Abstract
Objective:
To assess whether being able to quit smoking is an early marker of Parkinson disease (PD) onset rather than tobacco being “neuroprotective,” we analyzed information about ease of quitting and nicotine substitute use.
Methods:
For this case-control study, we identified 1,808 patients with PD diagnosed between 1996 and 2009 from Danish registries, matched 1,876 population controls on sex and year of birth, and collected lifestyle information. We estimated odds ratios and 95% confidence intervals with logistic regression adjusting for matching factors and confounders.
Results:
Fewer patients with PD than controls ever established a smoking habit. Among former smokers, those with greater difficulty quitting or using nicotine substitutes were less likely to develop PD, with the risk being lowest among those reporting “extremely difficult to quit” compared with “easy to quit.” Nicotine substitute usage was strongly associated with quitting difficulty and duration of smoking, i.e., most strongly among current smokers, followed by former smokers who had used nicotine substitutes, and less strongly among former smokers who never used substitutes.
Conclusions:
Our data support the notion that patients with PD are able to quit smoking more easily than controls. These findings are compatible with a decreased responsiveness to nicotine during the prodromal phase of PD. We propose that ease of smoking cessation is an aspect of premanifest PD similar to olfactory dysfunction, REM sleep disorders, or constipation and suggests that the apparent “neuroprotective” effect of smoking observed in epidemiologic studies is due to reverse causation.
According to epidemiologic studies, patients with Parkinson disease (PD) are less likely to smoke.1–3 Also, those who smoke for longer are at lower risk while lengthening time since quitting increases PD risk, causing some to argue that smoking may protect against PD and even prompting nicotine PD prevention trials.4 While this is counterintuitive given the otherwise adverse health effects of smoking, common biases including confounding, selection, or measurement error do not explain this finding. Alternatively, the possibility exists that biological mechanisms responsible for PD result in smoking avoidance or the ability to quit smoking more easily among those at risk of developing PD. That is, individuals who develop PD may not experience the positive reinforcement from, and possibly the addictive response to, nicotine many years before motor symptom development, and thus are able to quit more easily than smokers who never develop PD. This distinction is important because smoking may either provide leads for treatment/prevention of PD or be plainly no more than an early marker of insidious PD onset. To explore this, we examined difficulty quitting and use of nicotine substitutes in relation to PD risk in the Danish PASIDA (Parkinson's Disease in Denmark) Study. We hypothesize that (1) those who smoked and have greater difficulty quitting (vs those who found it easy) are less likely to develop PD; (2) use of nicotine substitutes is related to quitting difficulty; and (3) those who used nicotine substitutes (vs those who never used) have a decreased risk of PD.
METHODS
Study population.
We selected all 2,762 patients identified in the Danish National Hospital Register files between 1996 and 2010 at 10 large neurologic treatment centers in Denmark by diagnosis code for PD assigned by a neurologist. Patients were younger than 70 years at diagnosis before 2002 and younger than 80 years at diagnosis in 2002 and after to ensure that most eligible patients survived to time of interview. We density sampled between 5 and 10 controls matched on birth year, sex, and being alive and without a PD diagnosis at the time of case identification from the Danish Central Population Registry, a register of the whole Danish population. We collected interview and medical record data between January 2008 and December 2010. Patients and controls had to be able to speak Danish or English, and be alive and well enough to participate. From the list of 2,762 initially eligible patients, we excluded 179 subjects for whom medical record review before contact did not confirm idiopathic PD, thus we attempted to contact 2,583 patients. Of these, 497 declined to participate, and for 20, we were unable to retrieve a medical record to confirm the diagnosis. The remaining 2,066 patients with PD were interviewed, and medically trained staff supervised by a movement disorder specialist rigorously reviewed their complete medical records applying standard diagnostic criteria.5,6 After this review, we removed 238 patients who did not have idiopathic PD, leaving 1,828 confirmed cases, of whom 1,808 provided all data necessary for this analysis.
Of 3,626 eligible controls whom we initially contacted, 1,909 agreed to participate, were matched to a valid case, and completed an interview; 1,876 contributed to this analysis. Generally, we attempted to interview one control of 5 to 10 selected for each case after all cases from one hospital had been interviewed, but for 101 cases, we also matched by sex and birth year an already interviewed control whom we originally had matched to cases considered invalid after chart review. We required that controls did not have a diagnosis of PD at the date their respective case was identified in the Hospital Register. We used the occurrence of the first cardinal (motor) symptom on the medical records as the referent date for calculating time-dependent exposures and assigned controls the date of their respective case. When medical records did not contain a first symptom onset date, we used the first date of PD diagnosis instead.
Standard protocol approvals, registrations, and patient consents.
We obtained written informed consent from all subjects, and the University of California at Los Angeles (UCLA) Institutional Review Board and Danish Data Protection Agency approved the study protocol.
Smoking and nicotine substitute data collection.
We obtained information on demographics, education, and lifestyle habits, including lifelong smoking history and nicotine replacement, in a structured telephone interview. We allowed persons with speech or physical difficulties to respond by mail (approximately 17%). The questions on cigarette smoking included smoking status at interview, age at start and end of smoking allowing for multiple periods during which the participant may have started and stopped, and number of cigarettes smoked per day in each period. We defined “ever smoked” as having smoked at least 1 cigarette (or the equivalent amount of tobacco) per week for 6 months or more. A former smoker reported having smoked but was not smoking on the index date. In addition, we asked ever-smokers about their use of nicotine substitutes (ever/never and type: chewing gum, tablets, skin patch, nasal spray/inhaler) and former smokers to report how much difficulty they had experienced quitting (“How hard did you find it to quit smoking?” Answers: “Extremely difficult, I started and stopped several times”; “Hard, but successful the first time”; “Medium—it took some time getting used to it, but not a big deal”; “Remarkably easy to quit”; and “Other”).
Statistical analyses.
To guide analyses, we drew simplified directed acyclic graphs depicting assumptions and relationships between key exposure and outcome variables7 (see figure e-1 on the Neurology® Web site at Neurology.org).8–10 We used multivariable logistic regression analyses to estimate odds ratios (ORs) and 95% confidence intervals (CIs) and assess the following: (1) main effects of smoking and nicotine substitute use on PD as well as main effects of difficulty to quit smoking among former smokers on PD; (2) joint effects of smoking and nicotine substitute use on PD; and (3) associations between smoking-related variables and nicotine substitute use. In regression models with PD as the outcome, we adjusted for age at first cardinal symptom (years), year of birth (matching variable) and sex (matching variable), education (basic, vocational training, higher education), caffeinated drinks (never/ever), alcohol drinking (never/ever), residential location within 3 years before the time of diagnosis or index date (capital, provincial cities, rural, peripheral region), family history of PD (none, one, more than one family member), and in sensitivity analysis, we also stratified by sex.
In addition, we conducted some probabilistic bias analyses to formally address the potential for uncontrolled confounding of the PD-smoking association (for technical details, see the supplemental appendix).
RESULTS
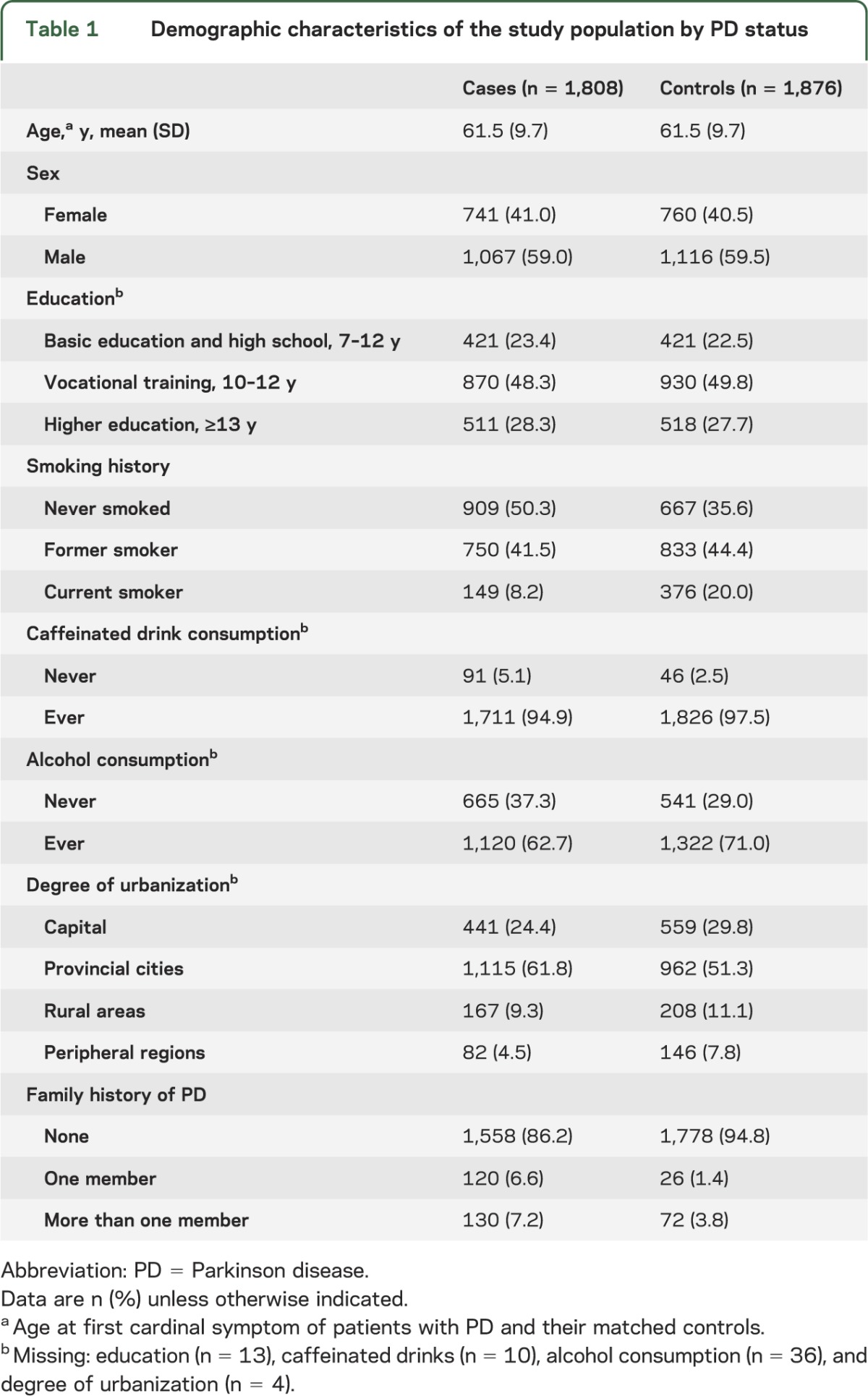
The average age at first cardinal symptom for cases was 61.5 years (SD = 9.7), and controls were matched on birth year and sex and had similar education as cases (table 1). More population controls than cases lived in rural areas or Copenhagen, and cases consumed less caffeine (high consumption OR = 0.57; 95% CI 0.47–0.68) and alcohol (high consumption OR = 0.70; 95% CI 0.57–0.85) and were more likely to report a family history of PD (OR = 2.83; 95% CI 2.16–3.72).
Table 1.
Demographic characteristics of the study population by PD status
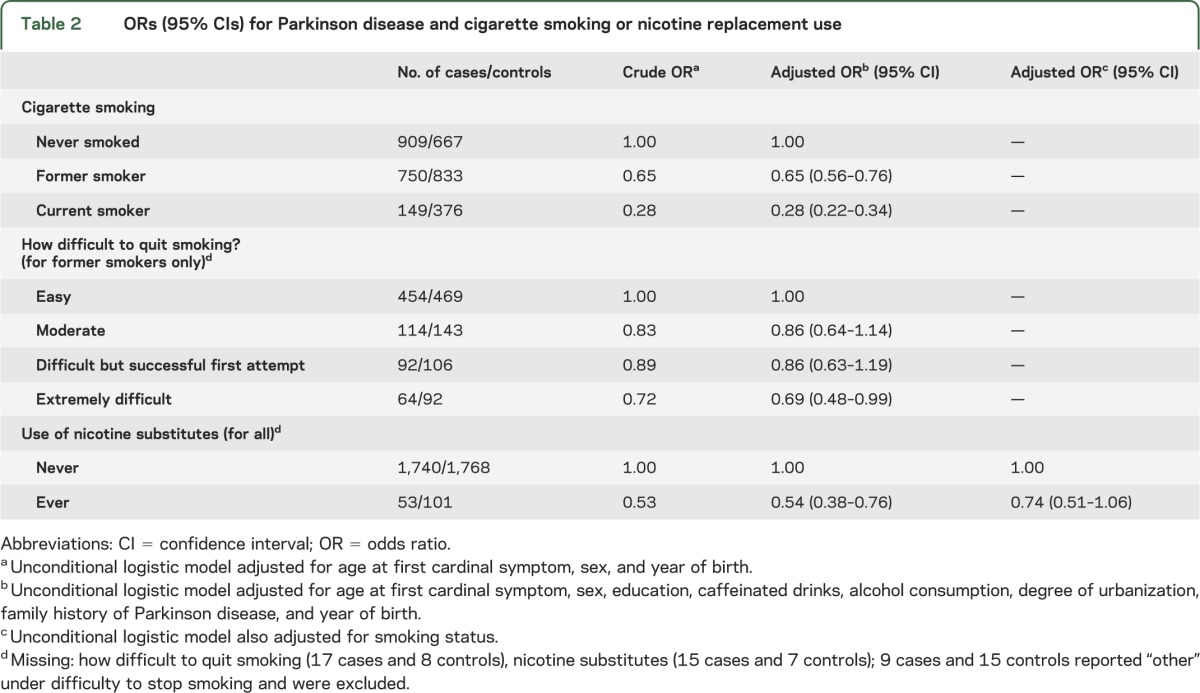
Patients with PD were less likely to ever smoke cigarettes and associations were strongest for current smokers followed by former smokers (table 2). Former smokers reporting that it was “extremely difficult to quit smoking” had a 31% decreased risk of developing PD compared with those reporting “quitting was easy”; and we observed a reduced risk of PD for those ever using nicotine substitutes (table 2). Few study participants ever used nicotine substitutes (3% cases; 5% controls) with nicotine gum being most common (2% cases; 4% controls) and an average duration of use of 4.4 years in cases and 2.8 years in controls. Among ever-smokers, 83% of patients with PD and 69% of controls quit smoking before the index date.
Table 2.
ORs (95% CIs) for Parkinson disease and cigarette smoking or nicotine replacement use
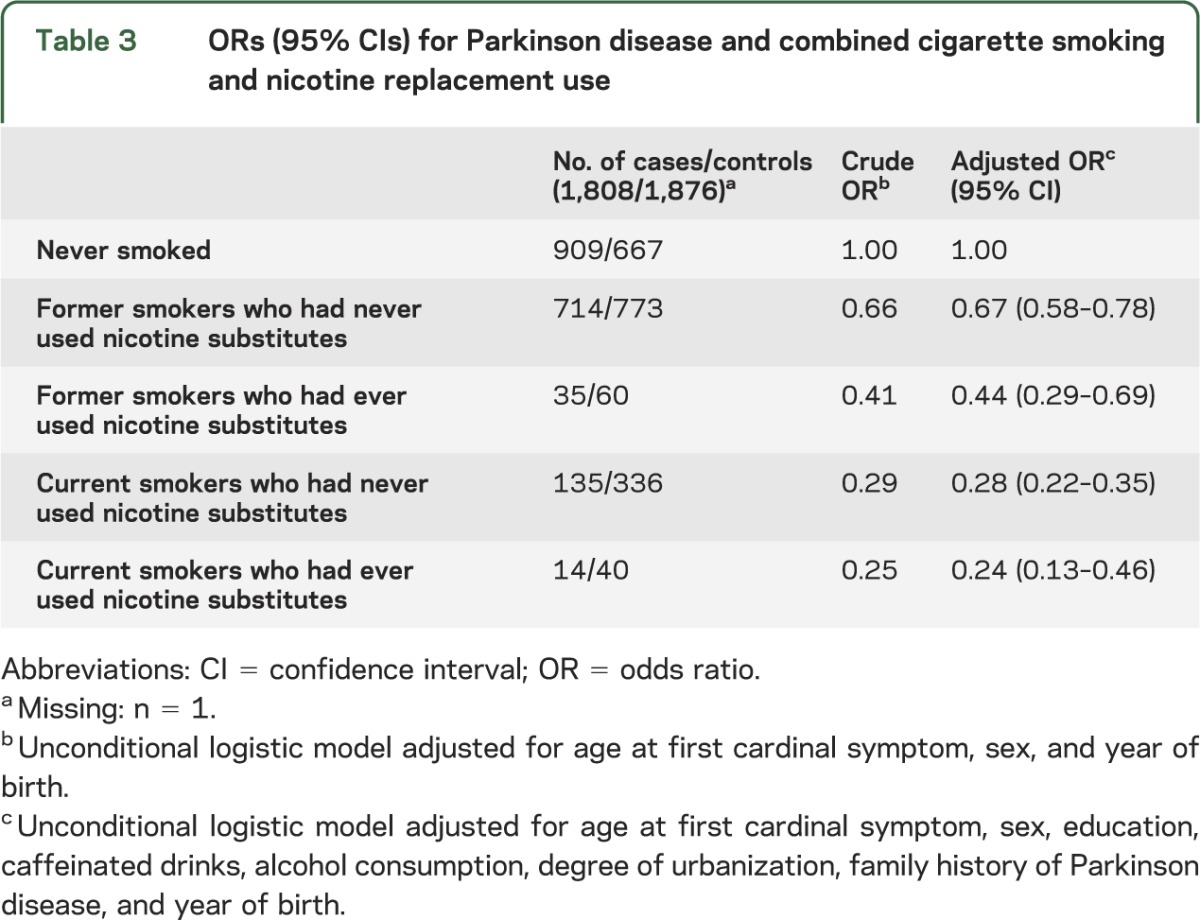
Examining the combined effect of smoking and nicotine substitutes, we observed the strongest associations with PD for current smokers with or without nicotine substitute use, followed by former smokers who reported having used nicotine substitutes (table 3). We saw the weakest association in former smokers who never used nicotine substitutes. In sensitivity analyses, we did not find more than minimal differences by sex and when excluding subjects whom interviewers considered unreliable.
Table 3.
ORs (95% CIs) for Parkinson disease and combined cigarette smoking and nicotine replacement use
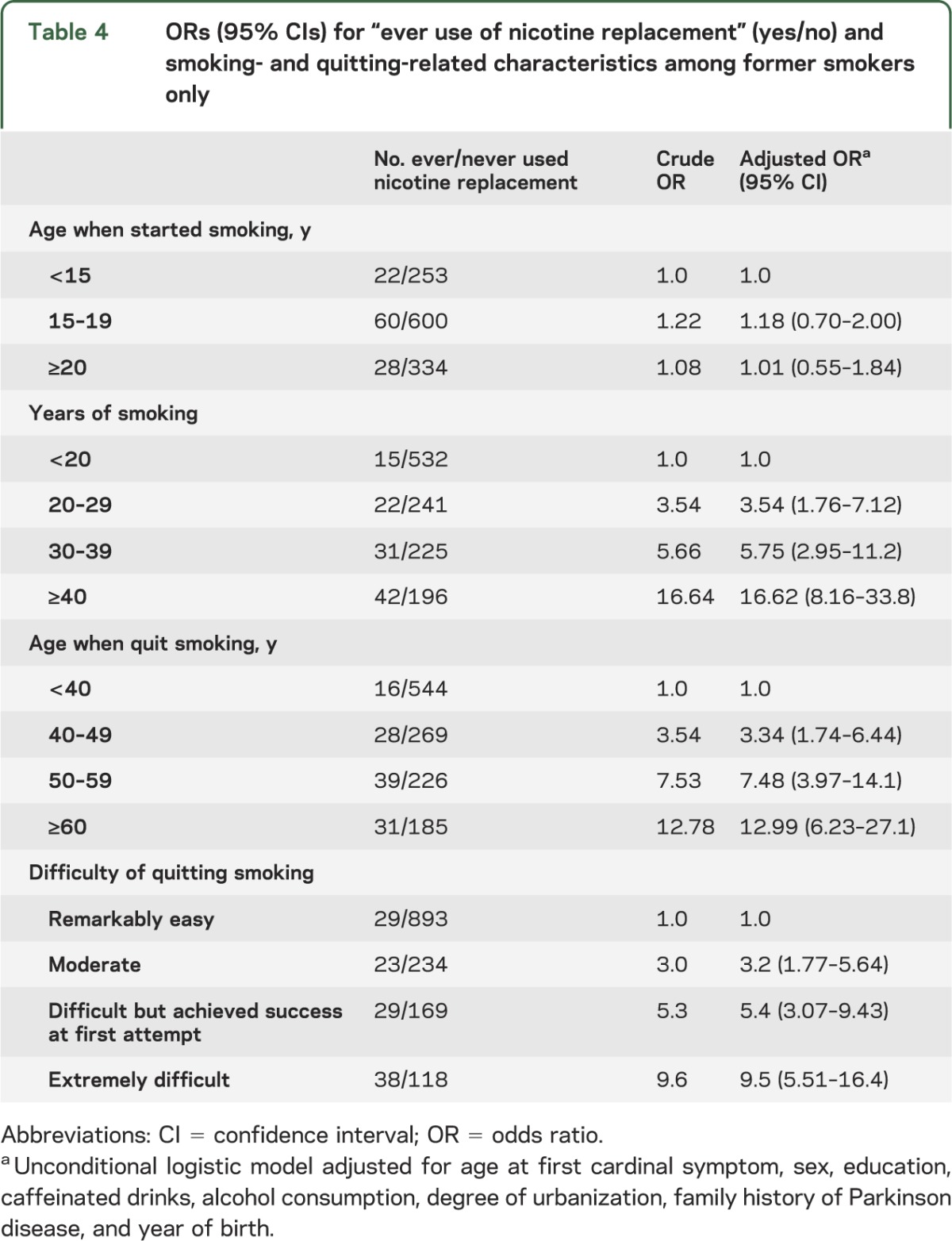
Among former smokers, participants who were more educated and lived in the capital or provincial cities were more likely to use nicotine substitutes than those living in peripheral and rural regions, but neither sex nor depression or alcohol consumption were related to nicotine substitute use (results not shown). Most importantly, participants reporting it was “extremely difficult” to quit smoking were much more likely to have ever used nicotine substitutes (more than 9 times) compared with those who found it “easy to quit” (table 4). Longer duration of smoking and quitting at older ages were also strongly associated with ever use of nicotine substitutes (e.g., more than 16-fold with ever use of nicotine substitutes and >40 years of smoking), while age at starting was unrelated (no association for smoking initiation at 20+ vs <15 years of age).
Table 4.
ORs (95% CIs) for “ever use of nicotine replacement” (yes/no) and smoking- and quitting-related characteristics among former smokers only
In table e-1, we present formal bias analyses illustrating that we need to invoke implausibly strong uncontrolled confounding to explain the reported smoking–PD associations.
DISCUSSION
We reexamined the role of smoking and, hence, nicotine in PD focusing on the difficulty to quit smoking and the related use of nicotine substitutes. As observed by others,3 patients with PD in our study were less likely to become habitual smokers, which previously gave rise to speculations about genetic or biological factors or “risk-avoiding” personality traits that may affect smoking initiation and PD risk. This hypothesis cannot be tested in our data, but we are able to explore quitting-related factors in a large group of smoking initiators who later nevertheless developed PD. Our hypothesis is that individuals at risk of developing PD have a much easier time quitting smoking and found that 17% of ever-smoking PD cases vs 31% of controls remained smokers. Ease of quitting results in fewer current smokers and a lower number of pack-years smoked among patients with PD and may explain previous epidemiologic findings without the need to invoke a “protective” role of smoking or nicotine. Indeed, we found that not only those who smoked but those (1) with the most difficulty quitting and/or (2) more often using nicotine substitutes were less likely to develop PD. Furthermore, the use of nicotine substitutes was strongly associated with quitting difficulty and heavy smoking. Finally, our bias analyses showed that risk factors that could cause confounding and account for the very strong “protective” effect of smoking reported in studies (suggesting an up to 70% reduction in PD risk3) require such factors to be very strong predictors of PD and it is implausible that they would be unknown. The only factors known to be as strongly associated with PD are prodromal symptoms of PD occurring years before diagnosis, such as olfactory dysfunction, REM sleep disorders, and constipation, with reported 5- to 9-fold relative risks for PD.11–13 Also, an autopsy study reported ORs as high as 11 for finding incidental Lewy bodies in the substantia nigra and the locus ceruleus of the deceased with the greatest recorded predeath olfactory impairment; Lewy body protein aggregates are characteristic of PD pathology, and loss of smell is a well-known prodromal sign of PD.14
In our large population-based, case-control study, we find a strong association between smoking and PD and a trend in risk with increasing smoking duration, and weaker smoking–PD associations among those who quit at younger ages. Generally, such findings have been interpreted as evidence for “protective” effects of smoking (or nicotine) on PD risk, with the caveat that “unknown or unmeasured causes” responsible for both a reduction in smoking and increase in PD remain alternative explanations. Here, we focus our attention on quitting difficulty and nicotine substitute use in smokers in an attempt to, for the first time, assess more explicitly influences related to changes in smoking behavior, which we refer to as a loss of nicotine “reward” or “sensitivity” that encourages quitting (note: in bias analyses, we thus expected lack of reward to be common in nonsmokers, less in quitters, and rare in long-time smokers). Our hypothesis that nicotine substitute use indicates high nicotine reward among those who attempt to quit was corroborated: we observed the smallest risk of developing PD in current smokers (at first cardinal symptom) who did or did not report nicotine substitute use followed by former smokers reporting to have used substitutes and finally those who quit smoking without using substitutes. Furthermore, we observed a trend in PD risk with the reported ease of quitting: former smokers reporting that it was extremely difficult to quit had a 31% reduced risk of developing PD compared with those who reported that quitting was easy. Nicotine substitute use and duration of smoking were both strongly predictive of quitting difficulty. Thus, both nicotine substitute use and higher quitting difficulty among controls suggest that when smokers decide to quit, nicotinic reward is less strong in those who later develop PD. Finally, while fewer patients with PD ever establish a smoking habit,3 those who do and nevertheless develop PD tend to quit smoking at an earlier age than control smokers (mean age at quitting for cases = 41 years; for controls = 44 years). These observations suggest that a mechanism associated with PD risk may influence smoking behavior or that less reward from nicotinic stimulation might be an event prodromal to PD in the same category as constipation, loss of smell, and REM sleep behavior problems for which very long latency periods and large relative risks for PD are reported in population studies.13
A major strength of our study is that we abstracted complete medical records for patients with PD, limiting misclassification of diagnosis. While patients search for explanations for their disease, it is harder to imagine that recall bias affects “risk reduction” from smoking, i.e., that underreporting of smoking differs by disease status. If, however, smokers who develop PD underreport smoking more often than controls, this would systematically bias the associations such that we would overestimate the “risk reduction” from smoking or vice versa. Our study population was necessarily elderly, and interview data collected late in life may have inaccuracies and some degree of nondifferential exposure misclassification. Patient participation was high (80.8%), but participation was moderate in controls (52.6%). Twice as many nonparticipants than participants had died as of March 2013 (13.0% vs 5.9% controls and 38% vs 18% cases). In addition, nonparticipants were more often hospitalized with diagnoses related to smoking, specifically ischemic heart disease or chronic obstructive pulmonary disease (19.4% vs 16.5% controls and 26% vs 17.8% cases). Thus, we cannot rule out selection (or survivor) bias in our study through the influence of smoking-related health status on participation. However, such bias would lead to an overestimation of the effect of smoking on PD because hospitalization for smoking-related diseases was greater for nonparticipating cases compared with controls.
Some authors speculated that personality traits related to smoking behavior may confound the association with PD such that genetic traits would increase susceptibility to PD but prevent smoking. A recent study reported that environmental factors such as school or peer influences shape nicotine, alcohol, and caffeine use behaviors in early adolescence, but as people age, environmental factors become less important while genetic factors gradually become more important.3,15 Large genome-wide association studies (GWAS) identified polymorphisms in genes encoding for the neuronal acetylcholine receptors and their subunits and nicotine-metabolizing enzymes that are related to smoking quantity.16–18 A New Zealand study created a “genetic risk score” by summing across GWAS-derived risk alleles related to smoking, and even though this score did not explain smoking initiation, higher-scoring individuals were more likely to convert to daily smoking, progress to heavy smoking, develop nicotine dependence, and failed in cessation attempts.19 Thus, multiple genetic factors may influence addictiveness and nicotine reward–related responses. For genetics to explain the reported strong smoking–PD associations, these complex traits need to be highly predictive of smoking and also strongly related to PD risk, which has not been shown.
Twin studies reporting that the twin with PD smoked less than the twin without PD also discredited a simple genetic trait hypothesis.20,21 Furthermore, among twin pairs, the one who developed PD stopped smoking on average 3.7 years earlier than the twin without PD; when both developed PD, the one affected first stopped smoking on average 1.3 years earlier.20 These observations fit the “loss of nicotine reward” hypothesis because one would expect the cotwin who develops PD first to quit smoking earlier. Notably, a similar sequence was observed for loss of smell in these twins: at baseline, the olfactory function of the twin who developed PD was impaired while the cotwin without PD had normal function, and cotwins who developed PD had a greater decline in smell than cotwins who did not develop PD.22 While these twin studies suggest that the smoking–PD associations are not easily attributable to a genetic trait shared by twins, they do not contradict, and even corroborate, the hypothesis that loss of nicotine reward may indicate insidious PD onset similar to loss of smell.
The simplest explanation for the smoking–PD association relates to the sensitivity of the brain reward system in response to nicotine; specifically, it has been suggested that loss or downregulation of nicotinic receptors may precede neurodegeneration.23,24 Postmortem studies of patients with PD have demonstrated a substantial loss of nicotinic receptors in the parietal cortex,25 frontal and temporal cortices, hippocampus, thalamus, striatum,26–28 substantia nigra pars compacta, and laterodorsal tegmental nucleus,24 leading to speculations that receptor loss might be responsible for cognitive, motoric, and behavioral deficits in PD.
Our analyses support the hypothesis that ease of smoking cessation is an early manifestation of premotor PD related to the loss of nicotinic rewards. Instead of tobacco being “neuroprotective,” we propose the possibility that smoking and PD are related through a reduction in nicotinic reward such that ease of quitting may act as an early prodromal marker.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Drs. Marie-Francoise Chesselet (UCLA) and Naomi Greene (UCLA) for helpful suggestions.
GLOSSARY
- CI
confidence interval
- GWAS
genome-wide association study
- OR
odds ratio
- PD
Parkinson disease
Footnotes
Editorial, page 1392
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Beate Ritz: study concept and design, obtained funding, designed and interpreted statistical analysis, wrote the manuscript. Pei-Chen Lee: conducted data analysis, wrote parts of the manuscript. Christina F. Lassen: designed and supervised field work, contributed to manuscript writing. Onyebuchi A. Arah: designed and conducted bias analysis, contributed to manuscript writing.
STUDY FUNDING
Supported by NIH (R01ES013717).
DISCLOSURE
The authors report no disclosure relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Hernan MA, Takkouche B, Caamano-Isorna F, Gestal-Otero JJ. A meta-analysis of coffee drinking, cigarette smoking, and the risk of Parkinson's disease. Ann Neurol 2002;52:276–284 [DOI] [PubMed] [Google Scholar]
- 2.Kiyohara C, Kusuhara S. Cigarette smoking and Parkinson's disease: a meta-analysis. Fukuoka Igaku Zasshi 2011;102:254–265 [PubMed] [Google Scholar]
- 3.Ritz B, Ascherio A, Checkoway H, et al. Pooled analysis of tobacco use and risk of Parkinson disease. Arch Neurol 2007;64:990–997 [DOI] [PubMed] [Google Scholar]
- 4.Quik M, Perez XA, Bordia T. Nicotine as a potential neuroprotective agent for Parkinson's disease. Mov Disord 2012;27:947–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol 1999;56:33–39 [DOI] [PubMed] [Google Scholar]
- 6.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 1992;55:181–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearl J. Causality: Models, Reasoning and Inference, 2nd ed. Cambridge, UK: Cambridge University Press; 2009 [Google Scholar]
- 8.Arah OA. The role of causal reasoning in understanding Simpson's paradox, Lord's paradox, and the suppression effect: covariate selection in the analysis of observational studies. Emerg Themes Epidemiol 2008;5:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arah OA, Sudan M, Olsen J, Kheifets L. Marginal structural models, doubly robust estimation, and bias analysis in perinatal and paediatric epidemiology. Paediatr Perinat Epidemiol 2013;27:263–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sudan M, Kheifets L, Arah OA, Olsen J. Cell phone exposures and hearing loss in children in the Danish National Birth Cohort. Paediatr Perinat Epidemiol 2013;27:247–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Postuma RB, Aarsland D, Barone P, et al. Identifying prodromal Parkinson's disease: pre-motor disorders in Parkinson's disease. Mov Disord 2012;27:617–626 [DOI] [PubMed] [Google Scholar]
- 12.Ross GW, Petrovitch H, Abbott RD, et al. Association of olfactory dysfunction with risk for future Parkinson's disease. Ann Neurol 2008;63:167–173 [DOI] [PubMed] [Google Scholar]
- 13.Ross GW, Abbott RD, Petrovitch H, Tanner CM, White LR. Pre-motor features of Parkinson's disease: the Honolulu-Asia Aging Study experience. Parkinsonism Relat Disord 2012;18(suppl 1):S199–S202 [DOI] [PubMed] [Google Scholar]
- 14.Ross GW, Abbott RD, Petrovitch H, et al. Association of olfactory dysfunction with incidental Lewy bodies. Mov Disord 2006;21:2062–2067 [DOI] [PubMed] [Google Scholar]
- 15.Kendler KS, Schmitt E, Aggen SH, Prescott CA. Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Arch Gen Psychiatry 2008;65:674–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tobacco and Genetics Consortium. Genome-wide meta-analyses identify multiple loci associated with smoking behavior. Nat Genet 2010;42:441–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu JZ, Tozzi F, Waterworth DM, et al. Meta-analysis and imputation refines the association of 15q25 with smoking quantity. Nat Genet 2010;42:436–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thorgeirsson TE, Gudbjartsson DF, Surakka I, et al. Sequence variants at CHRNB3-CHRNA6 and CYP2A6 affect smoking behavior. Nat Genet 2010;42:448–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xian H, Scherrer JF, Madden PA, et al. The heritability of failed smoking cessation and nicotine withdrawal in twins who smoked and attempted to quit. Nicotine Tob Res 2003;5:245–254 [PubMed] [Google Scholar]
- 20.Tanner CM, Goldman SM, Aston DA, et al. Smoking and Parkinson's disease in twins. Neurology 2002;58:581–588 [DOI] [PubMed] [Google Scholar]
- 21.Wirdefeldt K, Gatz M, Pawitan Y, Pedersen NL. Risk and protective factors for Parkinson's disease: a study in Swedish twins. Ann Neurol 2005;57:27–33 [DOI] [PubMed] [Google Scholar]
- 22.Marras C, Goldman S, Smith A, et al. Smell identification ability in twin pairs discordant for Parkinson's disease. Mov Disord 2005;20:687–693 [DOI] [PubMed] [Google Scholar]
- 23.Kenny PJ, Markou A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology 2006;31:1203–1211 [DOI] [PubMed] [Google Scholar]
- 24.Perry EK, Morris CM, Court JA, et al. Alteration in nicotine binding sites in Parkinson's disease, Lewy body dementia and Alzheimer's disease: possible index of early neuropathology. Neuroscience 1995;64:385–395 [DOI] [PubMed] [Google Scholar]
- 25.Perry EK, Smith CJ, Court JA, Perry RH. Cholinergic nicotinic and muscarinic receptors in dementia of Alzheimer, Parkinson and Lewy body types. J Neural Transm Park Dis Dement Sect 1990;2:149–158 [DOI] [PubMed] [Google Scholar]
- 26.Aubert I, Araujo DM, Cecyre D, Robitaille Y, Gauthier S, Quirion R. Comparative alterations of nicotinic and muscarinic binding sites in Alzheimer's and Parkinson's diseases. J Neurochem 1992;58:529–541 [DOI] [PubMed] [Google Scholar]
- 27.Lange KW, Wells FR, Rossor MN, Jenner P, Marsden CD. Cortical nicotinic receptors in Alzheimer's disease and Parkinson's disease. J Neurol Neurosurg Psychiatry 1991;54:373–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rinne JO, Myllykyla T, Lonnberg P, Marjamaki P. A postmortem study of brain nicotinic receptors in Parkinson's and Alzheimer's disease. Brain Res 1991;547:167–170 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


