Abstract
OBJECTIVE
To review the conceptual relationship between telehealth and translational research.
METHODS
Bibliographical search on telehealth was conducted in the Scopus, Cochrane BVS, LILACS and MEDLINE databases to find experiences of telehealth in conjunction with discussion of translational research in health. The search retrieved eight studies based on analysis of models of the five stages of translational research and the multiple strands of public health policy in the context of telehealth in Brazil. The models were applied to telehealth activities concerning the Network of Human Milk Banks, in the Telemedicine University Network.
RESULTS
The translational research cycle of human milk collected, stored and distributed presents several integrated telehealth initiatives, such as video conferencing, and software and portals for synthesizing knowledge, composing elements of an information ecosystem, mediated by information and communication technologies in the health system.
CONCLUSIONS
Telehealth should be composed of a set of activities in a computer mediated network promoting the translation of knowledge between research and health services.
Keywords: Translational Medical Research, Research in Health Services Health Research Policy, Milk Banks, Breast Feeding, Review
INTRODUCTION
Telehealth has been used with a broad scope in different countries, directly related to the myriad of health care practices.5 However, the meanings of telehealth vary according to emphasis: sometimes they tend toward technological discussion, sometimes towards the field of investigation or even towards the cyber-cultural dimension of health care, or program management. This multiplicity can be explained by the role of telehealth that is being highlighted.
In terms of programmatic and executive actions in Brazil, telehealth and translational research are the responsibility of government departments, including the Ministries of Science, Technology and Innovation, Health and Education. The inaugural landmark of the concept was the establishment of the Telemedicine University Network (RUTE), by the Ministry of Science, Technology and Innovation National Teaching and Research Network, in 2005. RUTE’s activities were, during the first stage (2006-2008), concentrated in public universities, as these institutions train health care professionals and are the locus of clinical and translational research in this country, as well as being those mainly responsible for regional permanent education in the Brazilian Unified Health System (SUS).44
According to Schmittdiel et al42 (2010), translational research in health care is a type of research that appeared mainly in order to decrease the distance between production of knowledge in laboratories and its effective use in the daily practice of medicine in health care services by innovative interventions for that population.
This study aims to contribute to understanding the specificity of the Brazilian experience, adding both the classic telehealth concept,a as well as definitions that broaden the telehealth scope to other health-related activities5,10,16 to the dimension of telehealth related to translational research.
The concept of translating knowledge originated in the actor-network theory of Latour et al (1994),30 in which translating or transferring means moving objectives, interests, devices and human beings. In its ethnography of laboratories, translating knowledge implies diversions from the route, inventing a thread between previously inexistent actors and, in some way, modifying the elements involved.29
Innovation in health care is, in principle, linked to producing new medicines. However, in a wider concept, innovation covers the whole process of implementing new ideas, products, services, processes, practice and policies,17 as well as including learning and constructing skills at different levels of aggregation, configuring assumptions of translational research.35
Translational research (TR) has two stages:
TR1 – transferring new laboratory acquired knowledge on disease mechanisms in order to develop new methods of diagnosis, treatment and prevention, as well as first tests in humans, and
TR2 – translating the results of these clinical studies into daily clinical practice and decision making in health.46,51
Scientists involved in clinical research create subdivisions to define TR1, involving: pre-discovery of the research; preclinical discovery; initial development stage; final development stage; stage IV approval of application and studies.25,b
In the presented models of analysis of the translational research most often applied in highly industrialized countries with high per capita gross domestic product (GDP), the pharmaceutical industry is present at the start of TR1. However, there are other important actors who are absent during the above mentioned stages. The university accompanies the first three subdivisions of TR1 such as, for example, the intense debate within the international consortium of governments of more than 18 countries coordinated by Francis Collins and the Celera Genomics company, by Craig Venter to conclude the first mapping of the human genome.
Stage IV, known as pharmacovigilance or drug safety, is the tenuous limit of TR2, which is developed in various pieces of translational research using the model proposed by Khoury & Gwinn et al,26 the so-called five stage model of translational research (FSMTR). Producing a new drug, the last point for “from workbench to hospital bed” translational research (TR1), in Brazil, the equivalent of gaining National Health Surveillance Agency (Anvisa) approval, is only the starting point of the second stage (TR2) of translation.27
In FSMTR, epidemiology is presented as the basis of translational research, using examples from the field of genome studies. From this focus, the five stages of this research are:
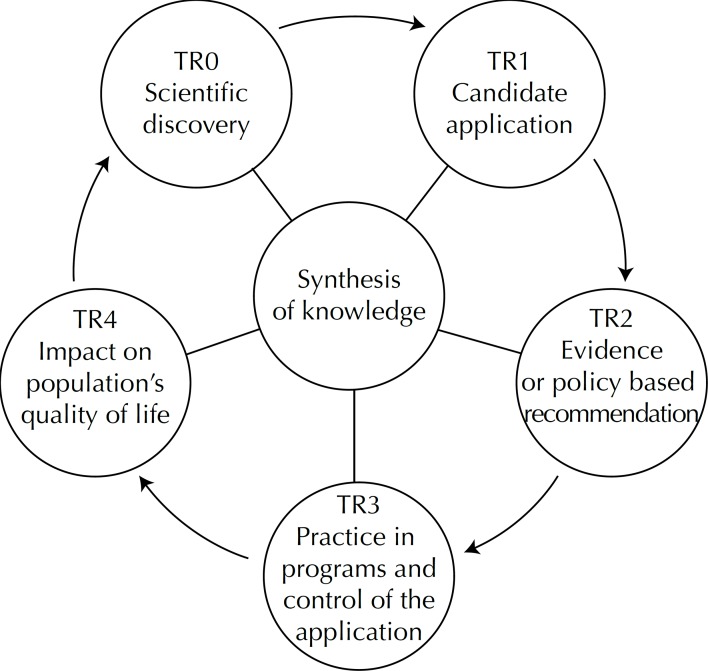
TR0 – scientific discovery from the research.
TR1 – from discovery to choice of application.
TR2 – the stage at which localized pilot studies are conducted, providing for the scale of the health care service. It includes ensuring the appropriateness of the candidate application (medication, clinical intervention, among others) until the policy for its adoption or, even, an evidence-based recommendation is consolidated.
TR3 – stage at which the policy or recommendation of the practical application of the programs is effectively disseminated in health care system and their controls.
TR4 – the stage covering the practice of the translational research and its impact on the population, directly involving health care program monitoring practices.
There is one more stage that links TR4 to TR0, thus closing the translational research cycle, in which the research solidifies future lines of health care research.
Central to the model is synthesis of knowledge, understood as a systematic and indispensable approach at all stages of translational research (Figure 1) to review evidence concerning peer knowledge of specific topics.26
Figure 1.
Model of the five stages of translational research.
The methods of synthesizing knowledge, such as meta-analysis, begin to become standardized in the development of evidence-based recommendation for practice (TR2) and can be seen in the Cochrane Collaborationc and in independent groups such as the US Preventive Services Task Forced and the Human Genome Epidemiology Network (HuGENet),e which synthesize data on genome research.
In the ambit of contemporary science, the health care practice paradigm has been modified based on human genome mapping and the discovery of genetic engineering.37,38 Moreover, the development of information and communication technologies is responsible for greater dissemination of scientific knowledge.11 Biology has become an informational, rather than taxonomic science, as rapid processing machines means scientists can create and apply algorithms to include models to explain complexity.4,f Genome studies, population genetics and quantitative genetics, making up the so-called genetic epidemiology7,12,13 require broad collaboration and multi-disciplinary and network teamwork. The new questions posed by this dynamic of scientific investigation bring dynamism into bioethics6 and it was these trends that inspired the Khoury group to systemize FSMTR.
The aim of this article was to review the telehealth concept in light of processes involving translational research in health care services, as there is a gap in the literature regarding the relationship between translational research practice in health and telehealth. To demonstrate telehealth in health care services, the theoretical framework of the stages of translational research was applied in observing Network of Human Milk Banks (HMBN) activities as part of the Telemedicine University Network with broad national and international coverage.
METHODS
The study had two different phases: a detailed, narrative review of telehealth and translational research; participant observation in the field to test the model of TR stages including multiple policy streams.
The detailed review aimed to identify conceptual elements of telehealth in the scientific community, linked to discussion of translational research in health care during the 2009 to 2012 period. A search strategy was established and the following four bibliographic databases were selected: Scopus, for its multi-disciplinary coverage; Cochrane Library for the Virtual Health Library (Cochrane VHL), in order to identify studies and reviews; LILACS (Latin American and Caribbean Literature on health sciences) and Medline. Three search terms were used: “telemedicine” OR “telehealth” AND “translational”.
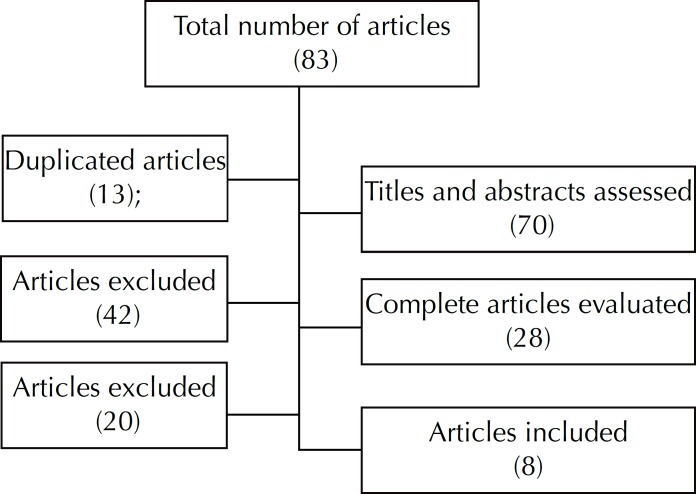
In Scopus, 20 works were recovered, seven of which were articles, four reviews, three conference summaries, two brief communications, one book, one chapter of a book, an editorial and a note. In the Cochrane VHL, 45 studies were found, all systematic reviews. In LILACS, the search was conducted using the key work telemedicine and it returned seven journal articles and one learning object. The Medline search retrieved 11 articles indexed in scientific journals.
Articles that were duplicated, without abstracts or missing hyperlinks were eliminated. Of the 83 studies found, 28 were assessed regarding their relevance to the topic and eight were used in the second part of the research. Systematic reviews were privileged, as in general they included previous discussion of terminology and an outlined objective for elimination of treatment of a specific health problem.
The second stage of this work involved an analyticaconceptual study based on identifying characteristics of the translational research cycle in government telehealth actions, the RUTE. The aim was to investigate aspects of possible relationships between telehealth and translational research, as well as the bibliographical analysis. For the analysis, the FSMTR was used as a reference to support the model analyzing the multiple streams framework in the manner established by Kingdon28 (2002).
RUTE, the field of observation in this study, was established in 2005 as an action aiming to implement and interconnected infrastructure between university hospitals and health care education units in Brazil. The RUTE has been strengthened by, still incipient, telehealth projects facilitating exchange between national groups of researchers by a high speed network connection. Moreover, it provides equipment to research groups in participating institutions and operationally supports multi-institutional discussion hubs, Special Interest Groups (SIG).43
In the RUTE universe, the initiative coordinated by the Instituto Nacional de Saúde da Mulher, da Criança e do Adolescente Fernandes Figueira (IFF/Fiocruz –Fernandes Figueira National Institute for Women’s, Children’s and Adolescents’ Health), was selected for its coverage. The IFF/Fiocruz brings together 30 points in the Network of Human Milk Banks and 23 countries in the Ibero-American Human Milk Bank Program, the world’s largest such network.8 The human milk bank network uses telehealth actions, stimulating the exchange of knowledge and permanent education so as to guarantee that all newborns have access to breast milk.g
In this context, the FSMTR was applied to observe the functionality of telehealth in the passage of breast milk in the public health network, from the donating to the recipient mother, including babies with low birth weight and with long hospitalizations in neonatal intensive care units.
FSMTR is a closed, cyclical system. In the case of clinical research, TR2, TR3 and TR4, easily identified in health care services, are connected to TR0 and TR1, so-called pre-innovation, which cover the pre-discovery to licensing stages of new medications. In the care practices provided by the health care system, this process can also be observed and systematized in the whole translational research cycle.
Before identifying more connections linking telehealth to translational research, it is important to emphasize one criticism of the FSMTR. For Hiatt23 (2010), the above mentioned model divides translational research into many stages, which may cause confusion in choosing monitoring and evaluation indicators. He therefore postulates that the central problem of health research is the need to put tested and efficient evidence-based interventions into practice in the shortest time possible. Another concern highlighted is the possibility of fragmenting the translational science process in the field of health care. The need for a trans-disciplinary approach to resolve this division is also highlighted.
The World Health Organization (WHO), allied to the International Telecommunication Union (2012),50 proposal of a global model to evaluate telehealth outlines the interlocutors involved in planning and executing monitoring and evaluation activities in each process. On the other hand, governments19 use adaptations of FSMTR to perfect their health care and health information system networks.
Hiatt’s23 proposal for a trans-disciplinary view of FSMTR, with the decisive contribution of studies of public policy, outlines political spaces and actors, detail micro-processes of stages and create a structured outline in order to understand telehealth as translational research in the context of SUS health care networks.
Kingdon’s multiple streams framework has been useful in investigation including specific themes in government policy agendas9 and, in this study, was used to respond to gaps in FSMTR highlighted by Hiatt’s criticism. The model divides analysis of policies into three streams: that of problems, that of alternatives and that of policies. Each stream follows a specific path, especially that of policy which, despite problems and available alternatives, follows its own dynamic and rules.
To recognize the problem, there are three relevant dimensions: indicators referring to the issue in question, public opinion and economic viability. When constructing alternatives, centrality of ideas is fundamental to including persuasion and diffusion. In the political stream, coalitions are constructed in the negotiation and bargaining process. Thus, the model seeks to comprehensively cover this process of drawing up public policies, identifying and studying these three streams in a differentiated manner.44
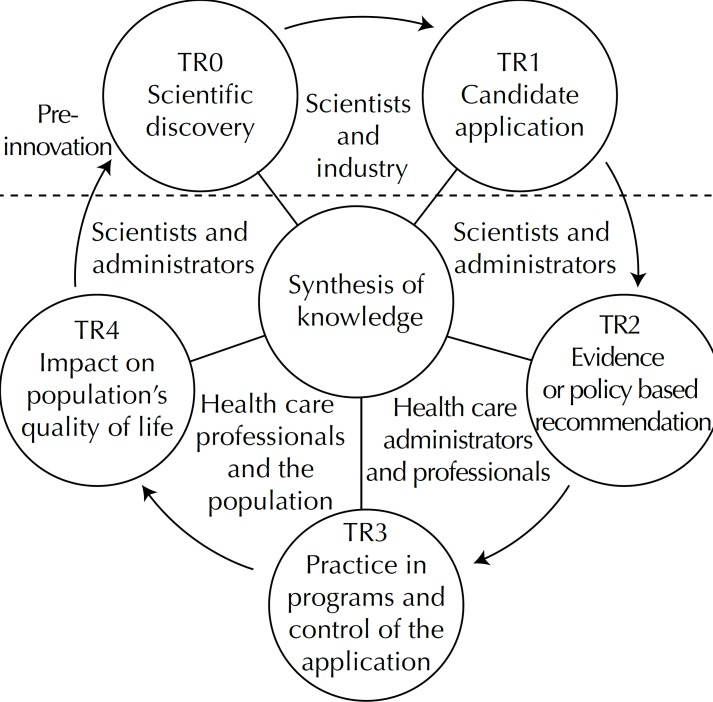
In the case of FSMTR, identifying the predominant interest groups during the transition from one stage to another could contribute to understanding the translational research cycle in health care services (Figure 2).
Figure 2.
Interest groups at the five stages of translational research, together with the multiple streams framework.
The scientific community, interacting with industry, is the protagonist in the passage from TR0 to TR1. In TR1 to TR2, the interest groups are predominantly made up of scientists and administrators. Health care professionals deal with administrators in the passage from TR2 to TR3. The population interact with health care professionals in diverse ways (as SUS users, by social control and in research and evaluations) in TR3 and in TR4.
In health, this interaction feeds into evaluation research in which social scientists, epidemiologists and researchers from a variety of disciplines interact. The results obtained support administrators and the scientific community itself in new discoveries, moving from TR4 to TR0 and closing the cycle.
RESULTS
Much polysemy was observed in the telehealth concept in the way the word was used in the area of health. When searching the four databases using the key words “telehealth” and “translational research” and the descriptor “telemedicine”, eight works were recovered. Studies citing translational research in health care analyzed tele-education in combatting specific public health problems such as, e.g., the prevalence of type 2 diabetes in ethnic minorities,22 distance monitoring of medication use by the elderly population39 and support for family members and carers of those suffering from dementia.34 Three articles dealt with the context in which FSMTR was conceived: discussion of the role of national institutes in the health care system.10,14,47
When applying FSMTR, combined with the actors identified in the multiple streams, it was possible to identify the problem (infant mortality), alternatives (preserve donated human milk and stimulate breastfeeding) and policies (constructing and regulating the HMB in high complexity hospitals, close to neonatal ICU) within the SUS.
It is worth explaining that the objective of the HMB network is the practice of breastfeeding, human milk and awareness of its properties by society in general as it meets the child’s nutritional needs and peculiarities of metabolism, with the value of exclusive breastfeeding for the first six months in no doubt.3
Even though they are common sense, it is worth highlighting the advantages of breast milk: better digestibility; balanced chemical composition; lack of principal allergens; protects child’s organism against infection; encourages babies’ IQ development and costs nothing. To summarize, breastfeeding is the best way to feed the baby, being the basis for biological and emotional effects in the child’s development.2
In various parts of the world, public health care strategies have been developed for mothers who, for one reason or another, do not breastfeed their child, including the Brazilian model of human milk banks (HMB).18 In this model, HMB operate using alternative technology, enabling low operational cost to be allied with technical rigor to ensure the quality of the human milk collected, stored and distributed.36,45 This milk is obtained from donors, healthy lactating women who produce more milk than needed for their own child and are willing to donate the surplus, without receiving any remuneration. This is passed on to premature babies, those with low birth weight and those hospitalized in neonatal ICU. One study reviewed concluded that telemedicine stimulated translational research, especially in neonatology.21
The HMB network exists in 23 countries and has used the RUTE since 2009. Web conferencing and video conferencing and other telehealth devices, including support of synchronous and asynchronous health information systems, are used to exchange knowledge and practice. The scale of telehealth service use, e.g., the need to compare microbiological samples of human milk from laboratories in different locations,h has increased.
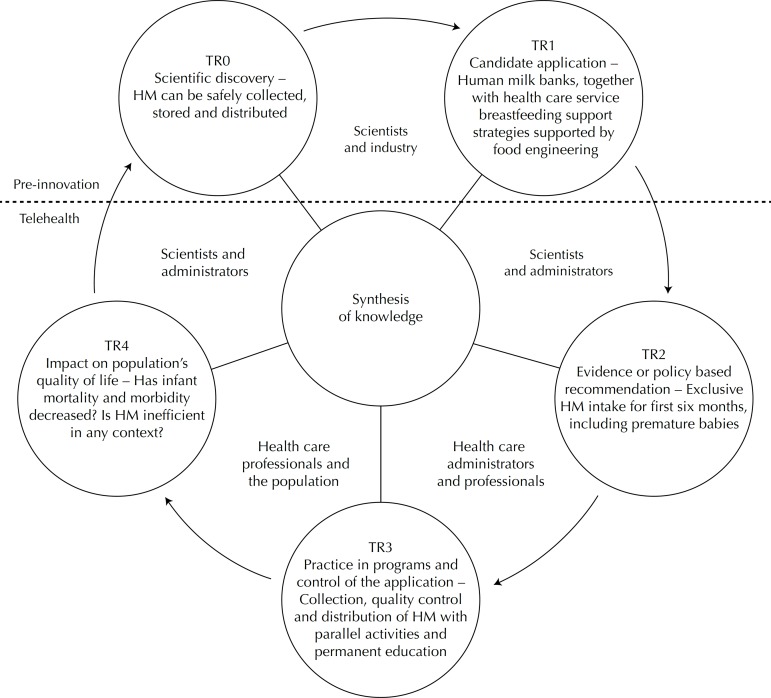
It was possible to apply FSMTR to the multiple streams in order to contextualize it in the SUS health care network. Some telehealth processes were observed integrated in this cycle (Figure 4).
Figure 4.
Flow chart of the process of selecting articles for detalied literature review (2009-2012).
Figure 3.
Model of the five stages of translational research in the context of multiple streams framework applied to the Human Milk Bank Network.
TR0 is a scientific discovery that has been perfected over a period of more than 50 years: the pasteurization process means human milk can, at low cost, be stored, conserving its properties, and purified of manipulation from the collection, storing and distribution stages.31 Telehealth is present at this stage as it provides audio-visual records of the techniques used in the milk bank and disseminates them via the information and technology networks for permanent education purposes.
At stage TR1, the IFF/Fiocruz Human Milk Bank National Reference Centre candidate application of the HMBN, was recognized by Anvisa,i which established HMB as a specialized service linked to maternity and children’s hospitals. The HMB was responsible for actions promoting, protecting and supporting breastfeeding, as well as collecting milk and selecting, classifying and processing, quality control and distribution activities, with commercialization of the products distributed being strictly prohibited.j The WHOk recommends exclusive breastfeeding for the first six months and continuing to breastfeed until two years of age (TR2). The evidence-based policy or directive providing human milk to babies, including premature babies, is considered a food security strategy aimed at achieving the UN Millennium Development Goal of reducing the 1990 infant mortality rate in under-five children by two thirds by 2015.l
Permanent and continuous education of health care professionals, and of the population, is an essential element in health activities involving collection, quality control and distribution of human milk (TR3). However, microbiological analysis of samples of human milk, definition of collection rounds, maintaining the cold chainm during transportation from donor’s house to the HMB, are potential objects of telehealth applications regarding quality control.
That UN Millennium Development Goal has been met in almost the whole of Brazil. Mortality and morbidity have decreased and the infant population’s state of health improved (TR4) with the health care services’ promotion of breastfeeding being a decisive contribution.49
There are many scientific possibilities that remain to be explored in relation to human milk, such as the discovery of a technique for multiplying stem cells found in human milk,40 made by researchers at a Brazilian public university,n,o facilitated by the context of donated human milk and by the presence of HMB integrated into the SUS health care network (TR4 – TR0).
Synthesis of knowledge appears in the HMBN internet portal. This is the principal reference for the HMBN community and brings together various activities related to telehealth and translational research, such as: facilitated access to systematic reviews, clinical trials; technical norms; international network management; virtual community; listings of research groups; minutes of video conferences; web conference rooms; information systems on the production of human milk and a direct channel to the population according to the trends of change in the care paradigm observed in the review, which emphasizes the formal aspects of modelling and implementing telehealth ubiquitously and multi-discipline integration to provide personalized health care.20
DISCUSSION
The models of the five stages of translational research and the multiple streams emphasize the strategic role of HMB coordinators in RUTE hospitals, as they are scientists or administrators who train professionals who will work in the SUS.
An example is the “Human Milk Debate” session, in 2011, a HMBN video conference in the Hospital Universitário of the Universidade de São Paulo (USP), in which the results of research on bone mineralization in newborns fed on human milk were presented to the community before journal publication.
On the other hand, the processes involved in the passage from TR3 to TR4 require social participation beyond the user-patient role, prioritized in the majority of primary epidemiological studies. The basic research is only characterized as translational when it has a high degree of effectiveness in the health care system and predicted improvements in health are achieved. To measure the degree of effectiveness of translational research in the health care system, its real performance (monitoring the system) needs to be documented, as well as the performance that health care science and technology can achieve in “ideal” conditions (efficacy of application generated by research). This comparative calculation is known as relative effectiveness.15
It is necessary to see how telehealth processes in the SUS could be essential facilitators in conducting the stages of translational research from the point of view of relative effectiveness, the concept adopted in the SUS Evaluation Program.48 Stages TR2, TR3 and TR4, in the triangle proposed by Glasgow19 (2012), extrapolate scientific means and rely on the intervention of different segments of society to become reality. Telehealth could even help in the cycle’s feedback loop, returning to TR0, as remote access technology to the databases produced by their respective platforms, when well-planned and interoperable, can be of great service to health surveillance and health care management. In this sense, the widespread tele-cardiology service covering more than 800 locations in Minas Gerais stands out.1
The strategy of coordinating centers of excellence all over the world to research cures for poverty-related disease is another example of research that mobilizes scientists, administrators and civil society organizations.32
With the support of social sciences, it is expected that the redefinition of telehealth can support choices of quality indicators for monitoring and evaluating health care systems. Telehealth encourages more rapid organization and synthesis of knowledge, facilitates knowledge exchange and accelerates implementation of innovations in the health care network.
CONCLUSION
Telehealth is not a synonym of video conferencing,24 wireless technology33 or an organizational component to establish information and communication technology or networks in health care units.41 Thus, it does not compete with other terms in the literature, such as telemedicine, ehealth or mhealth. Based on analysis of FSMTR, together with the multi-streams model, the opportunity to view telehealth as all computer-mediated network activity promoting translation of knowledge between research and health care services appears. Thus, a wide and structured concept emerges in the context of a health care system qualified by technological advances.
Seeking to broaden the World Medical Association and WHO concept of telehealth, which can be summarized as information and communication technology in health care, we propose redefining telehealth as health care practice and knowledge that, by mediation of technology, materializes in the health care information and knowledge ecosystem.10 In effect, telehealth grows ever closer to the translational research process as it enables individuals doing science to benefit those in health care services. Thus, this dimension is added to the cultural and institutional framework: cyberculture in health.
Identifying the cybercultural connections and processes of this ecosystem processes in which the research stages occur may help in evaluating telehealth and indicate better practice for health care systems.
Footnotes
Article based on the doctoral thesis of Silva AB, entitled: “Política pública, educação, tecnologia e saúde articuladas: como a telessaúde pode contribuir para fortalecer o SUS?”, presented to the Escola Nacional de Saúde Pública Sergio Arouca/Fiocruz, in 2013.
The authors declare that there is no conflict of interest.
According to the World Medical Association – WMA and the World Health Organization – WHO, telehealth is the use of information and communication technologies – ICT to offer health care and services at a distance. Cf. www.wma.net/en/30publications/10policies/t5/index.html and www.who.int/kms/initiatives/ehealth/en/
Clinical research involving human beings is, in general, classified into four stages: I to IV. In short, in stage I the medication is tested in small groups of healthy individuals; stage II involves a larger group suffering from the disease the medication is designed to combat; stage III involves multi-centric studies with thousands of patients and can lead to the medication being approved by the responsible government body; and, finally, stage IV in which commercial mass use of the drug is monitored
The Cochrane Collaboration [Internet]. Oxford (UK); c2014 [cited 2014 Mar 14]. Available from: http://www.cochrane.org/
U.S. Preventive Service Task Force [Internet]. Rockville, MD; 2010 [cited 2014 Mar 14]. Available from: http://www.uspreventiveservicestaskforce.org/
Centers for Disease Control and Prevention, Office of Public Health Genomics. Population Research: Human Genome Epidemiology Network - HuGENET [Internet]. Atlanta, GA; [updated 2013 Jan 29; cited 2014 Mar 14]. Available from: http://www.cdc.gov/genomics/hugenet/
The complex object is a non-linear and multi-faceted systemic-model object. It forms part of the system of partial totalities and can be understood as a system, as it incorporates partial totalities from the lower hierarchical level. This object can be understood at multiple levels of existence, as it operates at different levels of reality and is the source of multiple discourses, spilling over from the disciplinary outlines of science. For it to be constructed as a reference requires synthesis, producing synthetic models in which different discipline discourses intersect.
Silva AB, Souza KS. Estratégia de telessaúde do núcleo RUTE do IFF reúne países em prol da diminuição da mortalidade infantil prevista no Objetivo de Desenvolvimento do Milênio. In: Anais do 5º Congresso Brasileiro e Internacional de Telemedicina e Telessaúde Inovação e Sustentabilidade; 19-22 Nov 2011; Manaus. São Paulo: USP; 2011.
According to HMB National Reference Center, doubts come from states and countries regarding equipment which, in general, are not programmed to identify bacteria typical to the microbe ecology of HM and are concerned about impurities. Telehealth actions, such as remote configurations, can respond to these issues of HM quality control.
Agência Nacional de Vigilância Sanitária, Diretoria Colegiada. Resolução de Diretoria Colegiada – RDC nº 171, de 4 de setembro de 2006. Dispõe sobre o Regulamento Técnico para o funcionamento de Bancos de Leite Humano. [cited 2014 Jan 14]. Available from: http://portal.anvisa.gov.br/wps/wcm/connect/d02994804745973f9fa1df3fbc4c6735/RDC+N%C2%BA.+DE+171-2006.pdf?MOD=AJPERESS
Silva DA. Ensaios de proficiência para bancos de leite humano: formulação e avaliação de uma proposta para a Rede Brasileira de Bancos de Leite Humano [doctoral thesis]. Rio de Janeiro: Instituto Fernandes Figueira/Fiocruz; 2009.
World Health Organization. The WHO´s infant feeding recommendation. Geneva; 2002 [cited 2014 Jan 14]. Available from: http://www.who.int/nutrition/topics/infantfeeding_recommendation/en/index.html
Ban Ki-Moon. Children and the Millennium Development Goals: progress towards a world fit for children. New York: UNICEF;2007 [cited 2014 Jan 14]. Available from: http://www.unicef.org/publications/files/Children_and_the_MDGs.pdf
Maintaining the temperature of the transport so the product remains frozen between the point of origin and the destination, with no changes in temperature which would cause microbiological or physicochemical non-compliance.
Motta C. Cientistas obtêm células-tronco de leite materno. O Globo. 2011 out 10 [cited 2014 Jan 14]; Ciência. Available from: http://oglobo.globo.com/ciencia/mat/2011/10/25/cientistas-obtem-celulas-tronco-de-leite-materno-925660765.asp
Cryopraxis: Banco de Sangue de Cordão Umbilical. Rio de Janeiro; c2012 [cited 2014 Jan 14]. Available from: http://www.cryopraxis.com.br/home
REFERENCES
- 1.Alkmim MB, Figueira RM, Marcolino MS, Cardoso CS, Abreu MP, Cunha LR, et al. Improving patient access to specialized health care: the Telehealth Network of Minas Gerais, Brazil. Geneva: World Health Organization; 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almeida JAG, Maia PRS, Novak FR, Sydronio K. Bancos de leche humana y promoción de políticas públicas favorables a la salud materno-infantil. [citado 2014 Mar 17];Rev Cubana Salud Pública. 2006 Sep;32(3) revista en la Internet. Disponible en: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S0864-34662006000300012&lng=es. [Google Scholar]
- 3.Almeida JAG. Breastfeeding: a nature-culture hybrid. Rio de Janeiro: Editora Fiocruz; 2001. [Google Scholar]
- 4.Almeida N., Filho Almeida N, Filho, Barreto ML. Epidemiologia e saúde: fundamentos, métodos, aplicações. Rio de Janeiro: Guanabara Koogan; 2012. Epidemiologia e modelos de complexidade: perspectivas metodológicas; pp. 291–306. [Google Scholar]
- 5.Bashshur R, Shannon G, Krupinski E, Grigsby J. The taxonomy of telemedicine. Telemed J E Health. 2011;17(6):484–494. doi: 10.1089/tmj.2011.0103. [DOI] [PubMed] [Google Scholar]
- 6.Bergel SD. Bioética, genética y derechos humanos: la declaración de la Unesco. Rev Bioética. 1999;7(2):165–178. [Google Scholar]
- 7.Blanton RE, Silva LK, Melo PRS. Almeida N, Filho, Barreto ML. Epidemiologia e saúde: fundamentos, métodos, aplicações. Rio de Janeiro: Guanabara; 2011. Epidemiologia genética; pp. 342–349. [Google Scholar]
- 8.Cánepa MA. Un modelo de cooperación horizontal: la Red Iberoamericana de Bancos de Leche Humana (BLH) Madrid: OPAS; 2011. [Google Scholar]
- 9.Capella AC. Hochman G, Arretche M, Marques E. Políticas públicas no Brasil. Rio de Janeiro: Editora Fiocruz; 2007. Perspectivas teóricas sobre o processo de formulação de políticas públicas; pp. 87–124. [Google Scholar]
- 10.Carroll M, James JA, Lardiere MR, Proser M, Rhee K, Sayre MH, et al. Innovation networks for improving access and quality across the healthcare ecosystem. Telemed J E Health. 2010;16(1):107–111. doi: 10.1089/tmj.2009.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castells M. A Galáxia Internet: reflexões sobre a Internet, negócios e a sociedade. Rio de Janeiro: Jorge Zahar; 2003. [Google Scholar]
- 12.Cordell HJ, Clayton DG. Genetic association studies. Lancet. 2005;366(9491):1121–1131. doi: 10.1016/S0140-6736(05)67424-7. [DOI] [PubMed] [Google Scholar]
- 13.Devlin B, Roeder K. Genomic control for association studies. Biometrics. 1999;55(4):997–1004. doi: 10.1111/j.0006-341x.1999.00997.x. [DOI] [PubMed] [Google Scholar]
- 14.Doarn CR, Portilla LM, Sayre MH. NIH conference on the future of telehealth: essential tools and technologies for clinical research and care: a summary. Telemed J E Health. 2010;16(1):89–92. doi: 10.1089/tmj.2009.0151. [DOI] [PubMed] [Google Scholar]
- 15.Donabedian A, Bashshur R. An introduction to quality assurance in health care. New York: Oxford University Press; 2003. [Google Scholar]
- 16.Eysenbach G. What is e-health? J Med Internet Res. 2001;3(2):E20. doi: 10.2196/jmir.3.2.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gardner CA, Acharya T, Yach D. Health Aff. 4. Vol. 26. Millwood: 2007. Technological and social innovation: a unifying new paradigm for global health; pp. 1052–1061. [DOI] [PubMed] [Google Scholar]
- 18.Giugliani ERJ. J Pediatria. 3. Vol. 78. Rio J: 2002. Rede Nacional de Bancos de Leite Humano do Brasil: tecnologia para exportar; pp. 183–184. [DOI] [PubMed] [Google Scholar]
- 19.Glasgow RE, Green LW, Taylor MV, Stange KC. An evidence integration triangle for aligning science with policy and practice. Am J Prev Med. 2012;42(6):646–654. doi: 10.1016/j.amepre.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall JA, Blobel B. Paradigm changes in health lead to paradigm changes in pathology. Stud Health Technol Inform. 2012;179:38–50. doi: 10.3233/978-1-61499-086-4-38. [DOI] [PubMed] [Google Scholar]
- 21.Hall RW, Hall-Barrow J, Garcia-Rill E. Neonatal regionalization through telemedicine using a community-based research and education core facility. Ethn Dis. 2010;20(1) Suppl Suppl 1:136–140. [PMC free article] [PubMed] [Google Scholar]
- 22.Hawthorne K, Robles Y, Cannings-John R, Edwards AGK. Culturally appropriate health education for type 2 diabetes mellitus in ethnic minority groups. Cochrane Database Syst Rev. 2008;(3): doi: 10.1002/14651858.CD006424.pub2. [DOI] [PubMed] [Google Scholar]
- 23.Hiatt RA. Invited commentary: the epicenter of translational science. Am J Epidemiol. 2010;172(5):525–527. doi: 10.1093/aje/kwq212. [DOI] [PubMed] [Google Scholar]
- 24.Kailas A, Ingram MA. Wireless aspects of telehealth. Wireless Pers Commun. 2009;51(4):673–686. doi: 10.1007/s1277-009-9763-7. [DOI] [Google Scholar]
- 25.Kaitin KI. Deconstructing the drug development process: the new face of innovation. Clin Pharmacol Ther. 2010 Mar;87(3):356–361. doi: 10.1038/clpt.2009.293. Epub 2010 Feb 3. Review. Erratum in: Clin Pharmacol Ther. 2011 Jan;89(1):148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khoury MJ, Gwinn M, Ioannidis JPA. The emergence of translational epidemiology: from scientific discovery to population health impact. Am J Epidemiol. 2010;172(5):517–524. doi: 10.1093/aje/kwq211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khoury MJ, Gwinn M, Ioannidis JPA. Khoury et al respond to “The epicenter of translational science”: crossing all the T’s. Am J Epidemiol. 2010;172(5):528–529. [Google Scholar]
- 28.Kingdon JW. Agendas, alternatives, and public policies. 2. New York: Longman Publishing Group; 2002. Longman Classics in Political Science. [Google Scholar]
- 29.Latour B. Ciência em ação: como seguir cientistas e engenheiros sociedade afora. São Paulo: Editora UNESP; 2000. [Google Scholar]
- 30.Latour B. Jamais fomos modernos: ensaio de antropologia simétrica. Rio de Janeiro: Editora 34; 1994. [Google Scholar]
- 31.Maia PRS, Novak FR, Almeida JAG, Silva DA. Bases conceituais para uma estratégia de gestão: o caso da Rede Nacional de Bancos de Leite Humano. Cad Saude Publica. 2004;20(6):1700–1708. doi: 10.1590/S0102-311X2004000600029. [DOI] [PubMed] [Google Scholar]
- 32.Manderson L, Aagaard-Hansen J, Allotey P, Gyapong M, Sommerfeld J. Social research on neglected diseases of poverty: continuing and emerging themes. PLoS Negl Trop Dis. 2009;3(2): doi: 10.1371/journal.pntd.0000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masi C, Hamlish T, Davis A, Bordenave K, Brown S, Perea B, et al. J Clin Hypertens. 1. Vol. 14. Greenwich: 2012. Using an established telehealth model to train urban primary care providers on hypertension management; pp. 45–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moniz Cook ED, Swif K, James I, Malouf R, De Vugt M, Verhey F. Functional analysis-based interventions for challenging behaviour in dementia. Cochrane Database Syst Rev. 2012;2: doi: 10.1002/14651858.CD006929.pub2. [DOI] [PubMed] [Google Scholar]
- 35.Morel CM. Inovação em saúde e doenças negligenciadas [editorial] Cad Saude Publica. 2006;22(8):1522–1523. doi: 10.1590/S0102-311X2006000800001. [DOI] [PubMed] [Google Scholar]
- 36.Novak FR, Almeida JAG. J Pediatria. 3. Vol. 78. Rio J: 2002. Teste alternativo para detecção de coliformes em leite humano ordenhado; pp. 193–196. [DOI] [PubMed] [Google Scholar]
- 37.Oda L. Mudança de paradigma científico. Hist Cienc Saude Manguinhos. 2000;7(2):515–517. doi: 10.1590/S0104-59702000000300022. [DOI] [PubMed] [Google Scholar]
- 38.Offit K. Personalized medicine: new genomics, old lessons. Hum Genet. 2011;130(1):3–14. doi: 10.1007/s00439-011-1028-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patterson SM, Hughes C, Kerse N, Cardwell CR, Bradley MC. Interventions to improve the appropriate use of polypharmacy for older people. Cochrane Database Syst Rev. 2012;5: doi: 10.1002/14651858.CD008165.pub2. [DOI] [PubMed] [Google Scholar]
- 40.Patki S, Kadam S, Chandra V, Bhonde R. Human breast milk is a rich source of multipotent mesenchymal stem cells. Hum Cell. 2010;23(2):35–40. doi: 10.1111/j.1749-0774.2010.00083.x. [DOI] [PubMed] [Google Scholar]
- 41.Schmeida M, McNeal R. The telehealth divide: disparities in searching public health information online. J Health Care Poor Underserved. 2007;18(3):637–647. doi: 10.1353/hpu.2007.0068. [DOI] [PubMed] [Google Scholar]
- 42.Schmittdiel JA, Grumbach K, Selby JV. System-based participatory research in health care: an approach for sustainable translational research and quality improvement. Ann Fam Med. 2010;8(3):256–259. doi: 10.1370/afm.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silva AB, Amorim AC. A Brazilian educational experiment: teleradiology on web TV. J Telemed Telecare. 2009;15(7):373–376. doi: 10.1258/jtt.2009.090204. [DOI] [PubMed] [Google Scholar]
- 44.Silva AB, Moraes IHS. O caso da Rede Universitária de Telemedicina: análise da entrada da telessaúde na agenda política brasileira. Physis. 2012;22(3):1211–1235. doi: 10.1590/S0103-73312012000300019. [DOI] [Google Scholar]
- 45.Sousa PPR, Silva JA. Monitoramento da qualidade do leite humano ordenhado e distribuído em banco de leite de referência. Rev Inst Adolfo Lutz. 2010;69(1):7–14. [Google Scholar]
- 46.Sung NS, Crowley WF, Jr, Genel M, Salber P, Sandy L, Sherwood LM, et al. Central challenges facing the national clinical research enterprise. JAMA. 2003;289(10):1278–1287. doi: 10.1001/jama.289.10.1278. [DOI] [PubMed] [Google Scholar]
- 47.Topol EJ, Schork NJ, Smith JM. Digital medicine and the Scripps Translational Science Institute. Clin Transl Sci. 2011;4(1):8–9. doi: 10.1111/j.1752-8062.2010.00256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Viacava F, Ugá MAD, Porto S, Laguardia J, Moreira RS. Avaliação de desempenho de sistemas de saúde: um modelo de análise. Cienc Saude Coletiva. 2012;17(4):921–934. doi: 10.1590/S1413-81232012000400014. [DOI] [PubMed] [Google Scholar]
- 49.Victora CG, Aquino EML, Leal MC, Monteiro CA, Barros FC, Szwarcwald C. Maternal and child health in Brazil: progress and challenges. Lancet. 2011;377(9780):1863–1876. doi: 10.1016/S0140-6736(11)60138-4. [DOI] [PubMed] [Google Scholar]
- 50.World Health Organization . National eHealth Strategy Toolkit: overview. Geneva: 2012. [Google Scholar]
- 51.Woolf SH. The meaning of translational research and why it matters. JAMA. 2008;299(2):211–213. doi: 10.1001/jama.2007.26. [DOI] [PubMed] [Google Scholar]