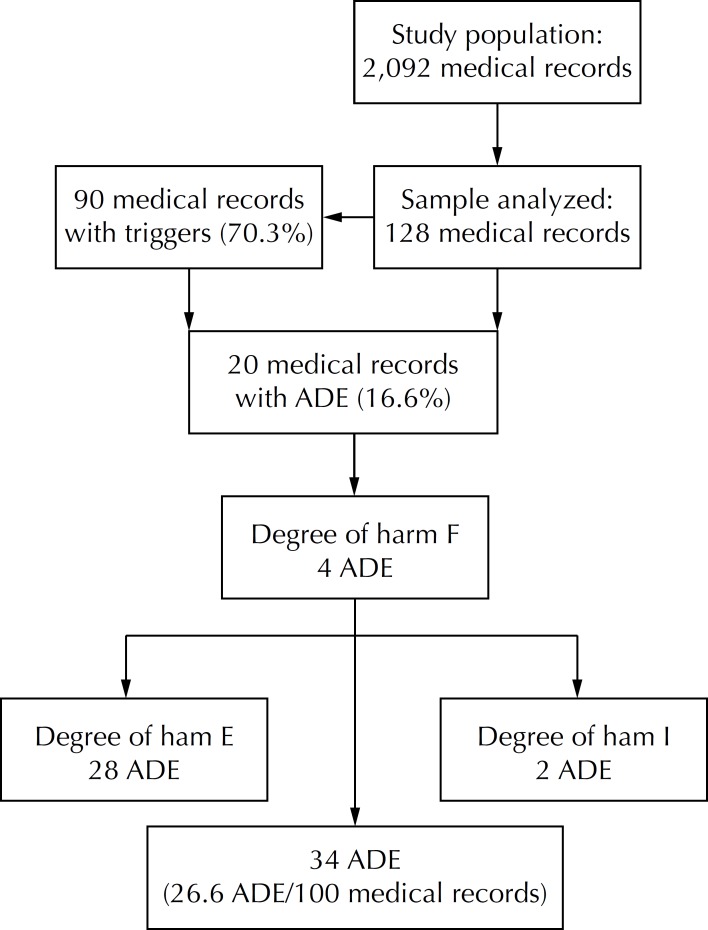
The triggers showed that 15.6% of the medical records contained an ADE. As there were patients who suffered two or more events, 34 ADE were found, 32 of which caused temporary harm to the patient and two of which contributed to the patients' death. The rate of events was estimated to be 26.6 ADE/100 medical records (Figure).
Figure.
Flowchart representing the study population and the adverse drug events identified using triggers in a federal hospital. Rio de Janeiro, RJ, Southeastern Brazil, 2007.
E = temporary harm to the patient and need for intervention; F = temporary harm to the patient and need for intervention or prolongation of hospitalization; I = death; ADE: Adverse Drug Events
The overall PPV was 14.4 (Table 2).
Table 2.
Frequency of triggers identified, of adverse drug events identified and Positive Predictive Value in a federal hospital. Rio de Janeiro, RJ, Southeastern Brazil, 2007.
| List of triggers | Trigger per 100 medical records | Adverse drug events per 100 medical records | Positive Predictive Valuea |
|---|---|---|---|
| Antihistamines (dexchlorpheniramine, loratadine, promethazine and epinephrine) | 7.0 | 0.0 | 0.0 |
| Coagulants (vitamin K1 and protamine) | 4.7 | 0.8 | 16.7 |
| Benzodiazepine antagonist (flumazenil) | 0.0 | 0.0 | – |
| Anti-emetic (bromopride, metoclopramide and ondansetron) | 58.6 | 7.0 | 12.0 |
| Opioid antagonist (naloxone) | 0.0 | 0.0 | – |
| Anti-diarrhea (loperamide) | 1.6 | 0.0 | 0.0 |
| Ion-exchange resin (calcium polystyrene sulfonate) | 0.8 | 0.0 | 0.0 |
| Blood sugar < 50 mg/dL | 2.3 | 0.8 | 33.3 |
| PTT > 100 seconds | 0.0 | 0.0 | – |
| INR > 6 | 0.0 | 0.0 | – |
| Leucocytes < 3.000 | 1.6 | 0.0 | 0.0 |
| Platelets < 50.000 | 0.8 | 0.0 | 0.0 |
| Use of digoxin and arrhythmia, bradycardia, nausea, vomiting, anorexia, or visual changes | 2.3 | 0.8 | 33.3 |
| Elevated serum creatinine | 8.6 | 1.6 | 18.2 |
| Excessive drowsiness, lethargy, fall, hypotension | 27.3 | 7.0 | 25.7 |
| Rash | 0.8 | 0.8 | 100.0 |
| Sudden interruption of medication | 63.3 | 7.8 | 12.4 |
| Transfer to a higher level of care | 5.5 | 0.0 | 0.0 |
| Total | 185.2 | 26.6 | 14.4 |
PPV = nº of medical records in which the trigger indicated ADE x 100/nº of medical records in which the trigger indicated ADE + nº of medical records in which the trigger did not indicate ADE. The denominator is the number of times the trigger occurred, indicating, or not, ADE. Example: anti-emetic: 7.0 x 100/58.6 = 12.0.
Of the 18 triggers, four (benzodiazepine antagonist; opioid antagonist; PTT above 100 seconds; INR above six) were not identified in any of the medical records evaluated. Another six (use of antihistamines or of antidiarrheal medication, or ion exchange resin; decreased leukocytes; decreased platelets; transfer to higher level of care complexity), although described in the records, did not identify ADE. Of the remaining eight (use of coagulants, or anti-emetics, or digoxin; blood sugar < 50 mg/dL, elevated serum creatinine, sedation, skin rash, sudden interruption of medication), the PPV ranged from 12.0% to 100.0%.
In total, there were 34 ADE, and the most commonly found were nausea and vomiting (ten events), somnolence (five) and hypoglycemia (four). Those remaining were: fainting (three) and hypotension, renal failure, pruritus, fall, and bleeding (two events each) (Table 3). The two most severe ADE occurred in the same patient, a 79-year-old male diagnosed with abdominal aortic aneurysm with no mention of rutpure (ICD-10 I71.6), hospitalized for 49 days. The patient suffered from hypotension and renal failure, which can be attributable to the use of captopril + spironolactone + isosorbide + furosemide and to the use of simvastatin, respectively. The use of these medications may have precipitated the patient's death.
Table 3.
Description of adverse drug events, medication implicated and degree of harm suffered by the patient, in a federal hospital. Rio de Janeiro, RJ, Southeastern Brazil, 2007.
| Adverse drug events | Number of cases | %a | Medications implicated | Degree of harm |
|---|---|---|---|---|
| Nausea and/or vomiting | 10 | 29.4 | Dipyrone | E |
| Ceftriaxone | E | |||
| Deslanoside | E | |||
| Tramadol | E | |||
| Cefazolin | E | |||
| Ciprofloxacin | E | |||
| Levofloxacin + tramadol | E | |||
| Indomethacin + levofloxacin | E | |||
| Metronidazole + tramadol + clindamycin + dipyrone + ranitidine | E | |||
| Drowsiness | 5 | 14.7 | Tramadol | E |
| Clonazepam | E | |||
| Metoclopramide | E | |||
| Diazepam | E | |||
| Promethazine | E | |||
| Hypoglycemia | 4 | 11.8 | Glibenclamide + metformin | E |
| Glibenclamide | E | |||
| Insulin | E | |||
| Lipothymy | 3 | 8.8 | Furosemide + dipyrone + metoclopramide | E |
| Propranolol + hydrochlorothiazide | F | |||
| Levothyroxine | E | |||
| Hypotension | 2 | 5.9 | Captopril + furosemide + amlodipine + atenolol | E |
| Captopril + spironolactone + isosorbide + furosemide | I | |||
| Renal failure | 2 | 5.9 | Simvastatin | I |
| Amphotericin B | E | |||
| Pruritus | 2 | 5.9 | Clindamycin | F |
| Vancomycin | E | |||
| Falls | 2 | 5.9 | Diazepam + dexchlorpheniramine | F |
| hydroxyzine | F | |||
| Hemorrhage | 2 | 5.9 | Enoxaparin | E |
| Arrhythmia | 1 | 2.9 | Digoxin + omeprazole + carvedilol | E |
| Diarrhea | 1 | 2.9 | Lactulose | E |
E = temporary harm to the patient and the need for intervention, F = temporary harm to the patient and the need for hospitalization or prolongation of hospitalization, I = death
The number of cases does not match the number of rows in the “ Medications implicated” column because there are repeated EAM-drug pairs (dipyrone-nausea = 2 instances hypoglycemia-glibenclamide + metformin = 2 cases, and hemorrhage-enoxaparin = 2 cases).
The 34 ADE were associated with 54 medications. The class of medicines most commonly involved were those that act on the cardiovascular system (27.8%); on the nervous system (22.2%); and on the digestive apparatus and metabolism (20.4%) (Table 4). The most commonly implicated medications were: tramadol (four events), dipyrone (four), glibenclamide (three) and furosemide (three).
Table 4.
Classes of medications related to an adverse drug event according to the first and second levels of the Anatomical Therapeutic Chemical classification in a federal hospital. Rio de Janeiro, RJ, Southeastern Brazil, 2007.
| Code - Anatomical Therapeutic Chemical classification group | n | Proportion (%) | |
|---|---|---|---|
| A - Digestive tract and metabolism | 11 | 20.4 | |
| A02 - Agents related to acid disorders | 2 | 3.7 | |
| A03 - Agents for function gastrointestinal disorders | 2 | 3.7 | |
| A06 - Laxatives | 1 | 1.9 | |
| A10 - Medications used in diabetes | 6 | 11.1 | |
| B - Blood and blood forming organs | 2 | 3.7 | |
| B01 - Antithrombotic medication | 2 | 3.7 | |
| C - Cardiovascular system | 15 | 27.8 | |
| C01 - Cardiac therapy | 3 | 5.6 | |
| C03 - Diuretics | 5 | 9.3 | |
| C07 - Beta-blocking agents | 3 | 5.6 | |
| C08 - Calcium channel blockers | 1 | 1.9 | |
| C09 - Agents acting on the renin-angiotensin system | 2 | 3.7 | |
| C10 - Agents modifying the lipid profile | 1 | 1.9 | |
| H - Systemic hormonal preparations, excluding sex hormones and insulins | 1 | 1.9 | |
| H02 - Corticosteroids for systemic use | 1 | 1.9 | |
| J - General anti-infectives for systemic use | 10 | 18.5 | |
| J01 - Anti-bacterials for systemic use | 9 | 16.7 | |
| J02 - Anti-mycotics for systemic use | 1 | 1.9 | |
| M - Musculoskeletal System | 1 | 1.9 | |
| M01 - Anti-inflammatory and anti-rheumatic drugs | 1 | 1.9 | |
| N - Nervous system | 12 | 22.2 | |
| N02 - Analgesics | 8 | 14.8 | |
| N03 - Anti-epileptics | 1 | 1.9 | |
| N05 - Psycholeptics | 3 | 5.6 | |
| R - Respiratory system | 2 | 3.7 | |
| R06 - Anti-histamine for systemic use | 2 | 3.7 | |
| Total | 54 | 100.0 | |
n: number of times the medication class was associated with an ADE
DISCUSSION
It was estimated that 15.6% of the hospitalized patients experienced ADE, a figure which is within the interval, albeit a wide interval, of the estimates of the recent revision. 2 Other studies have identified events using triggers or clinical or laboratory signs and have found a variety of values of 2%, 5 3.4% 10 and 24%. 7 There are several factors which may affect the estimates: among them, the implementation of interventions ensuring patient safety. ADE in the US fell by a factor of three in two years following educational interventions on medications. 6
Comparing the two groups of patients suggests that the mean length of hospitalization is higher among those who experience an ADE (35 days versus 11 days, p < 0.01), which coincides with findings by other authors. 5,9 The extended length of hospitalization is a burden to the health care system and the patients are at increased risk, inherent to the hospital environment.
Patients with comorbidities and more serious clinical situations have a higher proportion of ADE than others (19.6% versus 13.0%), although the difference was not statistically significant. More complex cases, with more disease and more interventions seem to have a higher chance of presenting complications and interactions between medications and diseases.
In general, there are relatively few cases of serious harm or death deriving from ADE. 7,9 It was estimated that 82.0% of events caused temporary harm and the need for some type of intervention to the patient. Even those events not considered serious, but that must cause discomfort to the patient, need to be recorded in the medical record.
Analgesics and antibiotics are among the medications most commonly involved in adverse events, 21 although cardiovascular medication also figures among the most commonly implicated classes. 9 These three groups account for 61.0% of medications involved in this study.
The most commonly encountered triggers were: sudden interruption of medication, prescribing anti-emetics and situations involving sedation, lethargy, falls or hypotension, coinciding with Rozich. 21 Given the frequency with which these triggers occur (above 27/100 medical records), it was possible to identify a greater number of ADE. Excessive sedation performed best: in ¼ of the cases in which it appeared, it indicated a possible ADE.
There are triggers which, although present in fewer medical records, were more efficient in identifying ADE: skin rash, blood sugar < 50 mg/dL and the use of digoxin with clinical signs or symptoms of digitalis intoxication. This meant higher PPV values and identified adverse events in proportions >1/3.
Comparing the performance of each trigger is difficult as the result can be affected by the sample size. Moreover, the performance of the trigger may vary over time and due to changes in diagnostic and therapeutic practices.
"Sudden interruption of the medicine" was a trigger identified in 63.0% of medical records. It can be affected by non-clinical factors unconnected with the patients' treatment, such as standardization and acquisition of pharmaceutical products, as well as by the quality of the data recording system. It allowed ADE to be identified in almost 8.0% of medical records and indicated that the quality of hospital management in the area of medication needs to be improved.
Using trigger tools to identify ADE enabled laboratory parameters printed on digitalized forms to be examined together with the sheets on clinical evolution from the medical record. The advantages of the objectivity of electronic information are associated with efficacy in the critical reading of the medical records, a long and subjective process.
This study had several positive points: extracting a representative sample from the hospital; standardizing procedures for identifying triggers and adverse events; using an electronic data entry program to reduce errors; double evaluation of the medical records and meetings to achieve consensus in order to increase validity in identifying cases.
Independent evaluation by two health care professionals was supported in a recent article. 24 Including more evaluators does not improve reliability. Zegers et al 24 studied the reliability of a retrospective review of medical records, performed by doctors, to identify adverse events in general, including those associated with medications. The reliability between the two independent reviewers was substantive (kappa 0.64; 95%CI 0.61;0.68) in the first stage; in the second stage, with another two doctors involved in reviewing each medical record, followed by a consensus meeting, the concordance was poor (kappa 0.25; 95%CI 0.05;0.45). Even so, more events were identified in the second stage.
One of the limitations of the study was that it was not able to distinguish between adverse reactions and errors. It would be beneficial to make this distinction, as it enables interventions reducing errors in prescribing, dispensing and administering medications to be planned. Estimates of systematic reviews using meta-analysis 13 are that avoidable adverse events related to medication affect 1.6% (95%CI 0.1;51.0) of hospitalized patients, which corresponds almost half of the events identified (45%, 95%CI 33.0;58.0). There seems to be a tendency to draw up studies that emphasize overall estimates of the frequency of events that harm the patient, irrespective of the causality associated with error, leaving the study of errors and their "preventability" to specific approaches.
As this was a retrospective study, the data were not complete and were recorded for ends other than evaluating care results. The hospital provided tertiary care with teaching activities, which guaranteed the quality of the medical records and record and identification of ADE triggers, which does not exclude the possibility that values were underestimated.
Formal instruments distinguishing between probable and possible reactions or establishing a causal relationship between the medication and the ADE were not used. Those elements traditionally used for this end were considered, such as the temporal relationship between cause and effect; previous knowledge of effect, using the literature; underlying diseases as alternative causes for explaining the adverse effects. Even so, the simultaneous use of multiple medications during hospitalization, the occurrence of drug interactions and incompletely recorded verbal prescriptions may affect assessments of causality. However, it is probable that overall estimates were not subject to relevant bias.
Extrapolating the results for the hospital as a whole and the proportional distribution of events should be viewed with caution, as the sample was calculated considering global incidence of expected ADE. Despite this caveat, extrapolation may suggest areas for intervention. Overall occurrence of adverse events would be 565 events per year. Among them, some clinically relevant effects have the potential to be reduced: 33 hemorrhages, 33 falls and 131 events involving drowsiness or fainting that could lead to falls.
A critical revision of anti-coagulant treatments should be considered, as complications involving hemorrhage, associated with inappropriate recommendation of anti-coagulants, are among the most common causes of avoidable adverse events. 14 In the same way, it is recommended that the use of psychotropic medication and the prescription of medications altering the functioning of the central nervous system be revised, as these are risk factors for falls.
Reviews do not enable any intervention to be made into the patients' treatment whilst in hospital, as they are a retrospective method, but they show the need to transform prescribing habits and to create strategies to decrease or avoid new events. Measures such as creating monitoring systems may contribute to improving drug treatments. Electronically automated laboratory parameters or the sudden interruption of treatment (triggers used herein), could be used prospectively to allow intervention in real time.
In Brazil, evaluating problems related to side effects from medication us just beginning. Evaluating health care quality and patient safety are areas which up to now have been investigated very little and studies on using triggers are scarce. 18,19 The following could make the method more cost-effective: decreasing the number of triggers; using those with higher PPV; using those with more clinical and epidemiological relevance in the hospital. It is possible to refine the definition of some triggers so as to decrease the number of false positives. 11
In this country, it is urgent that all the prescriptions of hospitalized patients be systematically electronically recorded, as in the majority of European countries and in the United States of America. This measure would enable the profile of the prescription to be identified and the role of adverse events in patient care and health care management to be evaluated more quickly and cheaply.
Using triggers to monitor patient care results requires commitment on the part of managers and engagement on the part of health care professionals working in teams directly connected with patient care. Improving quality takes place in an environment in which the rational use of medication and preventing errors in prescribing, dispensing and administering medication needs permanent attention and to be an integrated part of measures aiming at overall improvement in health care service quality.
Footnotes
WHO Collaborating Centre for Drug Statistics Methodology; Norwegian Institute of Public Health. ATC/DDD Index 2013. Oslo; 2013 [cited 2012 Dec 10]. Available from: http://www.whocc.no/atc_ddd_index/
The authors declare that there are no conflicts of interest.
REFERENCES
- 1.Arimone Y, Miremont-Salamé G, Haramburu F, Molimard M, Moore N, Fourrier-Réglat A, et al. Inter-expert agreement of seven criteria in causality assessment of adverse drug reactions. Br J Clin Pharmacol. 2007;64(4):482–488. doi: 10.1111/j.1365-2125.2007.02937.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cano FG, Rozenfeld S. Adverse drug events in hospitals: a systematic review. Cad Saude Publica. 2009;25(3):S360–S372. doi: 10.1590/S0102-311X2009001500003. [DOI] [PubMed] [Google Scholar]
- 3.Carrasco-Garrido P, Andrés López A, Hernández Barrera V, Ángel de Miguel G, Jiménez-García R. Trends of adverse drug reactions related-hospitalizations in Spain (2001-2006) BMC Health Serv Res. 2010;10:287. doi: 10.1186/1472-6963-10-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 5.Classen DC, Pestotnik SL, Evans RS, Burke JP. Computorized surveillance of adverse drug events in hospital patients. JAMA. 1991;266(20):2847–2851. doi: 10.1001/jama.1991.03470200059035. [DOI] [PubMed] [Google Scholar]
- 6.Cohen MM, Kimmel NL, Benage MK, Cox MJ, Sanders N, Spence D, et al. Medication safety program reduces adverse drug events in a community hospital. Qual Saf Health Care. 2005;14(3):169–174. doi: 10.1136/qshc.2004.010942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies EC, Green CF, Mottram DR, Pirmohamed M. Adverse drug reactions in hospital in-patients: a pilot study. J Clin Pharm Ther. 2006;31(4):335–341. doi: 10.1111/j.1365-2710.2006.00744.x. [DOI] [PubMed] [Google Scholar]
- 8.Dias MAE, Martins M, Navarro N. Rastreamento de resultados adversos nas internações do Sistema Único de Saúde. Rev Saude Publica. 2012;46(4):719–729. doi: 10.1590/S0034-89102012005000054. [DOI] [PubMed] [Google Scholar]
- 9.Dormann H, Muth-Selbach U, Krebs S, Criegee-Rieck M, Tegeder I, Schneider HT, et al. Incidence and costs of adverse drug reactions during hospitalisation: computerised monitoring versus stimulated spontaneous reporting. Drug Saf. 2000;22(2):161–168. doi: 10.2165/00002018-200022020-00007. [DOI] [PubMed] [Google Scholar]
- 10.Franklin BD, Birch S, Schachter M, Barber N. Testing a trigger tool as a method of detecting harm from medication errors in a UK hospital: a pilot study. Int J Pharm Pract. 2010;18(5):305–311. doi: 10.1111/j.2042-7174.2010.00058.x. [DOI] [PubMed] [Google Scholar]
- 11.Giordani F, Rozenfeld S, Oliveira DFM, Versa GLGS, Terencio JS, Caldeira LF, et al. Vigilância de eventos adversos a medicamentos em hospitais: aplicação e desempenho de rastreadores. Rev Bras Epidemiol. 2012;15(3):455–467. doi: 10.1590/S1415-790X2012000300002. [DOI] [PubMed] [Google Scholar]
- 12.Handler SM, Altman RL, Perera S, Hanlon JT, Studenski SA, Bost JE, et al. A systematic review of the performance characteristics of clinical event monitor signals used to detect adverse drug events in the hospital setting. J Am Med Inform Assoc. 2007;14(4):451–458. doi: 10.1197/jamia.M2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hakkarainen KM, Hedna K, Petzold M, Hägg S. Percentage of patients with preventable adverse drug reactions and preventability of adverse drug reactions: a meta-analysis. PLoS One. 2012;7(3): doi: 10.1371/journal.pone.0033236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardmeier B, Braunschweig S, Cavallaro M, Roos M, Pauli- Magnus C, Giger M, et al. Adverse drug events caused by medication erros in medical inpatients. Swiss Med Wkly. 2004;134(45-46):664–670.: doi: 10.4414/smw.2004.10801. [DOI] [PubMed] [Google Scholar]
- 15.Hougland P, Xu W, Pickard S, Masheter C, Williams SD. Performance of International Classification of Diseases, 9th Revision, Clinical Modification codes as an adverse drug event surveillance system. Med Care. 2006;44(7):629–636. doi: 10.1097/01.mlr.0000215859.06051.77. [DOI] [PubMed] [Google Scholar]
- 16.Kilbridge PM, Campbell UC, Cozart HB, Mojarrad MG. Automated surveillance for adverse drug events at a community hospital and an academic medical center. J Am Med Inform Assoc. 2006;13(4):372–377. doi: 10.1197/jamia.M2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuperman GJ, Bobb A, Payne TH, Avery AJ, Gandhi TK, Burns G, et al. Medication-related clinical decision support in computerized provider order entry systems: a review. J Am Med Inform Assoc. 2007;14(1):29–40. doi: 10.1197/jamia.M2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reis AMM, Cassiani SHB. Adverse drug events in an intensive care unit of a university hospital. Eur J Clin Pharmacol. 2011;67(6):625–632. doi: 10.1007/s00228-010-0987-y. [DOI] [PubMed] [Google Scholar]
- 19.Roque KE, Melo ECP. Adaptação dos critérios de avaliação de eventos adversos a medicamentos para uso em um hospital público no Estado do Rio de Janeiro. Rev Bras Epidemiol. 2010;13(4):607–619. doi: 10.1590/S1415-790X2010000400006. [DOI] [PubMed] [Google Scholar]
- 20.Rozenfeld S, Chaves SMC, Reis LGC, Martins M, Travassos C, Mendes W, et al. Efeitos adversos a medicamentos em hospital público: estudo piloto. Rev Saude Publica. 2009;43(5):887–890. doi: 10.1590/S0034-89102009005000051. [DOI] [PubMed] [Google Scholar]
- 21.Rozich JD, Haraden CR, Resar RK. Adverse drug event trigger tool: a practical methodology for measuring medication related harm. Qual Saf Health Care. 2003;12(3):194–200. doi: 10.1136/qhc.12.3.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneeweiss S, Hasford J, Gottler M, Hoffmann A, Riethling AK, Avorn J. Admissions caused by adverse drug events to internal medicine and emergency departments in hospitals: a longitudinal population-based study. Eur J Clin Pharmacol. 2002;58(4):285–291. doi: 10.1007/s00228-002-0467-0. [DOI] [PubMed] [Google Scholar]
- 23.Stausberg J, Hasford J. Drug-related admissions and hospital-acquired adverse drug events in Germany: a longitudinal analysis from 2003 to 2007 of ICD-10-coded routine data. BMC Health Serv Res. 2011;11:134. doi: 10.1186/1472-6963-11-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zegers M, Bruijne MC, Wagner C, Groenewegen PP, Wal G, Vet HCW. The inter-rater agreement of retrospective assessments of adverse events does not improve with two reviewers per patient record. J Clin Epidemiol. 2010;63(1):94–102. doi: 10.1016/j.jclinepi.2009.03.004. [DOI] [PubMed] [Google Scholar]