Abstract
Objective:
To assess the usefulness of transcranial Doppler CO2 reactivity (CO2R) for prediction of ipsilateral ischemic stroke in carotid artery stenosis and occlusion with a meta-analysis of prospective studies based on individual patient data.
Methods:
We searched Medline, Biosis Previews, Science Citation Index, The Cochrane Library, and EMBASE for studies in which patients with severe carotid artery stenosis or occlusion underwent Doppler CO2R testing (inhalation of CO2 or breath-holding) and were prospectively followed for ipsilateral ischemic stroke. Individual data from 754 patients from 9 studies were included. We used percentage cerebral blood flow velocity increase (pCi) during hypercapnia as the primary CO2R measure, and defined impaired reactivity as pCi <20% increase.
Results:
In a multiple regression model, impaired CO2R was independently associated with an increased risk of ipsilateral ischemic stroke (hazard ratio [HR] 3.69; confidence interval [CI] 2.01, 6.77; p < 0.0001). Risk prediction was similar for recently symptomatic vs asymptomatic patients. Using continuous values of pCi, a significant association between decreasing pCi and increasing risk of ipsilateral stroke was found: HR of 1.64 (95% CI 1.33, 2.02; p < 0.0001) per 10% decrease in pCi. For patients with asymptomatic internal carotid artery stenosis only (n = 330), a comparable stroke risk prediction was found: increasing HR 1.95 (95% CI 1.26, 3.04; p = 0.003) per 10% decrease in pCi.
Conclusions:
This analysis supports the usefulness of CO2R in risk prediction for patients with severe carotid artery stenosis or occlusion, both in recently symptomatic and asymptomatic patients. Further studies should evaluate whether treatment strategies in asymptomatic patients based on CO2R could improve patient outcomes.
Carotid recanalization in patients with asymptomatic internal carotid artery (ICA) stenosis offers a marginal clinical benefit when all patients are considered together.1 Given the advances in modern optimized medical therapy, identifying a subset of patients at high risk is thus of increasing importance.
Transcranial Doppler sonography (TCD) offers 2 prognostic tests that may allow risk stratification in ICA disease: detection of microembolic signal (MES) and determination of cerebrovascular reactivity (CVR). Detection of MES can identify asymptomatic patients with ICA stenosis at risk.2 CVR, which assesses the increase in cerebral blood flow in response to a vasodilatory stimulus, was a significant predictor of future stroke in some TCD studies,3–7 while other studies failed to show such an association.8,9
Previous conventional meta-analyses investigating CVR were limited by combining different methodologies of estimating cerebral blood flow (TCD vs nuclear medicine methods), different vasodilatory stimuli (CO2 vs acetazolamide), and by including heterogeneous patient risk profiles (symptomatic vs asymptomatic disease), cutoff values, and clinical outcome events.8,10
Individual patient data (IPD) meta-analysis overcomes such limitations through standardized definitions and analyses across studies and adjustment for variations in individual patient prognosis at baseline. This allows for more powerful investigations of subgroup effects and specific endpoints.11
We therefore performed an IPD meta-analysis to investigate the usefulness of TCD CO2 reactivity in the prediction of ipsilateral ischemic stroke in ICA stenosis or occlusion.
METHODS
Standard protocol approvals, registrations, and patient consents.
A study protocol for this meta-analysis of IPD was written in advance and sent to all investigators involved. This study was approved by the Ethics Committee of the University of Freiburg. We prepared the present report according to PRISMA guidelines.27
Study selection and data collection.
Eligible studies applied transcranial Doppler sonography of the middle cerebral artery with a vasodilatory CO2 challenge (hypercapnia or breath-holding) in symptomatic or asymptomatic patients with severe (≥70%) carotid artery stenosis (not scheduled for intervention) or occlusion (not scheduled for extracranial/intracranial bypass) and prospectively reported clinical outcome (stroke and TIAs) in relation to the measured parameter of CO2 reactivity (CO2R). Studies were excluded if they exclusively focused on acetazolamide as a stimulus or if they did not follow patients prospectively. There were no exclusions based on language or publication status of reports.
An experienced health science librarian (E.M.) identified suitable studies in July 2011 (the search was updated upon finalization of the manuscript on November 26, 2013) by a formal search in the following databases: Medline (since 1948), Medline In-Process & Other Non-Indexed Citations, Biosis Previews (since 1926), Cochrane Library: Cochrane Database of Systematic Reviews (issue 11/2013, Clinical Trials, issue 10/2013), Science Citation Index (since 1945), Embase, and Embase Alert (since 1974). Search terms, their synonyms, and, in databases with a thesaurus, appropriate controlled terms were combined. Combinations were according to the following aspects: cerebrovascular reactivity, CO2 reactivity, transcranial Doppler, and carotid artery. Reference lists of retrieved relevant articles were screened and experts in the field contacted for additional eligible studies. Detailed search strategies are available from the authors by request.
Two reviewers (M.R., A.H.) independently assessed trial eligibility based on titles, abstracts, full-text reports, and further information from investigators as needed. Because we built our own statistical models taking into account all relevant confounders and potential clustering effects, we focused in our assessment of the methodologic quality of included prognostic studies on 5 domains: representativeness of the population, loss to follow-up, prognostic factor measurement, outcome measurement, and confounding measurement12 (table e-1 on the Neurology® Web site at Neurology.org). Study quality was assessed independently by 2 reviewers (M.R., A.H.). Discrepancies between reviewers were resolved by consensus or third party arbitration (M.B.), if required.
Data collection, subgroups, and outcomes.
Individual patient data were requested from principal investigators of all eligible studies. All investigators were asked to provide a dataset including (1) baseline characteristics of each individual patient: age, sex, vascular risk factors, degree of ipsilateral and contralateral carotid stenosis, status of stenosis (symptomatic, asymptomatic), if applicable type and time of previous ischemic events in the territory supplied by the affected carotid artery; and (2) methodology of CO2R test: CO2R in terms of maximum % increase in cerebral blood flow velocity (CBFV) during the hypercapnic challenge, CO2R normalized to the amount of hypercapnia (% CBFV increase per mm Hg PetCO2 increase), and local cutoff values of CO2R3 clinical endpoints: ischemic stroke or TIA attributed to the territory of the affected carotid artery, death during follow-up, and carotid recanalization without a prior ischemic event. Data from each study were first checked against reported results and queries were resolved with the principal investigator.
In order to reanalyze IPD with a common continuous CO2R measure, we asked all authors to contribute (published or unpublished) data on maximum % increase in CBFV during the hypercapnic challenge in their patients (pCi = percentage CBFV increase).
As a main outcome across studies, individual data on ipsilateral ischemic events were gathered for all patients. Ischemic stroke was defined as the sudden onset of new neurologic symptoms attributable to the affected ICA and lasting longer than 24 hours in all studies. Separate data for minor and major stroke and neuroimaging results were not consistently available across studies. As a further outcome, ipsilateral TIA (hemispheric or retinal, symptoms lasting <24 hours) were assessed across all studies.
Statistical analysis.
Previous studies have reported percentage increase in CBFV and used a cutoff of 20%.3 Therefore, we used this prespecified cutoff for the primary analysis as well as continuous pCi. Multiple fractional polynomials (MFPs)13 were used to evaluate the functional form of continuous pCi and age (which was the other continuous covariable). We used the Stata programs stcox (for Cox proportional hazard regression model) as well as mfp and mfpi (for MFPs) with default settings, i.e., a p value of 0.05 was used for testing between MFP models.
We conducted the following prespecified sensitivity analyses: (1) considering interaction terms in multiple regression models between predictive value of pCi and (a) method of inducing hypercapnia (breath-holding vs CO2 inhalation), (b) presence or absence of recent symptoms, and (c) carotid occlusion vs severe stenosis; and (2) addition of covariables hypertension and diabetes in multiple regression models. A meta-regression was conducted for the predictive value of pCi of <20% (unadjusted estimates) and year of publication to evaluate a potential trend over time. Furthermore, a heterogeneity test was conducted for unadjusted pCi <20% predictions.
In addition, a linear mixed effect model with study as a random effect was used to identify baseline variables with an influence on the percentage increase of CBFV during the hypercapnic challenge.14
Cox proportional hazard models stratified by study (fixed effect model) were used to evaluate the prognostic value of percentage increase of CBFV during the hypercapnic challenge on the occurrence of TIA or stroke. The following covariables were considered in Cox regression models: age, sex, hypertension, diabetes, ICA stenosis ipsilateral, ICA stenosis contralateral, ischemic events during the last 3 months before study inclusion, and method of breath-holding.
RESULTS
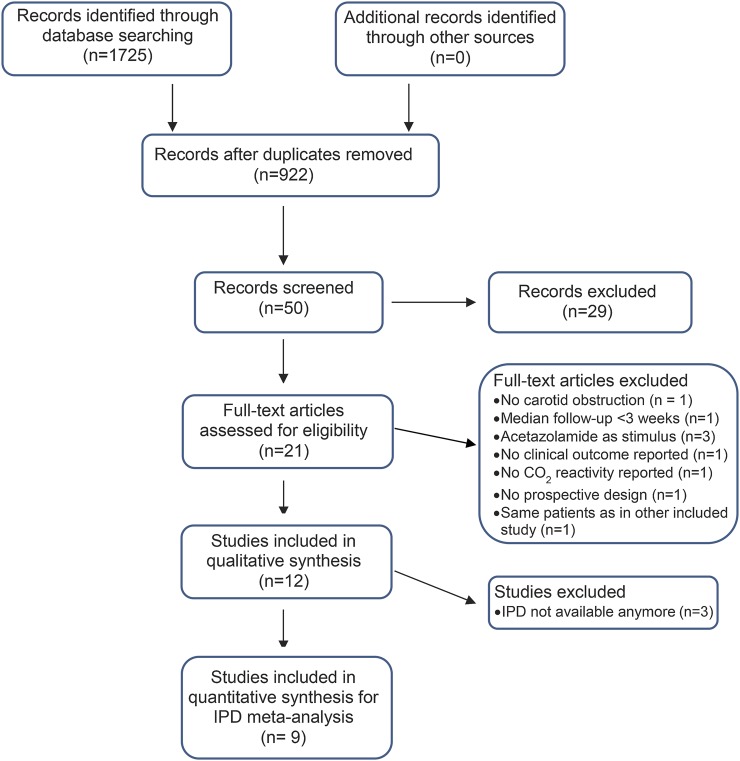
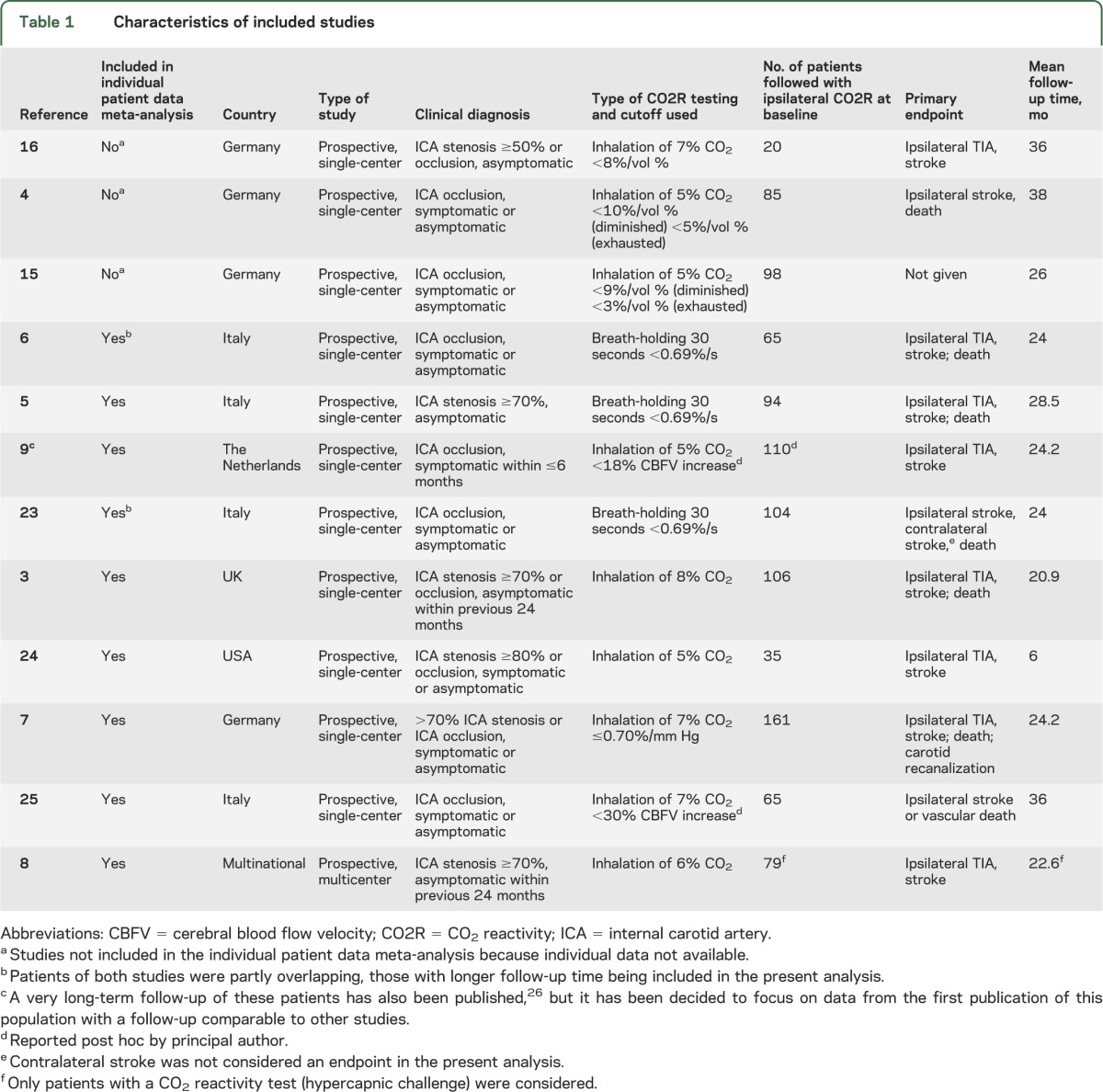
We identified 12 studies that met the inclusion criteria (figure 1). The median duration of follow-up was comparable across studies. There was, however, a considerable methodologic heterogeneity with respect to the type of CO2 challenge (inhalation of CO2 or breath-holding), the mode of calculating CO2R, and the cutoff used to define pathologic values (table 1). Clinical endpoints assessed were mostly ipsilateral ischemic events (stroke, cerebral TIA, and in some studies also retinal ischemia). The methodologic quality of all included studies was good; details are summarized in table e-1.
Figure 1. Flow diagram according to Preferred Reporting Items for Systematic reviews and Meta-Analyses guidelines27.
IPD = individual patient data.
Table 1.
Characteristics of included studies
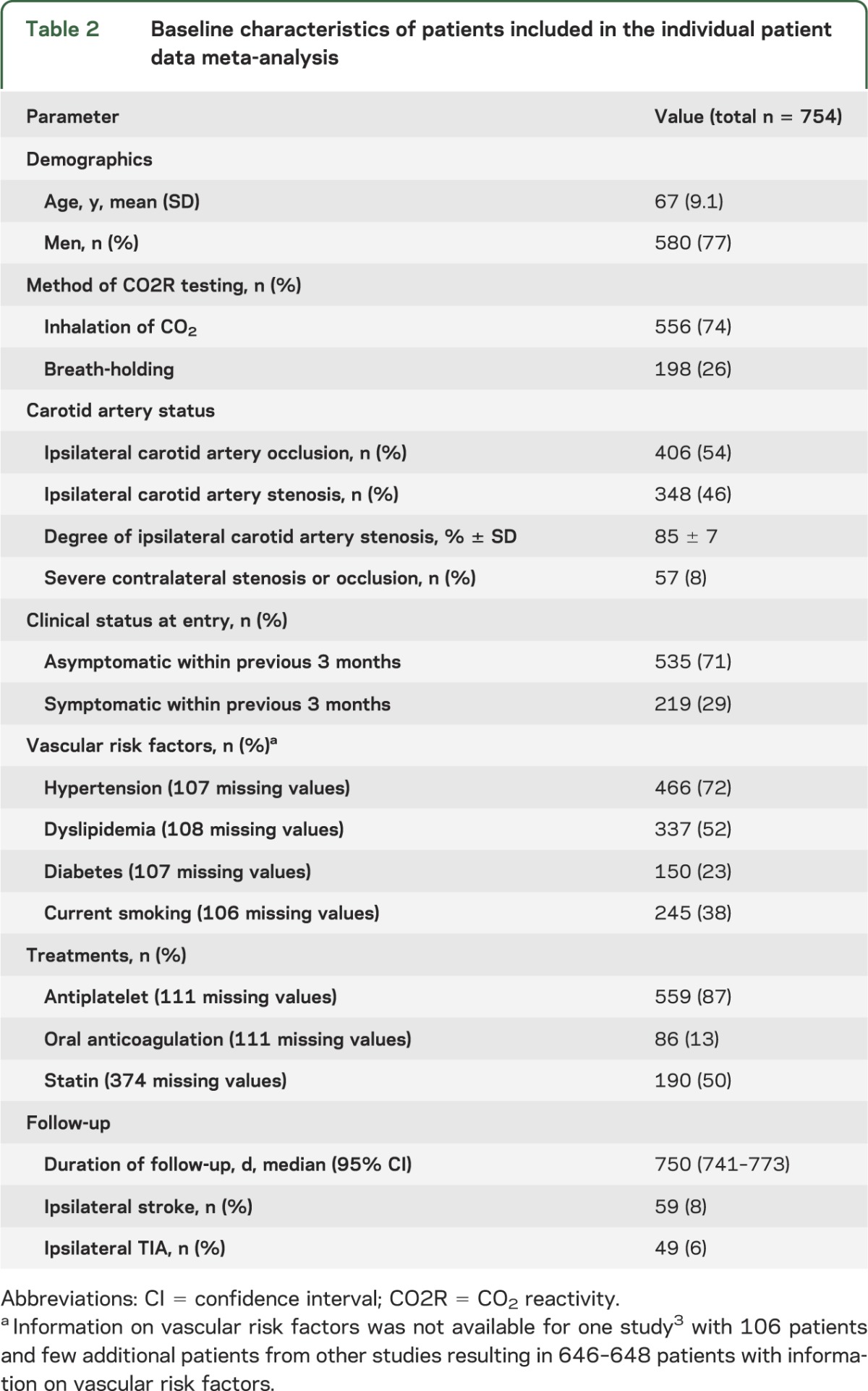
We were able to obtain IPD from 9 studies including a total of 762 patients. Data from 3 studies4,15,16 were no longer available. These studies mostly analyzed patients with ICA occlusion: 2 of them reported an association of impaired CO2R with stroke. Because information on pCi could not be obtained in 8 patients, the total sample is based on 754 individual patients prospectively followed for stroke or TIA. Baseline characteristics of all included patients are presented in table 2. During a median follow-up time of 750 days, 59 patients (8%) had an ipsilateral ischemic stroke and 49 patients (6%) an ipsilateral TIA.
Table 2.
Baseline characteristics of patients included in the individual patient data meta-analysis
CO2 reactivity at baseline.
In a multivariable model, the common ipsilateral CO2R measure pCi was lower in patients with ipsilateral ischemic symptoms within the previous 3 months, in current smokers, in patients with more severe ICA stenosis or occlusion, and in patients with a significant contralateral ICA stenosis (table e-2). It was slightly higher with increasing age. The method of induction of hypercapnia did not significantly influence pCi results. In the subset of data with information on statin therapy available (4 studies, n = 380), no significant influence of statin treatment on pCi was found.
Multiple regression model using prespecified cutoff values for pCi.
Impaired CO2R (pCi <20%) showed a highly significant and independent prognostic effect on the main endpoint of ipsilateral stroke and on the combined endpoint of ipsilateral stroke or TIA (table 3). Neither statistical heterogeneity nor a trend over time of study publication for the predictive effect of impaired CO2R was observed for either endpoint. Figure 2, A and B, shows incidence curves for the pooled patient group in the IPD analysis. Figure e-1 shows a forest plot for the prediction of ischemic events across studies.
Table 3.
Prognostic effect of impaired CO2 reactivity in severe carotid stenosis or occlusion
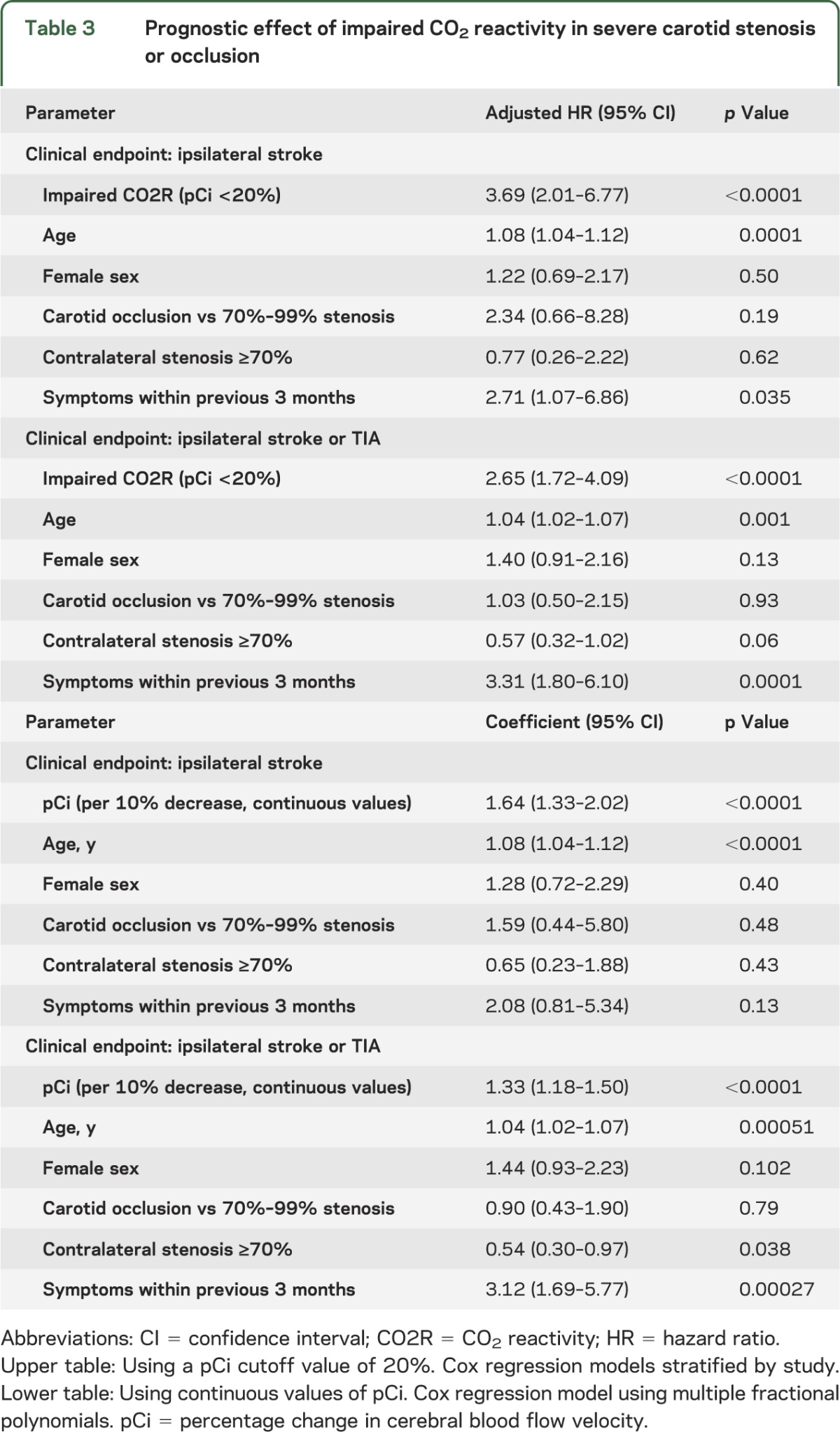
Figure 2. Relation between pCi and risk of ischemic stroke in carotid artery stenosis or occlusion.
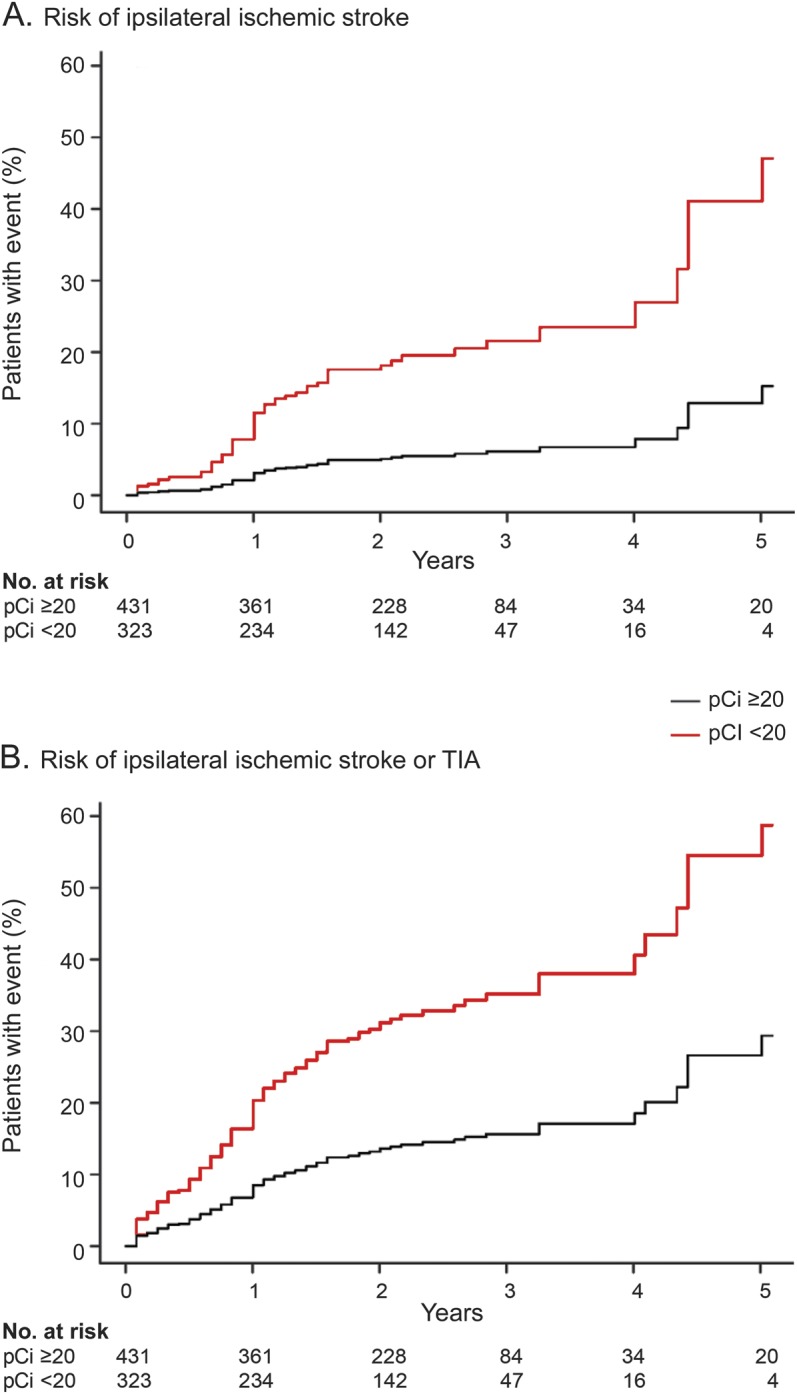
Estimated incidence of ischemic events over time in the pooled group of all patients including all covariates listed in table 3. (A) Risk of ipsilateral ischemic stroke. (B) Risk of ipsilateral ischemic stroke or TIA. Hazard ratio for impaired CO2 reactivity (pCi <20%) is slightly different from result in the Cox model used in table 3, which is additionally stratified by study (HR = 3.84 vs 3.69 for stroke, HR = 2.55 vs 2.65 for stroke or TIA; p < 0.00001 for all values). pCi = percentage increase in cerebral blood flow velocity (by hypercapnic stimulus).
In an interaction analysis, the specific predictive power of CO2R (relative risk increase) was not influenced by (1) the method used for induction of hypercapnia (breath-holding vs CO2 inhalation, p = 0.573 for interaction), (2) the presence or absence of recent symptoms (within the previous 3 months) (p = 0.293 for interaction), and (3) the presence of different degrees of ipsilateral stenosis (70%–89%, 90%–99%) and occlusion (p = 0.953 for interaction; detailed results of the respective proportional hazard regression models not shown).
A sensitivity analysis including the cofactors hypertension, diabetes, and smoking (data available for 647 patients, 8 studies) did not substantially change the independent predictive value of impaired CO2R for ipsilateral stroke (hazard ratio [HR] 2.54, 95% confidence interval [CI] 1.38, 4.69; p = 0.0029). Statin treatment (data available for 380 patients, 4 studies) was not associated with the risk of cerebral ischemic events and did not interact with predictive power of impaired CO2R (p = 0.90 for interaction).
Multiple regression model using continuous values of pCi.
A single patient with very extreme pCi value of −73% was excluded from MFP analyses to evaluate the functional form of continuous pCi. We found a significant influence for pCi on ipsilateral stroke risk (table 3). Overall, a decrease of pCi by 10% translates to an increased risk for stroke of 64%. A sensitivity analysis considering stroke and TIA as an outcome event yielded comparable HRs (table 3).
Whereas ischemic symptoms within the previous 3 months were associated with an increase in stroke risk, the relative risk increase inferred by CO2R for the risk of ischemic stroke was similar regardless of the presence or absence of recent symptoms (interaction term p = 0.924). A Cox regression model including an interaction term between continuous pCi and method of induction of hypercapnia (breath-holding or CO2 inhalation) showed a significant interaction between these 2 covariates with a steeper relation between pCi reduction and stroke risk increase for the breath-holding method.
Sensitivity analysis including the cofactors hypertension, diabetes, and smoking did not relevantly change the predictive value of 10% pCi decrease for ipsilateral stroke (HR = 1.49, 95% CI 1.19, 1.87; p = 0.0006).
Subanalysis in patients with asymptomatic severe ICA stenosis.
Asymptomatic patients with severe stenosis were defined as patients without ipsilateral symptoms in the previous 6 months (n = 330). This typical definition could be applied as data on delay to last symptoms were more detailed for patients with stenosis than occlusion. Overall, risk prediction for ipsilateral stroke in an adjusted multivariate model (corrected for age, sex, presence of severe contralateral stenosis) was comparable to that for the overall group of patients: for the dichotomized pCi <20%: HR = 2.90 (95% CI 1.02, 8.30; p = 0.047), for pCi as a continuous value: HR = 1.95 (95% CI 1.26, 3.04; p = 0.003) per 10% decrease in pCi.
DISCUSSION
This IPD meta-analysis included 754 patients with severe carotid artery stenosis or occlusion who underwent a TCD CO2R test and were prospectively followed for ischemic events. There was a strong and independent association between impaired CO2R with the risk of ipsilateral ischemic stroke and ischemic stroke or TIA. This association was similar for recently symptomatic and asymptomatic disease and for patients with severe carotid artery stenosis and occlusion. Of note, also in the specific subgroup of asymptomatic ICA stenosis, a highly predictive effect of impaired CO2R was found.
The use of IPD allows adjustment for patient characteristics across all studies. To increase comparability of studies and to identify a simple parameter for clinical risk prediction, we used the percentage CBFV increase (pCi) as a common CO2R measure across studies. This is the largest set of baseline data in patients to date, which enabled us to look for independent associations of impaired CO2R with clinical factors. Impaired CO2R was associated with contralateral severe stenosis or occlusion (indicating reduced potential of anterior crossover flow in this situation), recent symptoms (confirming the association between impaired CO2R and stroke risk), and current smoking.
The use of cutoff values may have reduced power and has some methodologic weaknesses.13,17 Therefore, a second analysis using continuous pCi values instead of cutoffs was performed to quantify the risk more accurately. The predictive power of pCi in this model was not relevantly affected by addition of vascular risk factors hypertension, diabetes, and smoking. This risk model needs validation in an external cohort. It might, however, be used in its present form as an indicator of the dimension of risk increase for experienced clinicians using Doppler CO2 reactivity techniques.
In the continuous statistical model, studies using the breath-holding method showed a steeper association between pCi status and stroke risk. The reasons for this difference are unclear and potential unmeasured confounders across various study sites cannot be ruled out. Finally, chance might have played a role since IPD from 2 studies that showed a clear association using CO2 inhalation could not be retrieved.4,15 Thus, inferences regarding the superiority of the breath-holding method cannot be drawn.
The TCD detection of microembolic signals is another promising test to predict stroke in patients with asymptomatic carotid artery stenosis.2 For practical reasons, the determination of CO2R, particularly using the breath-holding index, takes less time in comparison with detection of microemboli. Perhaps a combination of various ultrasound tests might improve the risk prediction. This has already been shown for the combination of plaque morphology and microemboli detection.18 Combining CO2R testing with plaque morphology or with the detection of microemboli may improve risk prediction further. This combined approach would address 2 pathophysiologic mechanisms of stroke in carotid artery disease: microembolism due to unstable plaque and poor hemodynamic compensation leading to impaired washout of emboli or pure hemodynamic ischemia. In the Asymptomatic Carotid Emboli Study, a relation between lower CO2R and increased number of embolic signals was found but the number of outcome events was too small to establish its clinical value.8
Limitations of this study are that there was no information on changes in the degree of stenosis and medication details during the follow-up period. Factors like carotid revascularization without an ischemic event and varying length of follow-up within studies might have interfered with risk analysis. Although the definition of ischemic stroke was homogenous across studies, specific details (severity, borderzone or embolic pattern) were not available and therefore could not be considered in this analysis. The 3 oldest studies could not be included because of missing data. Looking at the reported results and patient characteristics of these studies, it is unlikely that they might have relevantly changed the overall results of this IPD analysis. We had a priori decided to focus this IPD meta-analysis on the more frequently used CO2 stimulus and did not consider studies using acetazolamide as an alternative vasodilatory stimulus.19 This semi-invasive test may also be a risk indicator in carotid artery stenosis.20,21
Furthermore, CO2 inhalation and breath-holding can induce blood pressure increases and thus overestimate the actual vasomotor reactivity.22 In the present study, a correction for concomitant blood pressure increases was not possible since most of the studies did not monitor blood pressure during the hypercapnic challenge. However, any resultant rise in blood pressure would be expected to reduce, not increase, the association between impaired CO2R and stroke risk.
This meta-analysis based on IPD supports the usefulness of Doppler CO2R to predict the risk of ipsilateral ischemic stroke in patients with severe carotid stenosis or occlusion, with or without recent symptoms. There was no difference in prediction of clinical events; however, breath-holding may be easier to use in clinical practice than CO2 inhalation as it does not require the use of additional equipment. The CO2R method needs to be prospectively verified in a cohort receiving current optimal medical therapy. It should be evaluated whether treatment strategies in asymptomatic patients based on CO2R could improve patient outcomes.
Supplementary Material
GLOSSARY
- CBFV
cerebral blood flow velocity
- CI
confidence interval
- CO2R
CO2 reactivity
- CVR
cerebrovascular reactivity
- HR
hazard ratio
- ICA
internal carotid artery
- IPD
individual patient data
- MFP
multiple fractional polynomial
- pCi
percentage cerebral blood flow velocity increase during hypercapnia
- TCD
transcranial Doppler sonography
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Drs. Reinhard, Schwarzer, Briel, and Vernieri conceived the study. E. Motschall performed the literature search. Drs. Reinhard and Hetzel assessed the eligibility of identified studies. Dr. Schwarzer performed the final data analysis. The following authors were chief investigators of studies included in the IPD meta-analysis or essentially contributed in providing the individual patient data: Drs. Altamura, Palazzo, King, Bornstein, Petersen, Hetzel, Marshall, Klijn, Silvestrini, and Markus. Dr. Reinhard drafted the manuscript, which was revised by all authors named above for intellectual content.
STUDY FUNDING
No targeted funding reported.
DISCLOSURE
M. Reinhard, G. Schwarzer, M. Briel, C. Altamura, P. Palazzo, A. King, N. Bornstein, N. Petersen, E. Motschall, A. Hetzel, and R. Marshall report no disclosures relevant to the manuscript. C. Klijn is supported by a clinical established investigator grant of the Netherlands Heart Foundation (grant 2012 T077) and an ASPASIA grant from ZonMw, The Netherlands Organisation for Health Research and Development (grant 015.008.048). M. Silvestrini reports no disclosures relevant to the manuscript. H. Markus is supported by an NIHR Senior Investigator award. F. Vernieri reports no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Halliday A, Mansfield A, Marro J, et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet 2004;363:1491–1502 [DOI] [PubMed] [Google Scholar]
- 2.Markus HS, King A, Shipley M, et al. Asymptomatic embolisation for prediction of stroke in the Asymptomatic Carotid Emboli Study (ACES): a prospective observational study. Lancet Neurol 2010;9:663–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Markus H, Cullinane M. Severely impaired cerebrovascular reactivity predicts stroke and TIA risk in patients with carotid artery stenosis and occlusion. Brain 2001;124:457–467 [DOI] [PubMed] [Google Scholar]
- 4.Kleiser B, Widder B. Course of carotid artery occlusions with impaired cerebrovascular reactivity. Stroke 1992;23:171–174 [DOI] [PubMed] [Google Scholar]
- 5.Silvestrini M, Vernieri F, Pasqualetti P, et al. Impaired cerebral vasoreactivity and risk of stroke in patients with asymptomatic carotid artery stenosis. JAMA 2000;283:2122–2127 [DOI] [PubMed] [Google Scholar]
- 6.Vernieri F, Pasqualetti P, Passarelli F, Rossini PM, Silvestrini M. Outcome of carotid artery occlusion is predicted by cerebrovascular reactivity. Stroke 1999;30:593–598 [DOI] [PubMed] [Google Scholar]
- 7.Reinhard M, Gerds TA, Grabiak D, et al. Cerebral dysautoregulation and the risk of ischemic events in occlusive carotid artery disease. J Neurol 2008;255:1182–1189 [DOI] [PubMed] [Google Scholar]
- 8.King A, Serena J, Bornstein NM, Markus HS. Does impaired cerebrovascular reactivity predict stroke risk in asymptomatic carotid stenosis? A prospective substudy of the asymptomatic carotid emboli study. Stroke 2011;42:1550–1555 [DOI] [PubMed] [Google Scholar]
- 9.Klijn CJ, Kappelle LJ, van Huffelen AC, et al. Recurrent ischemia in symptomatic carotid occlusion: prognostic value of hemodynamic factors. Neurology 2000;55:1806–1812 [DOI] [PubMed] [Google Scholar]
- 10.Gupta A, Chazen JL, Hartman M, et al. Cerebrovascular reserve and stroke risk in patients with carotid stenosis or occlusion: a systematic review and meta-analysis. Stroke 2012;43:2884–2891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stewart LA, Clarke MJ. Practical methodology of meta-analyses (overviews) using updated individual patient data: Cochrane Working Group. Stat Med 1995;14:2057–2079 [DOI] [PubMed] [Google Scholar]
- 12.Hayden JA, Cote P, Bombardier C. Evaluation of the quality of prognosis studies in systematic reviews. Ann Intern Med 2006;144:427–437 [DOI] [PubMed] [Google Scholar]
- 13.Royston P, Sauerbrei W. Multivariable Model-Building, 1st ed. Hoboken: John Wiley & Sons; 2008 [Google Scholar]
- 14.Bates D, Maechler M, Bolker B. Linear mixed-effects models using S4 classes. Available at: www.CRAN.R-project.org/package=lme4. Accessed May 14, 2014
- 15.Widder B, Kleiser B, Krapf H. Course of cerebrovascular reactivity in patients with carotid artery occlusions. Stroke 1994;25:1963–1967 [DOI] [PubMed] [Google Scholar]
- 16.Rautenberg W, Hennerici M. Intracranial hemodynamic measurements in patients with severe asymptomatic extracranial carotid disease. Cerebrovasc Dis 1991;1:216–222 [Google Scholar]
- 17.Altman DG, Lausen B, Sauerbrei W, Schumacher M. Dangers of using “optimal” cutpoints in the evaluation of prognostic factors. J Natl Cancer Inst 1994;86:829–835 [DOI] [PubMed] [Google Scholar]
- 18.Topakian R, King A, Kwon SU, Schaafsma A, Shipley M, Markus HS. Ultrasonic plaque echolucency and emboli signals predict stroke in asymptomatic carotid stenosis. Neurology 2011;77:751–758 [DOI] [PubMed] [Google Scholar]
- 19.Garrett MC, Komotar RJ, Starke RM, et al. The efficacy of direct extracranial-intracranial bypass in the treatment of symptomatic hemodynamic failure secondary to athero-occlusive disease: a systematic review. Clin Neurol Neurosurg 2009;111:319–326 [DOI] [PubMed] [Google Scholar]
- 20.Gur AY, Bova I, Bornstein NM. Is impaired cerebral vasomotor reactivity a predictive factor of stroke in asymptomatic patients? Stroke 1996;27:2188–2190 [DOI] [PubMed] [Google Scholar]
- 21.Gur AY, Bornstein NM. Cerebral vasomotor reactivity of bilateral severe carotid stenosis: is stroke unavoidable? Eur J Neurol 2006;13:183–186 [DOI] [PubMed] [Google Scholar]
- 22.Dumville J, Panerai RB, Lennard NS, Naylor AR, Evans DH. Can cerebrovascular reactivity be assessed without measuring blood pressure in patients with carotid artery disease? Stroke 1998;29:968–974 [DOI] [PubMed] [Google Scholar]
- 23.Vernieri F, Pasqualetti P, Matteis M, et al. Effect of collateral blood flow and cerebral vasomotor reactivity on the outcome of carotid artery occlusion. Stroke 2001;32:1552–1558 [DOI] [PubMed] [Google Scholar]
- 24.Marshall RS, Rundek T, Sproule DM, Fitzsimmons BF, Schwartz S, Lazar RM. Monitoring of cerebral vasodilatory capacity with transcranial Doppler carbon dioxide inhalation in patients with severe carotid artery disease. Stroke 2003;34:945–949 [DOI] [PubMed] [Google Scholar]
- 25.Palazzo P, Tibuzzi F, Pasqualetti P, et al. Is there a role of near-infrared spectroscopy in predicting the outcome of patients with carotid artery occlusion? J Neurol Sci 2010;292:36–39 [DOI] [PubMed] [Google Scholar]
- 26.Persoon S, Luitse MJ, de Borst GJ, et al. Symptomatic internal carotid artery occlusion: a long-term follow-up study. J Neurol Neurosurg Psychiatry 2011;82:521–526 [DOI] [PubMed] [Google Scholar]
- 27.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.