Abstract
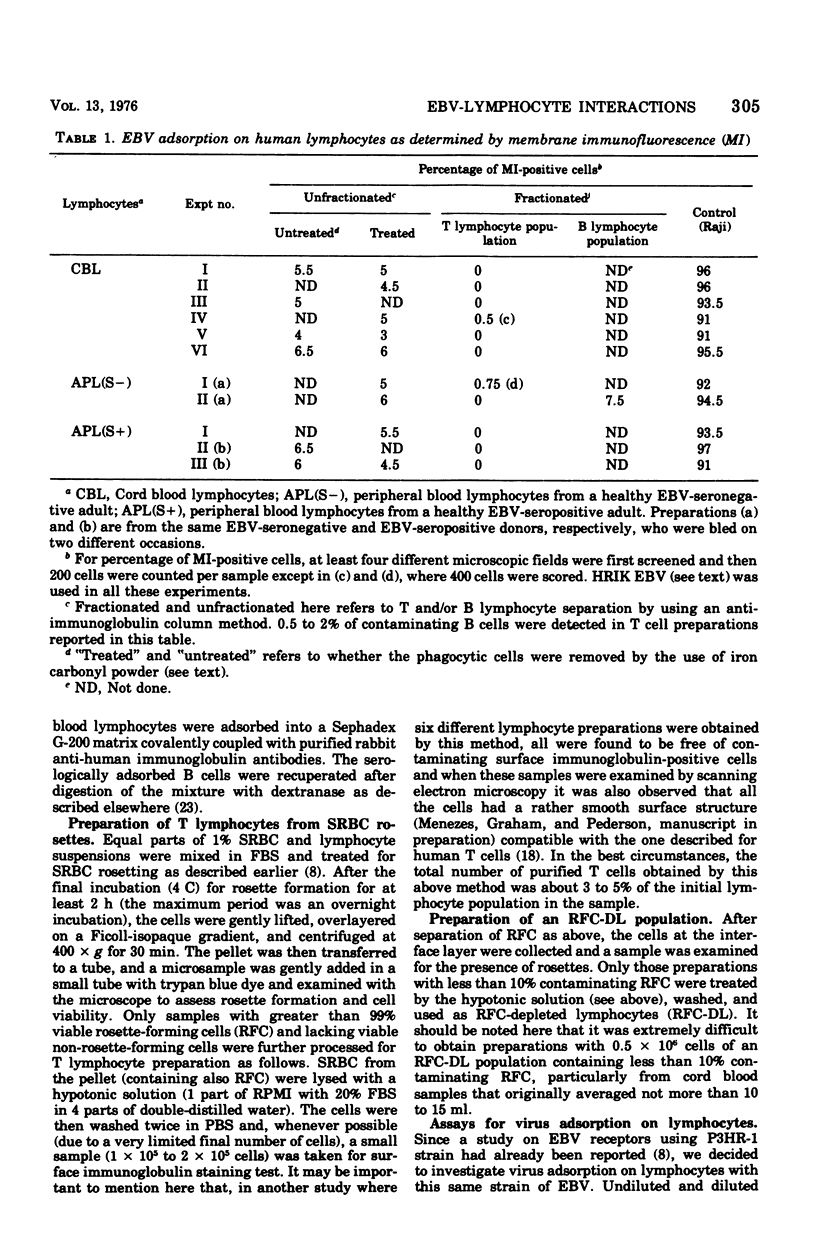
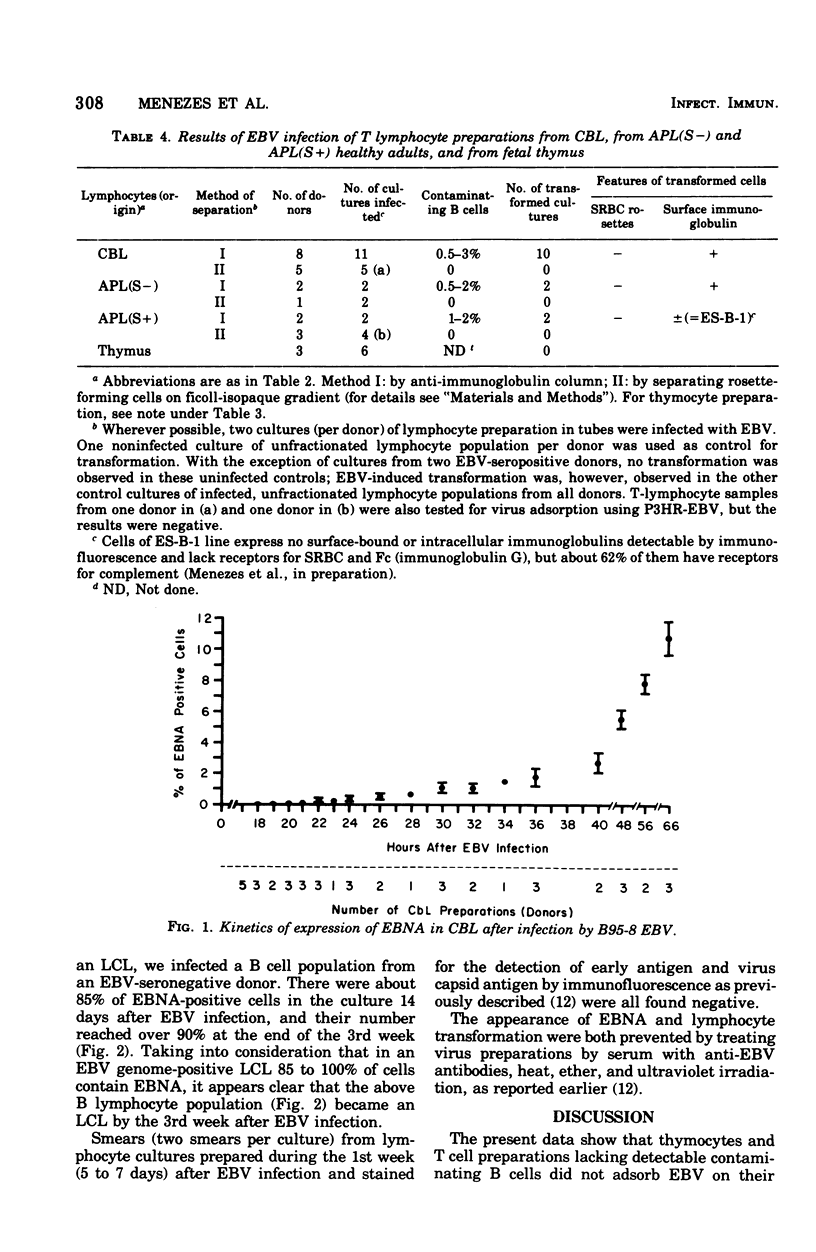
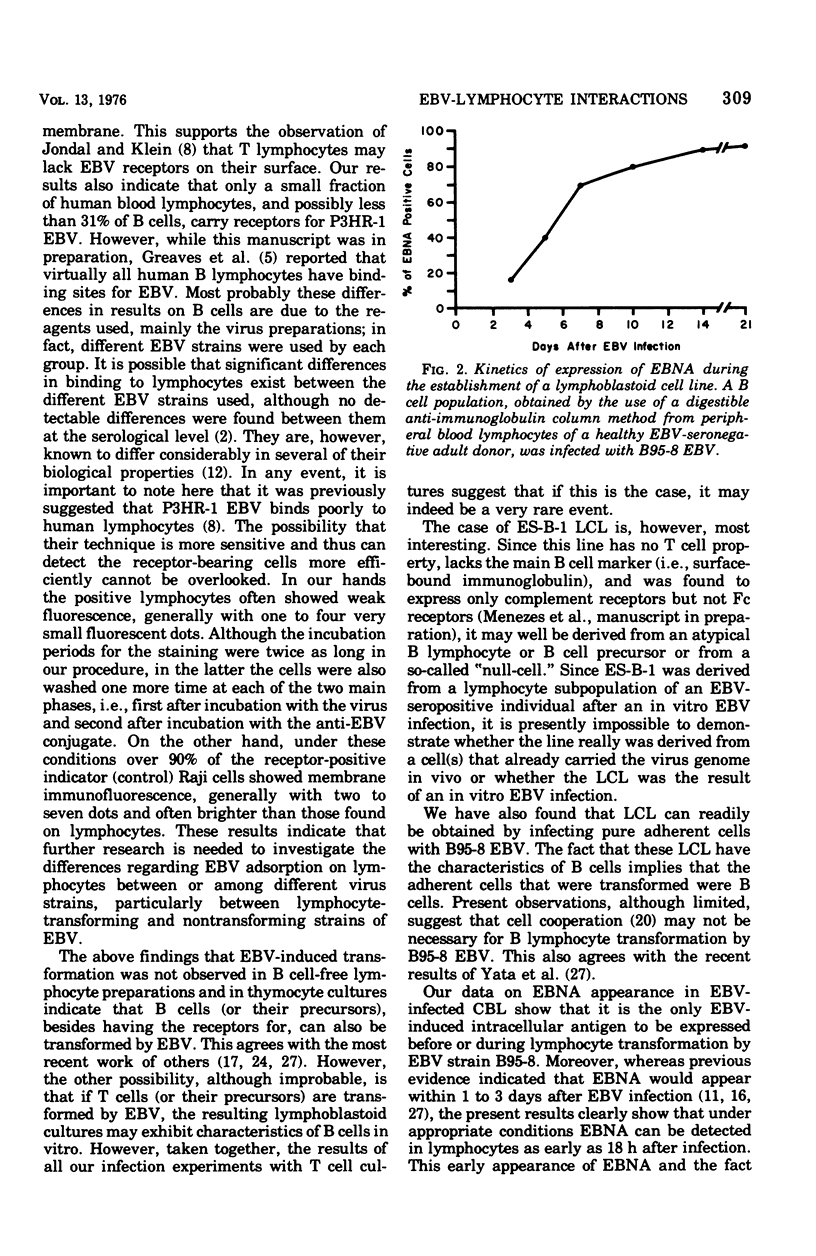
In order to further understand Epstein-Barr virus (EBV)-lymphocyte interactions, we investigated a chain of events including: (i) EBV binding to human lymphocyte subpopulations; (ii) the earliest appearance of EBV-determined nuclear antigen (EBNA) in the lymphocytes after EBV infection; and (iii) establishment of continuous lymphoblastoid cell lines (LCL) by infecting with EBV different types of lymphocyte preparations from the same as well as from different donors. By using direct membrane immunofluorescence assay, we found that only a small fraction of human peripheral blood and cord blood lymphocytes (CBL), and possibly less than 31% of the T cell-depleted lymphocyte population, carry receptors for P3HR-1 strain of EBV. The number of cells carrying receptors for EBV did not vary considerably among different blood lymphocyte populations from several normal donors. EBV adsorption on lymphocyte subpopulations showed that purified thymus-dependent (T) cells and thymocytes did not adsorb EBV, in contrast to T cell-depleted lymphocyte populations and lymphoid cells from fetal liver and spleen. In CBL infected with EBV strain B95-8, EBNA was detected by anti-complement immunofluorescence as early as 18 h after infection. This indicates that EBNA is the earliest detectable EBV-determined intracellular antigen to appear after infection and before or during lymphocyte transformation by EBV. Transformation was observed only in lymphocyte cultures containing detectable thymus-independent B cells but not in cultures of purified T cells. With one exception (es-b-1), all the EBV-transformed LCL from different origins carried surface-bound immunoglobulins (a B cell marker). These included also the 10 LCL obtained by infecting cultures of adherent cells from different donors. With regard to its surface markers, ES-B-1 appeared to be an exceptional EBV genome-carrying line, and it also lacked the ability to form spontaneous rosettes with sheep erythrocytes (a T cell marker). Therefore, it is possible that ES-B-1 was derived from an atypical B cell or B cell precursor or from a so-called "null cell" transformed by EBV.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Froland S. S., Natvig J. B. Identification of three different human lymphocyte populations by surface markers. Transplant Rev. 1973;16:114–162. doi: 10.1111/j.1600-065x.1973.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Gerper P., Whang-Peng J., Monroe J. H. Transformation and chromosome changes induced by Epstein-Barr virus in normal human leukocyte cultures. Proc Natl Acad Sci U S A. 1969 Jul;63(3):740–747. doi: 10.1073/pnas.63.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greaves M. F., Brown G. Epstein-Barr virus binding sites on lymphocyte subpopulations and the origin of lymphoblasts in cultured lymphoic cell lines and in the blood of patients with infectious mononucleosis. Clin Immunol Immunopathol. 1975 Mar;3(4):514–524. doi: 10.1016/0090-1229(75)90076-8. [DOI] [PubMed] [Google Scholar]
- Henle W., Diehl V., Kohn G., Zur Hausen H., Henle G. Herpes-type virus and chromosome marker in normal leukocytes after growth with irradiated Burkitt cells. Science. 1967 Sep 1;157(3792):1064–1065. doi: 10.1126/science.157.3792.1064. [DOI] [PubMed] [Google Scholar]
- Hinuma Y., Grace J. T., Jr Cloning of immunoglobulin-producing human leukemic and lymphoma cells in long-term cultures. Proc Soc Exp Biol Med. 1967 Jan;124(1):107–111. doi: 10.3181/00379727-124-31677. [DOI] [PubMed] [Google Scholar]
- Jondal M., Klein G. Surface markers on human B and T lymphocytes. II. Presence of Epstein-Barr virus receptors on B lymphocytes. J Exp Med. 1973 Dec 1;138(6):1365–1378. doi: 10.1084/jem.138.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jondal M., Wigzell H., Aiuti F. Human lymphocyte subpopulations: classification according to surface markers and-or functional characteristics. Transplant Rev. 1973;16:163–195. doi: 10.1111/j.1600-065x.1973.tb00120.x. [DOI] [PubMed] [Google Scholar]
- Klein G., Dombos L., Gothoskar B. Sensitivity of Epstein-Barr virus (EBV) producer and non-producer human lymphoblastoid cell lines to superinfection with EB-virus. Int J Cancer. 1972 Jul 15;10(1):44–57. doi: 10.1002/ijc.2910100108. [DOI] [PubMed] [Google Scholar]
- Leibold W., Flanagan T. D., Menezes J., Klein G. Induction of Epstein-Barr virus-associated nuclear antigen during in vitro transformation of human lymphoid cells. J Natl Cancer Inst. 1975 Jan;54(1):65–68. doi: 10.1093/jnci/54.1.65. [DOI] [PubMed] [Google Scholar]
- Menezes J., Leibold W., Klein G. Biological differences between Epstein-Barr virus (EBV) strains with regard to lymphocyte transforming ability, superinfection and antigen induction. Exp Cell Res. 1975 May;92(2):478–484. doi: 10.1016/0014-4827(75)90404-8. [DOI] [PubMed] [Google Scholar]
- Menezes J., Leibold W., Klein G., Clements G. Establishment and characterization of an Epstein-Barr virus (EBC)-negative lymphoblastoid B cell line (BJA-B) from an exceptional, EBV-genome-negative African Burkitt's lymphoma. Biomedicine. 1975 Jul;22(4):276–284. [PubMed] [Google Scholar]
- Miller G., Shope T., Lisco H., Stitt D., Lipman M. Epstein-Barr virus: transformation, cytopathic changes, and viral antigens in squirrel monkey and marmoset leukocytes. Proc Natl Acad Sci U S A. 1972 Feb;69(2):383–387. doi: 10.1073/pnas.69.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minowada J., Onuma T., Moore G. E. Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J Natl Cancer Inst. 1972 Sep;49(3):891–895. [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Moss D. J., Pope J. H. EB Virus-associated nuclear antigen production and cell proliferation in adult peripheral blood leukocytes inoculated with the QIMR-WIL strain of EB virus. Int J Cancer. 1975 Mar 15;15(3):503–511. doi: 10.1002/ijc.2910150316. [DOI] [PubMed] [Google Scholar]
- PULVERTAFT J. V. A STUDY OF MALIGNANT TUMOURS IN NIGERIA BY SHORT-TERM TISSUE CULTURE. J Clin Pathol. 1965 May;18:261–273. doi: 10.1136/jcp.18.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattengale P. K., Smith R. W., Gerber P. Selective transformation of B lymphocytes by E.B. virus. Lancet. 1973 Jul 14;2(7820):93–94. doi: 10.1016/s0140-6736(73)93286-8. [DOI] [PubMed] [Google Scholar]
- Polliack A., Lampen N., Clarkson B. D., De Harven E., Bentwich Z., Siegal F. P., Kunkel H. G. Identification of human B and T lymphocytes by scanning electron microscopy. J Exp Med. 1973 Sep 1;138(3):607–624. doi: 10.1084/jem.138.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope J. H., Horne M. K., Scott W. Transformation of foetal human keukocytes in vitro by filtrates of a human leukaemic cell line containing herpes-like virus. Int J Cancer. 1968 Nov 15;3(6):857–866. doi: 10.1002/ijc.2910030619. [DOI] [PubMed] [Google Scholar]
- Pope J. H., Scott W., Moss D. J. Human lymphoid cell transformation by Epstein-Barr virus. Nat New Biol. 1973 Dec 5;246(153):140–141. doi: 10.1038/newbio246140a0. [DOI] [PubMed] [Google Scholar]
- Reedman B. M., Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 1973 May;11(3):499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- Schlossman S. F., Hudson L. Specific purification of lymphocyte populations on a digestible immunoabsorbent. J Immunol. 1973 Jan;110(1):313–319. [PubMed] [Google Scholar]
- Schneider U., zur Hausen H. Epstein-Barr virus-induced transformation of human leukocytes after cell fractionation. Int J Cancer. 1975 Jan 15;15(1):59–66. doi: 10.1002/ijc.2910150108. [DOI] [PubMed] [Google Scholar]
- Wigzell H., Sundqvist K. G., Yoshida T. O. Separation of cells according to surface antigens by the use of antibody-coated columns. Fractionation of cells carrying immunoglobulins and blood group antigen. Scand J Immunol. 1972;1(1):75–87. doi: 10.1111/j.1365-3083.1972.tb03737.x. [DOI] [PubMed] [Google Scholar]
- Yata J., Desgranges C., Nakagawa T., Favre M. C., De-The G. Lymphoblastoid transformation and kinetics of appearance of viral nuclear antigen (EBNA) in cord-blood lymphocytes infected by Epstein-Barr Virus (EBV). Int J Cancer. 1975 Mar 15;15(3):377–384. doi: 10.1002/ijc.2910150303. [DOI] [PubMed] [Google Scholar]
- de Schryver A., Klein G., Hewetson J., Rocchi G., Henle W., Henle G., Moss D. J., Pope J. H. Comparison of EBV neutralization tests based on abortive infection or transformation of lymphoid cells and their relation to membrane reactive antibodies (anti-MA). Int J Cancer. 1974 Mar 15;13(3):353–362. doi: 10.1002/ijc.2910130311. [DOI] [PubMed] [Google Scholar]


