Abstract
OBJECTIVE
To analyze factors associated with persistence to breast cancer hormone therapy in order to contribute to the quality of care improvement.
METHODS
Retrospective longitudinal study, based on secondary data. A cohort of 5,861 women with breast cancer registered in different datasets of the Brazilian National Cancer Institute and the Brazilian Unified Health System were analyzed. All women were treated at this hospital, which provides free medication, and the follow-up period was from January 2004 to October 2010. Sociodemographic, behavioral, and clinical variables, as well as aspects of lifestyle and health care, were considered in the explanation of variations in the persistence to hormone therapy, applying the Kaplan-Meier method and the Cox proportional hazard model.
RESULTS
Overall persistence to hormone therapy was 79.0% at the end of the first year, and 31.0% in five years of treatment. The risk of discontinuing hormone therapy was higher among women under 35 years old, with more advanced disease (stages III and IV), alcohol drinkers, those undergoing chemotherapy, and for each additional hospitalization, exam performed, and month between diagnosis and beginning of treatment. In the opposite direction, the risk of discontinuity was lower among women who had at least finished high school, those with partner, with a family history of cancer, those who had undergone breast surgery, and who had outpatient visits to a Mastologist, and a Clinical Oncologist.
CONCLUSIONS
The majority of the women with breast cancer (69.0%) do not persist with hormone treatment for the five years recommended, increasing the risk of inadequate clinical results. The results show aspects of care that can provide better results.
Keywords: Breast Neoplasms; Patient Dropouts; Drug Therapy; Antineoplastic Agents, Hormonal, administration & dosage; Risk Factors; Health care; Women’s Health
INTRODUCTION
Endocrine or hormone therapy for breast cancer consists of using substances similar to hormones, or substances which suppress hormones in order to inhibit the tumor’s growtha and has, for decades, been associated with improved cancer free survival rates and mortality rates.b The treatment is long-term, with significant adverse effects,3 as the suppression of female hormones means, for some women, an additional mutilation of the image as a woman in addition to the mastectomy.18
There is interest in adherence and persistence to hormone treatment for breast cancer not only because of the difficulties related to long term treatment with many side effects. In addition to this, incorrect use of the medications could result in increased mortality and morbidity,18 and consequent increased demand for care resources, meaning increased costs.1
Hormone therapy is only recommended after the breast tumor is assessed as estrogen and progesterone receptor positive.6-8,a It is recommended that a daily hormone therapy pill be taken for five years.8,a There is evidence that women who take tamoxifen (the most commonly used type of hormone therapy globally) for a shorter period of time have a significantly increased risk of the cancer coming back or of mortality from breast cancer.3,6-8,a
Breast cancer is the most common tumor in the female population of Brazil (an estimated 52,830 new cases in 2013) and the primary cause of death from cancer (12,852 deaths in 2010).c
Despite its potential results, and although hormone therapy is available in the Brazilian Unified Health System (SUS), little is known in this country about women with breast cancer’s adherence and persistence to the treatment and the resulting clinical results. The International Society for Pharmacoeconomics and Outcome Research4 differentiates between the concepts of adherence and persistence, with persistence, the object of this article, defined as the time from starting until discontinuing the treatment.
The factors associated with adherence and persistence to hormone therapy for breast cancer have been widely studied, but with various contradictory or non-significant results.15 The most consistent results indicate an association between the worst rates of adherence and persistence with extremes of age (the oldest and youngest patients), increased budgetary costs, monitoring by general practitioner (versus by an oncologist), treatment in which the medication changes (switching to the aromatase inhibitor after treatment with tamoxifen) and side effects of the therapy. On the other hand, taking more medications, being referred to an oncologist and shorter diagnosis time were positively associated with adherence and persistence.15
For Murphy et al,15 many studies have focused on non-modifiable factors, making new research into modifiable factors associated with adherence and persistence to hormone therapy necessary. Thus, changes in the way care is organized may be relevant to helping patients follow their treatment for the recommended period.
The objective of this study was to analyze factors associated with persistence to hormone therapy for breast cancer, in order to improve care quality.
METHODS
This was a longitudinal study based on secondary data of women with breast cancer who had been prescribed hormone therapy. The women were all treated in the National Cancer Institute (INCA), a reference center for the Brazilian Ministry of Health in defining cancer health care policies in Brazil.d INCA is the largest provider of breast cancer treatment in the state of Rio de Janeiro (Southeastern Brazil), which has the highest incidence of the disease in the country.c
The study considers all women with breast cancer in the Hospital Cancer Register (RHC) between 2002 and 2008 and who started hormone treatment (tamoxifen and/or aromatase inhibitors anastrozole or letrozole) from January 2004 onwards, according to the INCA Pharmacy Department database, and to whom medication was dispensed at least twice until October 2010.
Integration and analysis of the information found in the following databases were performed as follows:
INCA Pharmacy Department dispensing database – data on medications dispensed including date, type of medication (tamoxifen letrozole and anastrozole) and quantity. Only patients who started hormone therapy after January 1, 2004 were considered as this database was only created in October 2003, including patients already undergoing treatment. The final date included for dispensing medication was October 29, 2010.
RHC – used to obtain sociodemographic and clinical variables of patients, as well as deaths. The inclusion criterion for the study of patients registered with a breast tumor between 2002 and 2008 was due to the availability of data at the time the study was carried out. The RHC is organized by tumor, meaning that patients with more than one primary malignant tumor (excluding recurrence or metastasis) may be registered more than once. For patients registered with multiple tumors, the most complete observation was used: the observation with the highest disease stage if the dates of diagnosis were the same or the first observation if the dates of diagnosis were different.
INCA Integrated Hospital System (SHI) and Absolute System – were used to identify the procedures undergone by the women with breast cancer. The SHI was established in 1998 and was used by INCA until 2004, when it was substituted by the Absolute System. The period considered was January 1, 2002 to October 29, 2010. While exploring the Absolute System, greater disaggregation of categories was observed than that of the SHI, requiring them to be made compatible.
Mortality Information System (SIM) – used to complement the information on deaths available in the RHC.
The registration number (from the medical record) of the women treated at INCA was used to integrate the databases, which was done hierarchically, beginning with the data from the Pharmacy and the RHC, with the others added later.
After uniting the databases, the difference between the start date of hormone therapy and the breast cancer diagnosis was found, with 198 cases with negative values verified, probably due to typing errors. Correction consisted of the following procedures: (1) when the date of starting treatment differed negatively from the diagnosis by fewer than three months, diagnosis and starting hormone treatment was considered to coincide – with a difference equal to zero; (2) if the negative difference between diagnosis and starting hormone therapy was more than three months and the second date of dispensing medication was consistent with the date of diagnosis, the first date on which medication was dispensed, and the quantity of medication dispensed on that occasion, was excluded and substituted with the second date. Using such procedures, it was possible to retain 185 of the cases for analysis, with a loss of 13 women due to complete lack of consistency in the data. A further 220 women were excluded as there was only one recorded date on which medication was collected.
Thus, data referring to 5,861 women remained in the analysis, making it unlikely that the exclusions for operational issues had introduced bias into the study given the non-systematic association with the variables in question.
This study adopted the recommendation of one pill taken daily for a period of five years. The medications, distributed without charge by INCA, were tamoxifen (TMX) and aromatase inhibitors (AIS).
Persistence was measured considering the time between starting hormone therapy and discontinuing or abandoning it for 60 days or more, counting from the last supply obtained. The results of assessing the sensitivity of this measure using 30 days of discontinuity did not differ greatly, justifying the choice of 60 days.
Women were classed as persistent (without discontinuing treatment) if they died, reached the end of the study or concluded 1,825 days (five years) of treatment without an interruption greater than 60 days. Those who interrupted treatment for 60 days or more, and for whom no information of death was obtained, were considered non-persistent (discontinued treatment). All of the women in the cohort began hormone therapy after January 1, 2004, with no left censoring in the observations included.
In the case of women for whom the quantity of pills dispensed corresponded to more than one per day for 1,825 days (five years) or who underwent hormone therapy for more than five years, the data were truncated to the observation period (1,825 days).
The independent variables in question were: (1) sociodemographic (RHC) – age at diagnosis, schooling, marital status; (2) clinical (RHC) – histological type of tumor, stage (stages 0, I and II being curable and III and IV incurable), laterality, family history of cancer, alcohol and tobacco consumption; and (3) health care related (SHI/ABSOLUTE) – type of hormone therapy (only TMX; only AIS – letrozole or anastrozole; both – TMX followed by AIS), surgery, chemotherapy, radiotherapy, hospitalizations, consultations with mastologist, clinical oncologist and other doctors, psychotherapy, multi-professional treatment support (MTS), including outpatient and nursing care, nutrition, physiotherapy, speech therapy, psychology, social, orthodontic or pharmaceutical care, diagnosis and therapeutic services (DTS) and time between diagnosis and the initiation of hormone treatment.
Statistical techniques to analyze survival were used to verify the factors associated with persistence to hormone treatment. Bivariate analysis based on the Kaplan-Meier technique was used in order to identify differences in the occurrence of discontinuity curves, over time, among the different strata of the independent variables. The Wilcoxon and log-rank tests were used to test the null hypothesis of there being no difference between the curves.
To discover the independent effect of the explanatory variables on time until discontinuity, Cox’s multivariate proportional hazard model was used, including variables related to p equal to or lower than 0.10. The proportional hazard assumption was tested by adding the model interaction terms of the explanatory variables and time, and those which were statistically significant (p ≤ 0.05) were incorporated to correct the assumption violation. The analyses were conducted using the SAS® statistic system, version 9.1.
The study was approved by the INCA Research Ethics Committee, Protocol 84/2010.
RESULTS
The women’s age at time of diagnosis varied between 21 and 103, with a mean age of 57.5 (standard deviation 3.6 years) and a median age of 56.6. Approximately 50.0% of the women were aged between 40 and 59 years old, and a minority (8.9%) were under 40.
Half of the women had low levels of education (illiterate or had incomplete elementary education); 10.0% had higher education. Of the total, 55.5% of the women had a family history of cancer, 46.5% had a partner at time of diagnosis, 27.4% consumed alcohol, 34.7% smoked, 40.5% were diagnosed at an advanced stage and 64.4% were treated with TMX alone.
Assuming an additional three month margin, added to the five-year period, 712 (12.15%) of the women exceeded the recommended treatment period. Of this total, 57.6% took TMX and AIS, 39.6% took only TMX and 2.8% only AIS.
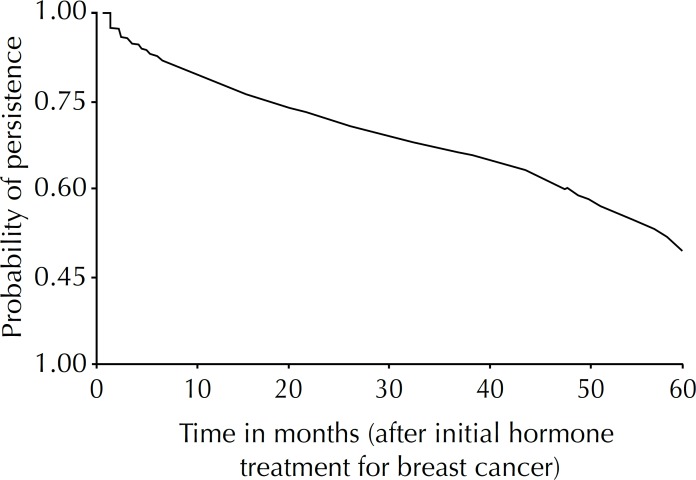
Overall persistence of women treated with hormone therapy for breast cancer was 79.0% at the end of the first year, 69.0% after two years, 60.0% after three years, 49.0% after four years and 31.0% after five years of treatment (Figure).
Figure.
Probability of persistence in hormone treatment for breast cancer in a cohort of women. Rio de Janeiro, RJ, Southeastern Brazil, 2004 to 2010. (N = 5,861)
Tables 1, 2 and 3 show significant difference (p ≤ 0.05) between the curves of persistence over time relative to the categories included in the variables: age at time of diagnosis, education, marital status, alcohol and tobacco consumption, stage, type of hormone therapy, surgery, chemotherapy (excluding hormone therapy), radiotherapy, treatment combination, frequency of chemotherapy, frequency of hospitalization, consultation with mastologist, consultation with oncologist, consultation with other doctors, psychotherapy, consultation with treatment support and DTS.
Table 1.
Persistence with hormone therapy, according to sociodemographic characteristics for women with breast cancer. Rio de Janeiro, RJ, Southeastern Brazil, 2004 to 2010. (N = 5,861)
| Sociodemographic characteristics | n | Prob. (%) of persistence until end of observation | Log-rank (p) | Wilcoxon (p) | |
|---|---|---|---|---|---|
| Age (years) | < 0.0001 | < 0.0001 | |||
| < 40 | 519 | 38.0 | |||
| 40 to 49 | 1,414 | 49.7 | |||
| 50 to 59 | 1,500 | 49.4 | |||
| 60 to 69 | 1,202 | 50.4 | |||
| ≥ 70 | 1,226 | 49.5 | |||
| Education | 0.0048 | 0.0263 | |||
| Illiterate/Incomplete elementary schoolchool | 2,943 | 47.5 | |||
| Complete elementary school | 1,032 | 46.1 | |||
| Complete secondary school | 1,259 | 50.9 | |||
| Higher education | 580 | 52.4 | |||
| No information | 47 | 53.2 | |||
| Marital status | 0.0009 | 0.0081 | |||
| Partner | 2,725 | 50.1 | |||
| No partner | 3,098 | 46.6 | |||
| No information | 38 | 47.4 | |||
Table 2.
Persistence with hormone therapy in women with breast cancer according to clinical and lifestyle characteristics. Rio de Janeiro, RJ, Southeastern Brazil, 2004 to 2010. (N = 5,861)
| Clinical characteristics | n | Prob. (%) of persistence until end of observation | Log-rank (p) | Wilcoxon (p) | |
|---|---|---|---|---|---|
| Family history of cancer | 0.1610 | 0.0632 | |||
| Yes | 3,251 | 50.0 | |||
| No | 2,426 | 46.9 | |||
| No information | 184 | 47.3 | |||
| Alcohol | 0.0017 | 0.0137 | |||
| Yes | 1,605 | 48.6 | |||
| No | 4,088 | 48.5 | |||
| No information | 168 | 47.0 | |||
| Smoker | 0.0622 | 0.0035 | |||
| Yes | 2,033 | 47.3 | |||
| No | 3,734 | 49.2 | |||
| No information | 94 | 45.7 | |||
| Histological type of tumor | 0.1853 | 0.2702 | |||
| IDC | 4,678 | 48.4 | |||
| Other | 1,183 | 49.0 | |||
| Laterality | 0.0772 | 0.1688 | |||
| Unilateral | 5,618 | 48.5 | |||
| Bilateral | 236 | 49.6 | |||
| No informaiton | 7 | 14.3 | |||
| Stage | < 0.0001 | < 0.0001 | |||
| Curable (0, I, II) | 3,286 | 55.5 | |||
| Non curable (III and IV) | 2,371 | 39.4 | |||
| No information | 204 | 41.7 | |||
IDC: infiltrating ductal carcinoma
Table 3.
Persistence with hormone therapy in women with breast cancer according to care-related characteristics. Rio de Janeiro, RJ, Southeastern Brazil, 2004 to 2010. (N = 5,861)
| Care-related characteristics | n | Prob. (%) of persistence until end of observation | Log-rank (p) | Wilcoxon (p) | |
|---|---|---|---|---|---|
| Type of hormone therapy | < 0.0001 | < 0.0001 | |||
| Only tamoxifen | 3,776 | 52.0 | |||
| Only aromatase inhibitors | 339 | 47.8 | |||
| Both | 1,746 | 41.1 | |||
| Surgery | < 0.0001 | < 0.0001 | |||
| Yes | 3,495 | 52.8 | |||
| No | 2,366 | 42.1 | |||
| Chemotherapy | < 0.0001 | < 0.0001 | |||
| Yes | 3,531 | 44.7 | |||
| No | 2,330 | 54.3 | |||
| Radiotherapy | 0.0005 | 0.0074 | |||
| Yes | 2,772 | 44.2 | |||
| No | 3,089 | 52.4 | |||
| Therapy combination | < 0.0001 | < 0.0001 | |||
| Only HT | 552 | 54.7 | |||
| HT and surgery | 899 | 58.0 | |||
| HT and CT | 579 | 41.5 | |||
| HT and RT | 503 | 40.2 | |||
| HT, CT and surgery | 1,059 | 52.3 | |||
| HT, RT and surgery | 376 | 63.6 | |||
| HT, CT and RT | 732 | 34.6 | |||
| HT, CT, RT and surgery | 1,161 | 45.8 | |||
| Frequency of chemotherapy | < 0.0001 | < 0.0001 | |||
| None | 2,330 | 54.3 | |||
| 1 to 3 procedures | 690 | 45.9 | |||
| 4 to 6 procedures | 1,777 | 56.8 | |||
| ≥ 7 procedures | 1,064 | 23.8 | |||
| Frequency of hospitalization | < 0.0001 | < 0.0001 | |||
| None | 961 | 63.6 | |||
| One | 2,794 | 51.0 | |||
| Two | 1,182 | 45.9 | |||
| ≥ 3 | 924 | 28.6 | |||
| Mastology (consultation) | < 0.0001 | < 0.0001 | |||
| None | 617 | 23.0 | |||
| 1 to 4 consultations | 1,001 | 20.2 | |||
| 5 to13 consultations | 2,971 | 60.0 | |||
| ≥ 14 consultations | 1,2 72 | 56.3 | |||
| Clinical oncology (consultation) | < 0.0001 | 0.0018 | |||
| None | 1,257 | 66.8 | |||
| 1 to 4 consultations | 1,674 | 57.4 | |||
| 5 to 12 consultations | 1,510 | 33.4 | |||
| ≥ 13 consultations | 1,420 | 37.9 | |||
| Other doctors (consultation) | < 0.0001 | < 0.0001 | |||
| ≤ 9 consultations | 604 | 27.7 | |||
| 10 to 22 consultations | 2,158 | 49.9 | |||
| 23 to 34 consultations | 1,635 | 55.7 | |||
| ≥ 35 consultations | 1,464 | 47.0 | |||
| Psychotherapy (consultation) | <0.0001 | < 0.0001 | |||
| None | 3,331 | 39.1 | |||
| 1 to 3 consultations | 1,971 | 59.9 | |||
| ≥ 4 consultations | 559 | 64.6 | |||
| Support treatment(consultation) | <0.0001 | < 0.0001 | |||
| None | 1,404 | 40.2 | |||
| 1 to 3 consultations | 2,010 | 42.4 | |||
| 4 to 7 consultations | 1,130 | 53.5 | |||
| ≥ 8 consultations | 1,317 | 62.3 | |||
| DTS (tests) | < 0.0001 | < 0.0001 | |||
| None | 1,112 | 39.4 | |||
| 1 test | 2,007 | 51.8 | |||
| 2 to 3 tests | 1,816 | 52.4 | |||
| ≥ 4 tests | 926 | 44.7 | |||
HT: hormone therapy; CT: chemotherapy; RT: radiotherapy; DTS: diagnosis and therapeutic services
The results of the bivariate model show less favorable curves of persistence to hormone therapy in women: aged under 40; with lower levels of education; single, with no family history of cancer; who consume alcohol; smokers; at non-curable stage; with combined hormone therapy (TMX and AIS); who did not have surgery; treated with chemotherapy; who had radiotherapy; and with more hospitalizations (Tables 1, 2 and 3).
The factors associated with discontinuing treatment also stand out: having no or few consultations with a mastologist or therapy support, and not having psychotherapy.
When testing the assumptions of proportionality of immediate risk in Cox’s multivariate model (Table 4), the variables stage III, alcohol consumption, chemotherapy and mastology show statistically significant interactions in relation to time, and were incorporated.
Table 4.
Multivariate analysis of factors associated with discontinuity in hormone therapy in women with breast cancer. Rio de Janeiro, RJ, Southeastern Brazil, 2004 to 2010. (N = 5,861)
| Variable | Unadjusted immediate risk ratio | Adjusted immediate risk ratio | ||
|---|---|---|---|---|
| Estimate | 95%CI | Estimate | 95%CI | |
| Time between diagnosis and initiation HT (months) | 1.00 | 1.00;1.00 | 1.00 | 1.00;1.00 |
| Age < 40 | 1.36 | 1.21;1.53 | 1.25 | 1.11;1.41 |
| Complete secondary school | 0.95 | 0.87;1.04 | 0.91 | 0.83;0.99 |
| Higher education | 0.87 | 0.77;0.99 | 0.88 | 0.77;1.00 |
| With partner | 0.89 | 0.83;0.96 | 0.92 | 0.85;0.99 |
| Stage III | 1.45 | 1.35;1.56 | 2.36 | 2.08;2.67 |
| Stage IV | 3.03 | 2.71;3.40 | 3.21 | 2.82;3.64 |
| Consume alcohol (yes) | 1.12 | 1.03;1.21 | 1.29 | 1.15;1.46 |
| Surgery (yes) | 0.83 | 0.77;0.89 | 0.80 | 0.74;0.87 |
| Chemotherapy (yes) | 1.18 | 1.10;1.28 | 1.29 | 1.14;1.46 |
| Hospitalizations (number) | 1.18 | 1.15;1.21 | 1.12 | 1.09;1.15 |
| Family history of cancer (yes) | 0.99 | 0.96;1.01 | 0.96 | 0.94;0.99 |
| Mastologist consultation (yes) | 0.30 | 0.26;0.32 | 0.44 | 0.39;0.51 |
| Oncologist consultation (yes) | 1.17 | 1.05;1.30 | 0.82 | 0.74;0.92 |
| DTS (number of tests) | 1.01 | 1.01;1.01 | 1.01 | 1.00;1.01 |
| Time x Stage III | 1.00 | 1.00;1.01 | 0.98 | 0.98;0.99 |
| Time x Consume alcohol | 1.00 | 1.00;1.00 | 0.99 | 0.99;1.00 |
| Time x Chemotherapy | 1.00 | 1.00;1.00 | 0.99 | 0.99;1.00 |
| Time x Mastology | 0.97 | 0.96;0.97 | 0.98 | 0.98;0.99 |
HT: hormone therapy; DTS: diagnosis and therapeutic services
Reference categories of the variables categorized: age ≥ 40; education: illiterate, incomplete elementary school, complete elementary school, no information; marital status: no partner and no information; stage: stage 0, I, II and no information; alcohol consumption: no; surgery: no; chemotherapy: no; family history of cancer: no; mastology: no consultation; oncologist: no consultation.
The multiple model estimates indicate that immediate risk of discontinuing treatment is 25.0% higher among women aged under 40, and 22.0% higher in women with stage IV cancer, compared with stages 0, I and II and no information (Table 4).
With the effect diminishing over time, indicated by the term of interaction, it was observed that the hazard of discontinuity was 136.0% higher among women with stage III cancer, compared with those with stage 0, I, II and no information, 29.0% greater among those who consumed alcohol and 29.0% higher in women who had had chemotherapy.
The patient’s hazard of discontinuing treatment increased by 12 percentage points with each hospitalization and by approximately 1 percentage point for each extra test conducted.
On the other hand, the hazard of discontinuing was shown to be 8.0% lower in women with a partner and 9.0% and 12.0% lower among those who had completed secondary and further education, respectively, compared with those with lower levels of education. It was 4.0% lower among those with a family history of cancer, compared with those with no such history, and 20.0% lower in patients who had undergone surgery, compared with those who had not (Table 4).
Table 4 shows that consulting a clinical oncologist decreased hazard of discontinuing by 18.0% and consulting a mastologist decreased it by 56.0%, although it should be noted that, for this variables, the effect decreases over time.
DISCUSSION
In this study, the estimate of discontinuity in hormone therapy for breast cancer at the end of the first year corroborates the results of other studies on TMX and AIS use.13,16,20 The estimate after five years of treatment was close to the 73.0% found by Nekhlyudov et al16 and higher than the 62.7% estimated by Guth et al.9 It is difficult to compare the diverse studies available due to differing definitions of persistence (intervals of 60, 90, 120 and 180 days), eligibility criteria (patients with early tumors, only the young, only the older adults and others), in the method of analysis (logistic regression, Kaplan-Meyer, among others), in the total period of following (1, 3½, 4½ or 5 years) and medication use (only TMX or AIS or both).
Regarding the persistence estimates, it is necessary to relativize the results, as the concept adopted corresponds to interrupting treatment for 60 days or more. The women classed as non-persistent may return to complete the treatment for the recommended period after the episode of discontinuity. However, women who have gaps in the first year of their endocrine breast cancer treatment do not re-start treatment and these percentages climb in subsequent years.16
On the other hand, the method used in this and other studies considers collecting medication from the pharmacy as a proxy variable for medication use, which could lead to overestimating levels of persistence. It is, however, assumed that such a bias is mitigated in estimates made based on secondary data incorporating large populations.e
Hormone therapy for breast cancer is only recommended for patients with tumors that have proved sensitive to estrogen or progesterone. However, it was not part of this study’s objective to evaluate appropriate indication of hormone therapy.
Another limitation is the lack of individual data on side effects, which may affect estimates of persistence with hormone therapy,9 as suppressing the hormones brings on early menopause and affects sexuality in some women. Moreover, it can be associated with a significantly increased risk of endometrial cancer, pulmonary embolism, venous thrombosis, arthralgia, fractures and cardiac events.3,9
Regarding the sociodemographic variables, this study corroborated the observation that younger women are less persistent2,10,15 in treatment, although, at the other extreme,15 there was no difference between middle-aged and older adult women. Non-persistence in younger patients may be explained by the side effects of the medication on women’s sexuality.
Marital status was also shown to be associated with persistence, although with results that sometimes agree10 and sometimes disagree13 with the findings. Marital status could be related to the idea of having social support, a variable that various studies12 have shown to be positively associated with persistence in treatment.
Concerning education, it is difficult to compare international results, as school levels in different countries do not correspond and there is a lack of national data on the topic. Despite this, it is plausible that there is greater persistence among patients with higher education.
No studies were found associating persistence with family history of cancer, alcohol consumption and smoking, although these variables have been associated with adherence to hormone treatment.14
The relationship between persistence and stage tumor is also difficult to compare between studies, as many of them are restricted to early tumors. In concordance with the results of this study, there was greater discontinuity among women with more lymph nodes involved.5 However, Kimmick et al13 estimated greater persistence in women with local (versus regional) stage cancer, and Nekhlyudov et al16 found no significant association.
The results of this research show a trend for lower rates of persistence among women who undergo more procedures, except those concerning surgery and multi-professional care. Other studies also show an association between greater discontinuity and having chemotherapy,5 having had more oncologist consultations and more days of hospitalization/year,16 in contrast to other, which estimate higher persistence in women who receive chemotherapy11 and radiotherapy.11,17
A finding which was consistent in both the bi- and multivariate analysis was the link between greater persistence and having seen a mastologist and having had surgery (versus not). Surgery and consulting a mastologist are recommended for patients whose cancer is at an early stage, which has been shown to be associated with more persistent behavior. Thus, the early stage is an additional advantage in facing the treatment of breast cancer, as it increases the probability of the women persisting with a treatment associated with better results.
Worse persistence was observed in women who did not see a psychotherapist or multi-professional support team. It is believed that these results reflect to some extent the relationship between depression and lower persistence,19 and are in consonance with the recommendationsb,d on the need to treat cancer from an integrated and multi-professional perspective as this diagnosis has a multi-faceted impact. It affects daily life, physical appearance and self-esteem, femininity and psychological health, and also imposes work limitations on some women.
The indications for the medications dealt with in this study are based on consensus and on updated clinical guidelines, recommending the use of TMX for a five-year period as the standard treatment for pre-menopausal women with endocrine-positive tumors.8 AIS is contraindicated for this group,6,8 unless the patient has a history of thrombophilia.8
For post-menopausal women, previously, some specialists recommended changing to AIS after two to three years taking TMX,7 depending on bone density assessments, due to the increased risk of losing bone density with this treatment.6 However, this strategy, compared with TMX alone, does not appear to be so widespread, at the moment, 50.0% of specialists still prefer to prescribe AIS (when available and not contraindicated) at some point in the treatment, being more in favor of indicating AIS when lymph nodes are involved.8 Most believe that specific patients can be treated with TMX alone, and that those receiving AIS can be switch to TMX if intolerant to AIS.8
Backed up by more recent clinical guidelines,8 showing that the advantages of combined treatment (TMX followed by AIS) are not so great, in the population studied TMX used alone had the additional benefit of increasing the probability of persisting with treatment and improving expected results.
The lower probability of persistence on the part of women who took both (TMX and AIS) does not suggest that switching medication due to side effects of the initial medication.
Regarding the length of treatment, the majority of specialists previously supported additional use of AIS for a period, in the case of post-menopausal patients and those with positive lymph nodes, after completing TMX treatment.6 Brazilian recommendations have always been five years of treatment, regardless of the scheme used.a In more recent times, specialists have deemed five years of AIS as sufficient, and the majority are opposed to extending this, even in cases with positive lymph nodes and in younger post-menopausal patients (< 55 years of age).8
It was found that hormone treatment was used for periods of more than five years, which is not in concordance with nationala and international recommendations.8
Persistence is understood as behavior that is sensitive to factors of socioeconomic and clinical dimensions, of the treatment regime, of the disease, of the patient-health care professional relationship and of the organization of the health care services.19 This article concentrates on the perspective of highlighting some aspects of care that contribute to this. Actions that encourage early diagnosis and treatment, a multi-professional approach, provision of psychotherapy, encouraging social support and coordinating care for the subgroup of women at higher risk of abandoning treatment are recommended practices, but should be reinforced in the treatment of breast cancer.
This study shows that 69.0% of women with breast cancer do not persist with hormone treatment until the end and presents factors associated with discontinuity in the Brazilian context. Such factors may guide reformulations in care, aiming to increase rates of persistence and, consequently decrease the risk of worse results for this subgroup of women and contribute to decreasing unnecessary spending.
HIGHLIGHTS
Hormonal treatment for breast cancer is associated with improved mortality and cancer-free survival rates. The focus of this research was to study factors related to persistence in hormonal treatment, aiming to contribute to improved care for women suffering from breast cancer.
The differential of this study was that it included modifiable factors, underlining how changes in the way care is organized can help patients complete their treatment for the recommended period.
It is hoped that these results may contribute to reorienting oncological care and cancer control practices in Brazil. They indicate the introduction of new rationalities in national cancer care policies.
The results show differences in the persistence of women receiving hormone treatment for breast cancer related to sociodemographic and clinical characteristics and to the interventions applied, as well as estimating the significant quantity of women (69.0%) who did not complete their hormonal treatment for breast cancer, jeopardizing a clinical response appropriate to the expected patterns.
Treating chronic disease requires changes in patients’ behavior, and this can affect whether they complete the prescribed treatment. On the other hand, aspects of the health care service (professional-patient relationship and management of side effects, among others) can attenuate or aggravate the problem of abandoning long-term treatment. These factors can cause temporary or definitive interruptions to treatment, including in patients with a good prognosis.
Professor Rita de Cássia Barradas Barata Scientific Editor
Footnotes
Article based on the doctoral thesis of Brito C, entitled: “Adesão e persistência à terapia endócrina para o câncer de mama, fatores preditores e resultados relacionados”, presented to the Escola Nacional de Saúde Pública Sergio Arouca, Fundação Oswaldo Cruz, in 2011.
The authors declare that there is no conflict of interest.
Barner JC. Medication adherence: focus on secondary database analysis: ISPOR Student Forum Presentation, 2010 [cited 2012 Mar 20]. Available from: http://www.ispor.org/student/teleconferences/ISPORStudentForumPresentation022410.pdf
Ministério da Saúde, Secretaria de Atenção à Saúde. Portaria nº 741, de 19 de dezembro de 2005. Definir as unidades de assistência de alta complexidade em oncologia, os centros de assistência de alta complexidade em oncologia (CACON) e os centros de referência de alta complexidade em oncologia e suas aptidões e qualidades. Diario Oficial Uniao. 23 dez 2005 [cited 2012 Dec 12]. Available from: http://bvsms.saude.gov.br/bvs/saudelegis/sas/2005/prt0741_19_12_2005.html
Ministério da Saúde, Secretaria de Atenção à Saúde, Departamento de Regulação, Avaliação e Controle, Coordenação Geral de Sistemas de Informação. SIA/SUS Sistema de Informações Ambulatoriais. Oncologia: manual de bases técnicas. Brasília (DF); 2011 [cited 2013 Jan 6]. Available from: http://www1.inca.gov.br/inca/Arquivos/manual_oncologia_13edicao_agosto_2011.pdf
Ministério da Saúde, Instituto Nacional do Câncer José Alencar Gomes da Silva. Estimativa 2012: incidência de câncer no Brasil. Rio de Janeiro: INCA; 2011 [cited 2012 Nov 10]. Available from: http://www.inca.gov.br/estimativa/2012/estimativa20122111.pdf
World Health Organization. National cancer control programs: policies and managerial guidelines. 2.ed. Geneva; 2002 [cited 2011 May 15]. Available from: http://whqlibdoc.who.int/hq/2002/9241545577.pdf
REFERENCES
- 1.Avorn J, Monette J, Lacour A, Bohn RL, Monane M, Mogun H, et al. Persistence of use of lipid-lowering medications: a cross-national study. JAMA. 1998;279(18):1458–1462. doi: 10.1001/jama.279.18.1458. [DOI] [PubMed] [Google Scholar]
- 2.Barron TI, Connolly R, Bennett K, Feely J, Kennedy MJ. Early discontinuation of tamoxifen: a lesson for oncologists. Cancer. 2007;109(5):832–839. doi: 10.1002/cncr.22485. [DOI] [PubMed] [Google Scholar]
- 3.Breast International Group (BIG) 1-98 Collaborative Group. Thürlimann B, Keshaviah A, Coates AS, Mouridsen H, Mauriac L, Forbes JF, et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N Engl J Med. 2005;353(26):2747–2757. doi: 10.1056/NEJMoa052258. [DOI] [PubMed] [Google Scholar]
- 4.Cramer JA, Roy A, Burrell A, Fairchild CJ, Fuldeore MJ, Ollendorf DA, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–47. doi: 10.1111/j.1524-4733.2007.00213.x. [DOI] [PubMed] [Google Scholar]
- 5.Fink AK, Gurwitz J, Rakowski W, Guadagnoli W, Silliman RA. Patient beliefs and tamoxifen discontinuance in older women with estrogen receptor – positive breast cancer. J Clin Oncol. 2004;22(16):3309–3315. doi: 10.1200/jco.2004.11.064. [DOI] [PubMed] [Google Scholar]
- 6.Goldhirsch A, Wood WC, Gelber RD, Coates AS, Thürlimann B, Senn H-J. Progress and promise: highlights of the International Expert Consensus on the Primary Therapy of Early Breast Cancer 2007. Ann Oncol. 2007;18(7):1133–1144. doi: 10.1093/annonc/mdm271. [DOI] [PubMed] [Google Scholar]
- 7.Goldhirsch A, Ingle JN, Gelber RD, Coates AS, Thürlimann B, Senn HJ. Thresholds for therapies: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2009. Ann Oncol. 2009;20(8):1319–1329. doi: 10.1093/annonc/mdp322. Panel members. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22(8):1736–1747. doi: 10.1093/annonc/mdr304. Panel members. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Güth U, Myrick ME, Schötzau A, Kilic N, Schmid SM. Drug switch because of treatment-related adverse side effects in endocrine adjuvant breast cancer therapy: how often and how often does it work? Breast Cancer Res Treat. 2011;129(3):799–807. doi: 10.1007/s10549-011-1668-y. [DOI] [PubMed] [Google Scholar]
- 10.Hershman DL, Kushi LH, Shao T, Buono D, Kershenbaum A, Tsai WY, et al. Early discontinuation and nonadherence to adjuvant hormonal therapy in a cohort of 8.769 early-stage breast cancer patients. J Clin Oncol. 2010;28(27):4120–4128. doi: 10.1200/JCO.2009.25.9655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hershman DL, Shao T, Kushi LH, Buono D, Tsai WY, Fehrenbacher L, et al. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res Treat. 2011;126(2):529–537. doi: 10.1007/s10549-010-1132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huiart L, Bouknik A, Rey D, Tarpin C, Cluze C, Bendiane MK, et al. Early discontinuation of tamoxifen intake in younger women with breast cancer: is it time to rethink the way it is prescribed? Eur J Cancer. 2012;48(13):1939–1946. doi: 10.1016/j.ejca.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 13.Kimmick G, Anderson R, Camacho F, Bhosle M, Hwang W, Balkrishnan R. Adjuvant hormonal therapy use among insured, low-income women with breast cancer. J Clin Oncol. 2009;27(21):3445–3451. doi: 10.1200/JCO.2008.19.2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin JH, Zhang SM, Manson JE. Predicting adherence to tamoxifen for breast cancer adjuvant therapy and prevention. Cancer Prev Res. 2011;4(9):1360–1365. doi: 10.1158/1940-6207.CAPR-11-0380. Phila. [DOI] [PubMed] [Google Scholar]
- 15.Murphy CC, Bartholomew LK, Carpentier MY, Bluethmann SM, Vernon SW. Adherence to adjuvant hormonal therapy among breast cancer survivors in clinical practice: a systematic review. Breast Cancer Res Treat. 2012;134(2):459–478. doi: 10.1007/s10549-012-2114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nekhlyudov L, Li L, Ross-Degnan D, Wagner AK. Five-year patterns of adjuvant hormonal therapy use, persistence, and adherence among insured women with early-stage breast cancer. Breast Cancer Res Treat. 2011;130(2):681–689. doi: 10.1007/s10549-011-1703-z. [DOI] [PubMed] [Google Scholar]
- 17.Owusu C, Buist DS, Field TS, Lash TL, Thwin SS, Geiger AM, et al. Predictors of tamoxifen discontinuation among older women with estrogen receptor-positive breast cancer. J Clin Oncol. 2008;26(4):549–555. doi: 10.1200/JCO.2006.10.1022. [DOI] [PubMed] [Google Scholar]
- 18.Pellegrini I, Sarradon-Eck A, Soussan PB, Lacour AC, Largillier R, Tallet A, et al. Women’s perceptions and experience of adjuvant tamoxifen therapy account for their adherence: breast cancer patients’ point of view. Psychooncology. 2010;19(5):472–479. doi: 10.1002/pon.1593. [DOI] [PubMed] [Google Scholar]
- 19.Ruddy K, Mayer E, Partridge A. Patient adherence and persistence with oral anticancer treatment. CA Cancer J Clin. 2009;59(1):56–66. doi: 10.3322/caac.20004. [DOI] [PubMed] [Google Scholar]
- 20.Weaver KE, Camacho F, Hwang W, Anderson R, Kimmick G. Adherence to adjuvant hormonal therapy and its relationship to breast cancer recurrence and survival among low-income women. Am J Clin Oncol. 2013;36(2):181–187. doi: 10.1097/coc.0b013e3182436ec1. [DOI] [PMC free article] [PubMed] [Google Scholar]