Abstract
Objective:
We studied the frequency of auras in generalized epilepsy (GE) using a detailed semistructured diagnostic interview.
Methods:
In this cross-sectional study, participants with GE were drawn from the Epilepsy Phenome/Genome Project (EPGP). Responses to the standardized diagnostic interview regarding tonic-clonic (grand mal) seizures were then examined. This questionnaire initially required participants to provide their own description of any subjective phenomena before their “grand mal seizures.” Participants who provided answers to these questions were considered to have an aura. All participants were then systematically queried regarding a list of specific symptoms occurring before grand mal seizures, using structured (closed-ended) questions.
Results:
Seven hundred ninety-eight participants with GE were identified, of whom 530 reported grand mal seizures. Of these, 112 (21.3%) reported auras in response to the open-ended question. Analysis of responses to the closed-ended questions suggested that 341 participants (64.3%) experienced at least one form of aura.
Conclusions:
Auras typically associated with focal epilepsy were reported by a substantial proportion of EPGP subjects with GE. This finding may support existing theories of cortical and subcortical generators of GE with variable spread patterns. Differences between responses to the open-ended question and closed-ended questions may also reflect clinically relevant variation in patient responses to history-taking and surveys. Open-ended questions may underestimate the prevalence of specific types of auras and may be in part responsible for the underrecognition of auras in GE. In addition, structured questions may influence participants, possibly leading to a greater representation of symptoms.
An aura is defined as a subjective experience of a focal seizure.1,2 The current International League Against Epilepsy classification describes these as seizures without impairment of consciousness and with specific autonomic, motor, psychic, sensory, or other phenomena.3 Auras or lateralized clinical features are conventionally considered indications that a seizure is focal rather than generalized in onset. However, prior research questions the validity of this conventional interpretation.4–6 Distinguishing focal epilepsy (FE) and generalized epilepsy (GE) syndromes typically occurs early in a patient's diagnostic evaluation and significantly affects therapeutic decisions. We used a semistructured interview to obtain information about auras in a large cohort of patients with GE.
METHODS
Participant eligibility.
The study included the sibling and parent-child pairs with GE of unknown cause (including those with idiopathic GEs [IGEs]) and FE in the Epilepsy Phenome/Genome Project (EPGP). EPGP was a study conducted from May 2007 to April 2014 whose goal was to recruit, perform detailed phenotyping on, and collect DNA from more than 3,750 participants with epilepsy. There were 27 participating clinical centers in the United States, Canada, Argentina, Australia, and New Zealand. At each site, detailed information was collected on epilepsy phenotype, family history, electrophysiologic characteristics, neuroimaging findings, demographic variables, and response to medications. Eligibility requirements included enrollment age 4 weeks to 60 years, age at first unprovoked seizure 40 years or younger, and a clear diagnosis of epilepsy, i.e., a lifetime history of 2 or more unprovoked seizures or one seizure with epileptiform EEG activity. Individuals with only febrile seizures or acute symptomatic seizures (i.e., precipitated by acute metabolic or structural CNS insults) were excluded, as were those with a history of acquired CNS injury before onset of epilepsy. To be classified as GE, participants had to have generalized-onset seizures, normal neuroimaging if done (although not required), and an EEG showing generalized epileptiform activity with a normal posterior dominant rhythm for age. Exceptions were occasionally made to include a subject in the GE group without a positive EEG only when all 6 members of the Phenome Core agreed that one or more of the clinical features (e.g., morning myoclonus, photosensitivity, or responsiveness to valproate) were diagnostic and no focal features were present. To be classified as nonacquired FE, neuroimaging was required to be normal or show evidence of mesial temporal sclerosis or focal cortical dysplasia. Individuals with mesial temporal sclerosis or focal cortical dysplasia were not excluded because these lesions are not clearly a result of exogenous injury. Patients with nonacquired FE were also required to have focal EEG abnormalities or unambiguous clinical semiology consistent with focal seizures. Participants with benign rolandic epilepsy diagnosed by clinical presentation were not required to have neuroimaging. Participants with diagnoses of both GE and FE were not included in this study.
Study conduct.
Subjects were administered a detailed, semistructured diagnostic interview to ascertain seizure types, seizure semiology, seizure frequency, age at onset, diurnal pattern of seizure occurrence (e.g., nocturnal only, upon awakening), and history of status epilepticus. The interview was modified from a previously validated instrument.7,8
Participants who reported a history of at least one “grand mal” (generalized tonic-clonic [GTC]) seizure were identified and their responses to the diagnostic interview regarding these seizures were examined. The interview initially asked subjects to provide their own description of any subjective phenomena experienced before their grand mal seizures, with their answers recorded verbatim. These open-ended questions consisted of the following: “In your own words, can you describe how you feel or what happens before the grand mal seizure? If you had to choose one symptom, what symptom would you say occurs most frequently before your grand mal seizure(s)? What would you say is the very first thing that usually happens or you feel in your grand mal seizure?” Participants who provided answers to these questions were considered to have an aura. Responses to these open-ended questions were independently classified by 2 epileptologists (P.D., J.B.) into categories using the Partial Seizure Symptom Definitions developed by Choi et al.9 After independent reviews, the 2 reviewers discussed cases on which they disagreed with a third reviewer (D.J.C.) and arrived at a consensus.
All subjects were then asked a series of structured (closed-ended) questions about specific types of subjective experiences before their grand mal seizures and were required to answer “yes,” “no,” or “don't know,” with multiple affirmative answers allowed. For example, “Before the seizures start, or at the beginning of the seizures, do you have or have you ever been told you had numbness, tingling, pain, or other unusual feelings on only one side of the body?”
The durations of auras were not specified. However, experiences that were consistent with prodromal or premonitory symptoms were excluded, such as, “feeling increasingly sick throughout the day.” Prodromal or premonitory symptoms have been defined as symptoms that precede seizures by at least 30 minutes; their correlation with the underlying epilepsy syndrome is not clear.10 Reports of recognized generalized seizure clusters were excluded, such as “back-to-back absence seizures” or “a buildup of myoclonic jerks leading up to a grand mal.” Nonspecific symptoms such as dizziness, fatigue, or lapses in awareness were also excluded because these were believed to be inconsistent with the conventional aura definition and difficult to distinguish from incipient impairment of consciousness. Behaviors consistent with oral and limb automatisms were also excluded because these likely also reflected evolving seizure activity.
These findings were compared with responses provided by participants with a total of 434 participants who had a history of tonic-clonic seizures associated with FE.
Standard protocol approvals, registrations, and patient consents.
All research was approved by the institutional review board of each clinical center, and all participants provided written informed consent. Phenotypic data reside in a centralized data repository, and collected DNA is stored in the National Institute of Neurological Disorders and Stroke Human Genetics DNA and Cell Line Repository at the Coriell Institute for Medical Research.11
RESULTS
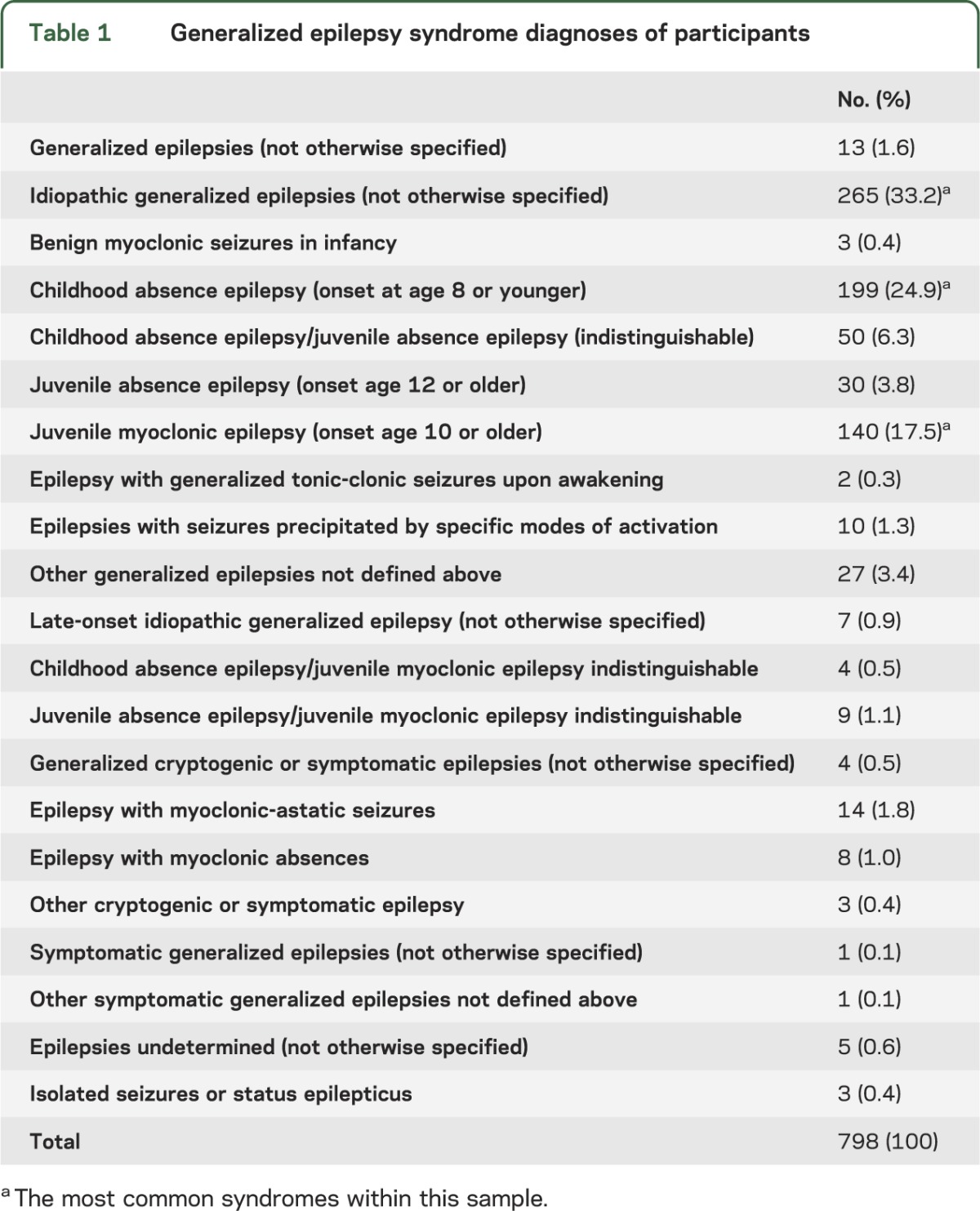
A total of 798 participants with only GE were identified. Details of the epilepsy syndromes are provided in table 1. The most common syndromes within this sample were IGEs not otherwise specified (33.2%), childhood absence epilepsy (24.9%), and juvenile myoclonic epilepsy (JME) (17.5%). Among all participants, 530 (66.4%) reported grand mal seizures.
Table 1.
Generalized epilepsy syndrome diagnoses of participants
Utilizing the open-ended question, 112 (21.3%) of the participants reported auras before their grand mal seizures. Symptoms such as a rising epigastric sensation, fear, pain, numbness, unusual taste, and visual disturbances were reported. The most frequently reported auras in response to the open-ended questions were cephalic sensations including ictal headache and bilateral and unilateral limb shaking or stiffening (table 2). Nine participants (8.0%) reported more than one aura.
Table 2.
Comparison of reported symptoms with open-ended and closed-ended questions
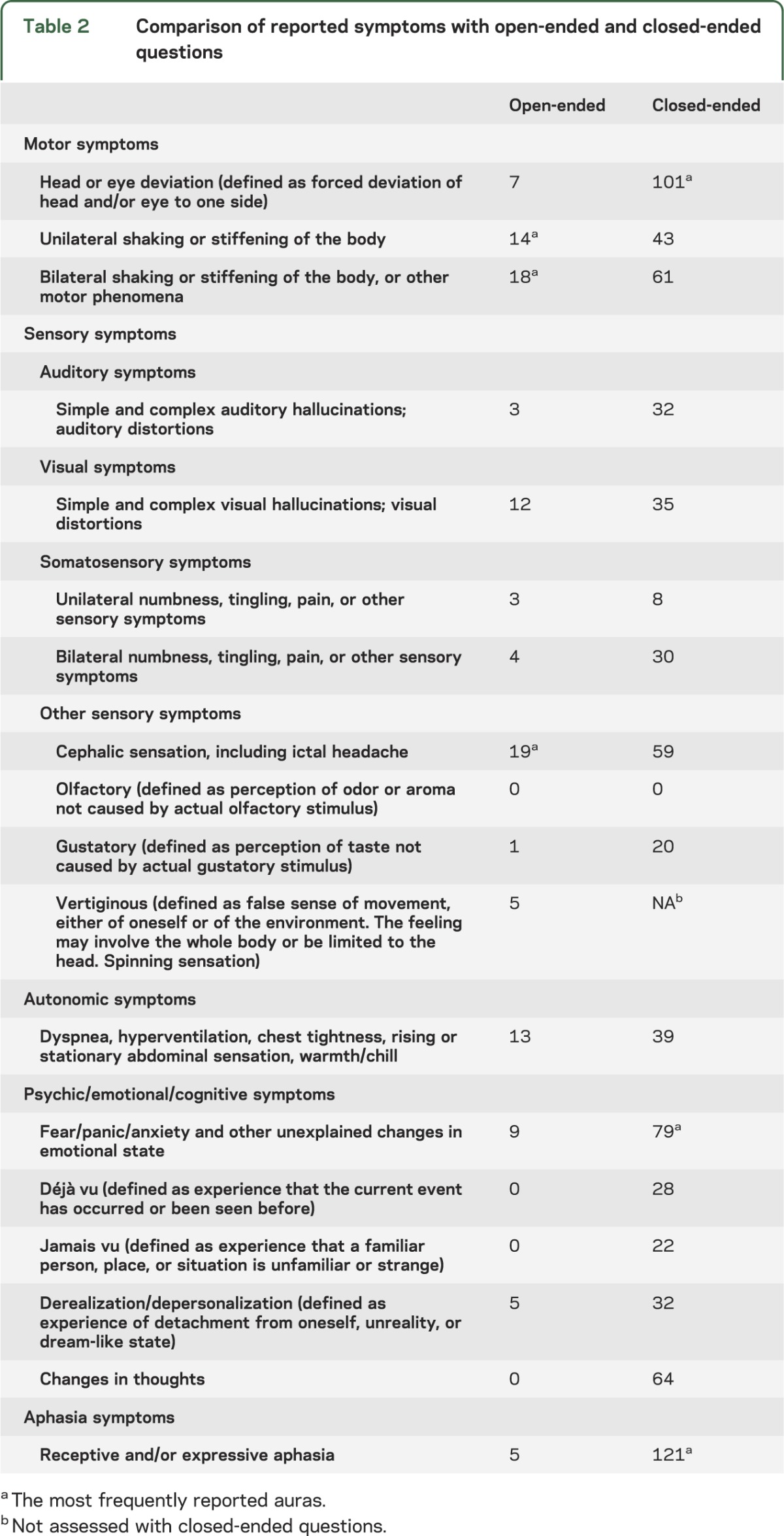
In response to the closed-ended questions, 341 (64.3%) of the participants with grand mal seizures reported at least one form of aura. The most common auras reported were version of the head or other body parts, receptive or expressive aphasia, and fear/panic/anxiety and other unexplained changes in emotional state (table 2). Two hundred six (60.4%) of those who reported auras in response to closed-ended questions reported more than one aura. Auras characterized by unusual tastes, visual or auditory phenomena, déjà vu, and jamais vu were also frequently reported. No subject reported olfactory auras.
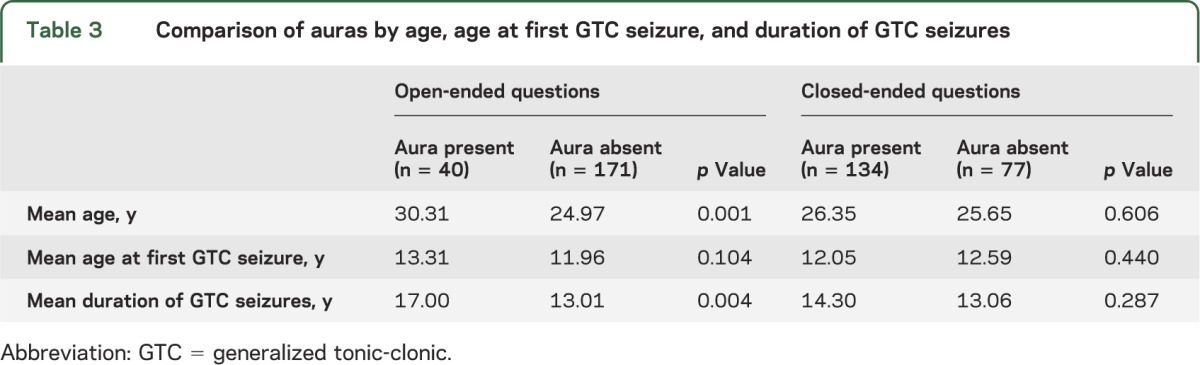
The proportion of participants who reported auras did not differ by sex utilizing either open-ended questions (p = 0.33, Fisher exact test) or closed-ended questions (p = 0.78). Using open-ended questions, participants who reported auras were older (p = 0.001, t test) and had a longer duration of experiencing GTC seizures (p = 0.004), but no difference was noted with the age at first GTC seizure (p = 0.104). Using closed-ended questions, no differences were observed between groups (table 3).
Table 3.
Comparison of auras by age, age at first GTC seizure, and duration of GTC seizures
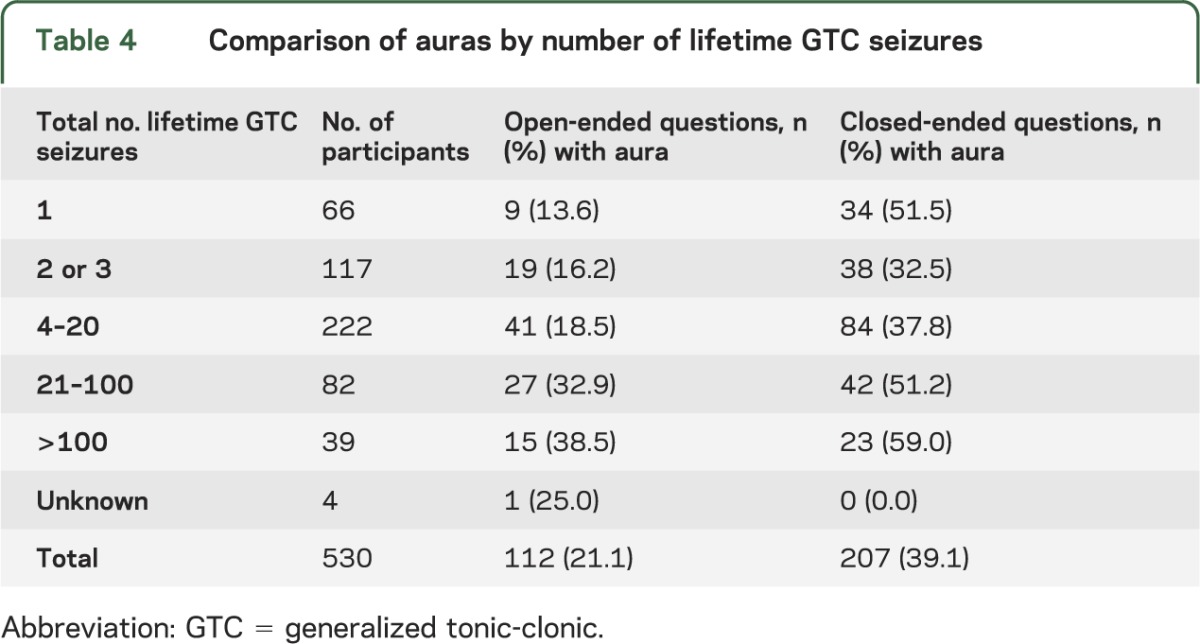
Participants quantified their number of lifetime grand mal seizures as follows: 1, 2 or 3, 4–20, 21–100, >100, or unknown. As the number of total lifetime GTC seizures increased, there was an overall increase in the percentage of participants that reported an aura. This was observed between all groups using either open-ended questions (p = 0.002, χ2) or closed-ended questions (p = 0.001) (table 4). When this was stratified for sex, a significant difference was observed in women (p = 0.001, χ2) but not in men (p = 0.50) with open-ended questions. Using closed-ended questions, significant differences were also observed in women (p = 0.01, χ2) but not in men (p = 0.34). Our data showed that women were also more likely to report auras and had a greater number of lifetime grand mal seizures.
Table 4.
Comparison of auras by number of lifetime GTC seizures
To place the rate of reported auras in context, we reviewed the same data for EPGP participants with FE who were interviewed utilizing the same instruments. Of 604 participants with FE, 434 (71.9%) reported a history of tonic-clonic seizures. Of the 434 participants with a history of tonic-clonic seizures, 173 (39.9%) reported auras in response to similar open-ended questions while 297 (68.4%) reported auras in response to closed-ended questions. The proportion of participants who reported symptoms before their grand mal seizures was significantly greater among those with FE than those with GE based on the open-ended question (p < 0.001, Fisher exact test), but not based on the closed-ended questions (p = 0.1938).
DISCUSSION
Nearly 65% of EPGP participants with GE reported auras before tonic-clonic seizures when asked about specific symptoms with structured interview questions. Even utilizing open-ended spontaneous descriptions of their seizures, almost a quarter (21.3%) reported auras. Auras typically associated with FE such as gustatory, visual, auditory phenomena, déjà vu, jamais vu, and versive eye or head movements were frequently reported in this cohort.
Similar to our findings, a prior report showed a high prevalence of auras in 154 patients (70%) with IGE, where the frequency of aura symptoms did not distinguish between patients with IGE and FE.4 Auras were reported in 13% of subjects with GE in a large study evaluating 3 population-based twin registries.6 Another study found sensory, psychic, and autonomic auras reported by 20 of 37 patients (54%) with JME.5
Our data demonstrate similar rates of aura reporting when using structured questions for both GE and FE. For both GE and FE, a higher rate of reported auras was seen with closed-ended questions, which likely reflects the difference between spontaneous vs cued recall. Although it may be argued that respondents could be influenced to provide affirmative responses by the presence of specific alternatives with closed-ended questioning,12 nonresponses to open-ended questions may be attributable to a lack of eloquence or expression rather than lack of relevance.13 We believe that these findings are important and clinically relevant because clinicians tend to use open-ended forms of questioning initially when eliciting patient histories and use closed-ended questions to obtain additional clinical information. The types of closed-ended questions used may be biased by the clinician's hypothesis; questions investigating the presence or absence of auras may not be asked if the clinician suspects a diagnosis of GE and a history of auras is not spontaneously reported. Any report of auras should be further substantiated with additional questions to determine whether these are stereotyped and occur consistently with all or most tonic-clonic seizures.
Reporting of auras was associated with a greater number of lifetime grand mal seizures and a longer duration of grand mal seizures. We postulate this may be because these facilitate greater familiarity with the experience of auras and an improved ability to articulate and report this. Further study will be required to elucidate this relationship. Our data showed that women were also more likely to report auras and had a greater number of lifetime grand mal seizures; these findings may be attributable to older age and a greater proportion of female vs male parents in the EPGP study population, compared with probands and siblings.14
This EPGP study population presented additional limitations for this study. Participants were recruited primarily from tertiary epilepsy centers, presumably for management or diagnostic evaluation. This may result in a potential bias for subjects with more refractory epilepsy.
Focal clinical features may occur at the onset or during GTC seizures in patients with GE.4,15–19 Focal clinical features such as forced lateralized head turning at seizure onset, and asymmetries and asynchronies of limb movements during the clonic phase also occur in IGE.15 In patients with JME, unilateral and asymmetric myoclonic jerks can occur. Furthermore, patients with JME may perceive asymmetries in myoclonic seizures that are actually symmetric.16,17 In a study of 26 patients with JME, 14 (54%) demonstrated focal semiologic or electroencephalographic features, or both. Notably, the “figure 4” sign (extension of one arm and flexion of the contralateral arm at the elbow), which is conventionally viewed as a lateralizing sign in secondarily generalized seizures, was noted in 19.2%18 of patients with JME. Four patients reported auras of numbness and tunnel vision before seizures.18 “Benign versive” or circling seizures have also been shown to occur in patients with absence epilepsy and JME.17 Subjective sensations of fear and derealization, as well as variable degrees of impaired consciousness, are reported by patients with absence epilepsy.19
The presence of auras and other lateralized features associated with GE may support cortical and subcortical generator theories of GE with variable spread patterns involving discrete cortical networks. Sustained lateralization at seizure onset, with features clinically indistinguishable from focal-onset frontal lobe seizures, supports the postulate of frontal lobe hyperexcitability in some patients with GE.20 Versive eye or head movements may also reflect asymmetries in cortical architecture, connections, and networks that are unrelated to epilepsy. Meticulous testing reveals that many absence seizures are characterized by behavioral arrest rather than true impairment of consciousness; eventual impairment of consciousness depends on the degree and nature of seizure evolution, comparable with focal seizures arising from the premotor frontal lobe.21 In addition, neurotransmitter synthesis and activity varies between brain regions, which may restrict and modulate seizure activity and propagation.22
Focal EEG findings also occur in GE. Among patients with JME, 20% to 55% have focal epileptiform discharges either preceding or independent of their typical generalized discharges or asymmetry in the amplitude of generalized discharges. These findings tend to be intermittent and shifting in laterality.15,23–25 Dense-array EEG analysis of patients with absence epilepsy demonstrated ictal onsets, often unilateral, arising from the dorsolateral frontal or orbital frontal lobe followed by stereotyped evolution to involve both mesial frontal and orbital frontal structures.26
Auras and other “focal” features in patients with GE can lead to the misdiagnosis of FE.4,5,16,18,19,26 It is also possible that auras in GE are underreported, and may be more likely to be elicited with systematic questioning. For most patients with epilepsy, an initial goal of clinical evaluation is distinguishing GE from FE. The presence of auras tends to bias clinicians in favor of a diagnosis of FE despite other clinical and EEG features consistent with GE. Recognition that auras are common in patients with GE should help prevent the misdiagnosis of FE based solely on reports of isolated auras suggesting localized seizure onset (e.g., déjà vu, gustatory hallucination). Dyscognitive symptoms such as lapses in awareness and changes in speech, thoughts, or comprehension are also frequently reported by patients with GE.6 Although these may represent unrecognized absence seizures, the clinicians may attribute these to dyscognitive symptoms conventionally associated with focal seizures, especially in the setting of a normal EEG or a generalized EEG with asymmetric features.
Misdiagnosis may have considerable therapeutic implications, particularly with the selection of antiepileptic drugs. For example, the prescription of carbamazepine can exacerbate seizures in patients with GE.27 Immediate diagnosis of JME may encourage the use of valproate, which often provides excellent control of this seizure syndrome.5 Diagnostic uncertainty may prompt greater use of broad-spectrum antiepileptic drugs and encourage referral to comprehensive epilepsy centers for definitive diagnosis, perhaps with long-term video EEG monitoring for characterization.
Future studies to examine the electrographic and clinical correlations may shed light on the mechanisms underlying focal features in GE. It is not clear whether patients with GE who have auras or lateralized motor features at seizure onset have higher rates of lateralized or asymmetric EEGs than other patients with GE. In examining the genetics of GE, it will also be valuable to study differences among those who do and do not report auras. Finally, understanding the pathophysiology of auras in generalized seizures (e.g., thalamic vs neocortical vs thalamocortical onsets) may highlight potential targets for pharmacologic and neuromodulatory therapies.
Supplementary Material
ACKNOWLEDGMENT
The authors acknowledge the recruitment contributions of the EPGP Community Referral Network (CRN). The CRN consists of health care professionals not paid by the EPGP grant who refer eligible families to EPGP. A list of individual contributors can be found at www.epgp.org.
GLOSSARY
- EPGP
Epilepsy Phenome/Genome Project
- FE
focal epilepsy
- GE
generalized epilepsy
- GTC
generalized tonic-clonic
- IGE
idiopathic generalized epilepsy
- JME
juvenile myoclonic epilepsy
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Patricia Dugan, MD: responsible for the accuracy of the data analysis, conduct of the research, and has access to all of the data in the study. Chad Carlson, MD, Judith Bluvstein, MD, Derek Chong, MD, MS, Daniel Friedman, MD, and Heidi Kirsch, MD, MS: each made a substantive intellectual contribution to the submitted manuscript regarding analysis and interpretation of data and manuscript revision. EPGP investigators: study group.
STUDY FUNDING
Supported by National Institute of Neurological Diseases and Stroke grant U01 NS053998, as well as planning grants from the Finding a Cure for Epilepsy and Seizures Foundation and the Richard Thalheimer Philanthropic Fund.
DISCLOSURE
P. Dugan, C. Carlson, J. Bluvstein, and D. Chong report no disclosures relevant to the manuscript. D. Friedman receives salary support from The Epilepsy Study Consortium, a nonprofit organization dedicated to improving the lives of patients with epilepsy, and devotes 15% of his time to work done for the consortium. The consortium receives payments from a large number of pharmaceutical companies for consulting activities. All payments are made to the consortium and not to Dr. Friedman directly. Several companies also support the consortium's biennial Antiepileptic Drug Trials Symposium. Because there are so many companies contributing, the amount from each company toward Dr. Friedman's salary is minimal and is reviewed annually by NYU's conflict of interest committee. H. Kirsch's salary is partially supported by the NIH. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Johanson M, Valli K, Revonsuo A, Wedlund JE. Content analysis of subjective experiences in partial epileptic seizures. Epilepsy Behav 2008;12:170–182 [DOI] [PubMed] [Google Scholar]
- 2.Schachter S. Seizure disorders. Med Clin North Am 2009;93:343–351 [DOI] [PubMed] [Google Scholar]
- 3.Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia 2010;51:676–685 [DOI] [PubMed] [Google Scholar]
- 4.Boylan LS, Labovits DL, Jackson SC, Starner K, Devinsky O. Auras are frequent in idiopathic generalized epilepsy. Neurology 2006;67:343–345 [DOI] [PubMed] [Google Scholar]
- 5.Vazquez B, Devinsky O, Luciano D, Alper K, Perrine K. Juvenile myoclonic epilepsy: clinical features and factors related to misdiagnosis. J Epilepsy 1993;6:233–238 [Google Scholar]
- 6.Nakken KO, Solaas MH, Kjeldsen MJ, Friis ML, Pellock JM, Corey LA. The occurrence and characteristics of auras in a large epilepsy cohort. Acta Neurol Scand 2009;119:88–93 [DOI] [PubMed] [Google Scholar]
- 7.Ottman R, Hauser WA, Stallone L. Semistructured interview for seizure classification: agreement with physicians' diagnoses. Epilepsia 1990;31:110–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ottman R, Lee JH, Hauser WA, et al. Reliability of seizure classification using a semistructured interview. Neurology 1993;43:2526–2530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi H, Winawer MR, Kalachikov S, Pedley TA, Hauser WA, Ottman R. Classification of partial seizure symptoms in genetic studies of the epilepsies. Neurology 2006;66:1648–1653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schulze-Bonhage A, Kurth C, Carius A, Steinhoff B, Mayer T. Seizure anticipation by patients with focal and generalized epilepsy: a multicenter assessment of premonitory symptoms. Epilepsy Res 2006;70:83–88 [DOI] [PubMed] [Google Scholar]
- 11.Nesbitt G, McKenna K, Mays V, Carpenter A, Miller K, Williams M; The EPGP Investigators. The epilepsy phenome/genome informatics platform. Int J Med Inform 2013;82:248–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schuman H, Presser S. The open and closed question. Am Sociol Rev 1979;44:692–712 [Google Scholar]
- 13.Roberts M, Stewart B, Tingley D, et al. Structural topic models for open-ended survey responses. Am J Pol Sci 2014:1–19 [Google Scholar]
- 14.Winawer M, Connors R; EPGP Investigators. Evidence for a shared genetic susceptibility to migraine and epilepsy. Epilepsia 2013;54:288–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niaz FE, Abou-Khalil B, Fakhoury T. The generalized tonic-clonic seizure in partial versus generalized epilepsy: semiologic differences. Epilepsia 1999;40:1664–1666 [DOI] [PubMed] [Google Scholar]
- 16.Oguni H, Mukahira K, Oguni M, et al. Video-polygraphic analysis of myoclonic seizures in juvenile myoclonic epilepsy. Epilepsia 1994;35:307–316 [DOI] [PubMed] [Google Scholar]
- 17.Ferrie CD. Idiopathic generalized epilepsies imitating focal epilepsies. Epilepsia 2005;46:91–95 [DOI] [PubMed] [Google Scholar]
- 18.Usui N, Kotagal P, Matsumoto R, Kellinghaus C, Luders HO. Focal semiologic and electroencephalographic features in patients with juvenile myoclonic epilepsy. Epilepsia 2005;46:1668–1676 [DOI] [PubMed] [Google Scholar]
- 19.Panayiotopoulos CP, Chroni E, Daskalopoulos C, Baker A, Rowlinson S, Walsh P. Typical absence seizures in adults: clinical, EEG video-EEG findings and diagnostic/syndromic considerations. J Neurol Neurosurg Psychiatry 1992;55:1002–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casaubon L, Pohlmann-Eden B, Khosravani H, Carlen PL, Wennberg R. Video-EEG evidence of lateralized clinical features in primary generalized epilepsy with tonic-clonic seizures. Epileptic Disord 2003;5:149–156 [PubMed] [Google Scholar]
- 21.Craiu D, Magureanu S, van Emde Boas W. Are absences truly generalized seizures or partial seizures originating from or predominantly involving the pre-motor areas? Some clinical and theoretical observations and their implications for seizure classification. Epilepsy Res 2006;70S:S141–S155 [DOI] [PubMed] [Google Scholar]
- 22.Blumenfeld H. From molecules to networks: cortical/subcortical interactions in the pathophysiology of idiopathic generalized epilepsy. Epilepsia 2003;44:7–15 [DOI] [PubMed] [Google Scholar]
- 23.Lombroso CT. Consistent EEG focalities detected in subjects with primary generalized epilepsy monitored for two decades. Epilepsia 1997;38:797–812 [DOI] [PubMed] [Google Scholar]
- 24.Aliberti V, Grunewald RA, Panayiotopoulos CP, Chroni E. Focal electroencephalographic abnormalities in juvenile myoclonic epilepsy. Epilepsia 1994;35:297–301 [DOI] [PubMed] [Google Scholar]
- 25.Jayalakshmi SS, Srinivasa Rao B, Sailaja S. Focal clinical and electroencephalographic features in patients with juvenile myoclonic epilepsy. Acta Neurol Scand 2010;122:115–123 [DOI] [PubMed] [Google Scholar]
- 26.Holmes MD, Brown M, Tucker DM. Are “generalized” seizures truly generalized? Evidence of localized mesial frontal and frontopolar discharges in absence. Epilepsia 2004;45:1568–1579 [DOI] [PubMed] [Google Scholar]
- 27.Perucca E, Gram L, Avanzini G, Dulac O. Antiepileptic drugs as a cause of worsening seizures. Epilepsia 1998;39:5–17 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


