Abstract
Hypoxia-inducible factor 1 (HIF-1) is a transcription factor that controls vascular responses to hypoxia and ischemia. In this study, mice that were heterozygous for a null allele at the locus encoding the HIF-1α subunit (HET mice) and their wild type (WT) littermates were subjected to thermal injury involving 10% of body surface area. HIF-1α protein levels were increased in burn wounds of WT but not of HET mice on day 2. Serum levels of stromal-derived factor 1α, which binds to CXCR4, were increased on day 2 in WT but not in HET mice. Circulating angiogenic cells were also increased on day 2 in WT but not in HET mice and included CXCR4+Sca1+ cells. Laser Doppler perfusion imaging demonstrated increased blood flow in burn wounds of WT but not HET mice on day 7. Immunohistochemistry on day 7 revealed a reduced number of CD31+ vessels at the healing margin of burn wounds in HET as compared to WT mice. Vessel maturation was also impaired in wounds of HET mice as determined by the number of α-smooth muscle actin-positive vessels on day 21. The remaining wound area on day 14 was significantly increased in HET mice compared to WT littermates. The percentage of healed wounds on day 14 was significantly decreased in HET mice. These data delineate a signaling pathway by which HIF-1 promotes angiogenesis during burn wound healing.
Introduction
Millions of individuals in the United States suffer burn injury each year (1, 2). With current therapy, both cosmetic and functional outcomes are often unsatisfactory (3). Novel strategies designed to heal burn wounds with tissue regeneration rather than fibrous scarring would have tremendous potential benefit for individuals suffering burn injury. Restoring the vascular bed of the dermis after a burn is an important potential component of tissue regeneration (4). However, our limited understanding of the underlying molecular mechanisms regulating burn wound healing is a major obstacle to the development of novel therapies.
Hypoxia-inducible factor 1 (HIF-1) promotes angiogenesis and vascular remodeling in response to hypoxia or ischemia (5). HIF-1 is a heterodimeric protein that is composed of a constitutively expressed HIF-1β subunit, and an O2-regulated HIF-1α subunit (6). Under normoxic conditions, the HIF-1α subunit is ubiquitinated and degraded, whereas under hypoxic conditions, HIF-1α accumulates, dimerizes with HIF-1β, and activates the transcription of downstream target genes encoding multiple angiogenic growth factors and cytokines of potential importance in wound healing, including vascular endothelial growth factor (VEGF), placental growth factor, angiopoietins 1 and 2, platelet-derived growth factor B, stromal-derived factor 1 (SDF-1), and stem cell factor (7–12). Transgenic expression of a constitutively active form of HIF-1α from a keratinocyte-specific promoter was associated with increased dermal vascularization (13). The dermal hypervascularity was not associated with hyperpermeability, which was observed when VEGF was expressed from the same promoter (14).
Angiogenic cytokines activate endogenous endothelial cells and also recruit circulating angiogenic cells (CACs), a term used here to denote a heterogeneous population of cells that have potential to participate in various aspects of angiogenesis. They include endothelial progenitor cells, which incorporate into the endothelium of new or remodeling vessels, as well as myeloid, mesenchymal, and hematopoietic stem cells, which promote vascular growth and remodeling through production of angiogenic cytokines (15–18). CACs are released from blood vessels, bone marrow, and other sites in response to the production of angiogenic cytokines at the site of tissue injury. Following mobilization into the circulation, CACs home to the wound, where they participate in angiogenesis. Recent studies of patients who have suffered thermal injury have analyzed subsets of CACs in peripheral blood using flow cytometry to identify cells that express VEGF receptor 2 (VEGFR2) and progenitor cell markers (CD133, CD144) and that lack expression of myeloid cell markers (CD15, CD45). VEGFR2+/CD15− cells appeared in the peripheral blood of individuals suffering burns within 12 h after injury (19). CD45−/CD133+/CD144+/VEGFR2+ cells were significantly increased in the peripheral blood at 24 h after burn wounding as compared to healthy controls (20).
Stimulating CACs to mobilize from distant sites and home to the wound may be a major mechanism by which HIF-1 promotes angiogenesis and vascular remodeling in response to tissue ischemia (9, 11, 21, 34). Expression of a constitutively active form of HIF-1α in mouse skin following electroporation-assisted gene transfer was sufficient to mobilize CACs and to improve healing of excisional wounds in diabetic mice (22). Topical application of chemical inducers of HIF-1 also promoted excisional wound repair in diabetic mice (23).
Unlike the prior work described above, our study utilized a burn wound model. An excisional wound has clean, non-necrotic borders, while the burn wound has central necrosis that must be cleared as healing extends in from the margins. The burn wound faces the double challenge of repair and regeneration from the periphery along with the clearance of necrotic tissue from more central areas. Furthermore, the margins of the burn wound are not clearly demarcated as thermal injury extends beyond the tissue that is immediately in contact with the heated element that produces the burn. We have recently developed a mouse model of graded thermal injury and demonstrated that more severe wounds are associated with delays in CAC mobilization, wound vascularization, and wound healing (24). We have utilized this model to study burn wound healing in mice that are heterozygous for a null (knockout) allele at the locus encoding HIF-1α.
Materials and Methods
Mouse strain
The Hif1atm1jhu mouse strain was generated by homologous recombination to replace exon 2 of the Hif1a gene with a neoR expression cassette in the J1 line of embryonic stem cells (derived from 129/Sv mice), followed by injection of the genetically modified cells into blastocysts that were isolated from C57BL/6J mice (25). The strain has been maintained by HET x WT matings for the last 12 years and the mice segregate the Hif1atm1jhu allele on an outbred 129 x B6 genetic background.
Mouse burn wound model
All procedures were approved by The Johns Hopkins University Animal Care and Use Committee. Burn wounds were performed on 8-week-old male WT and HET littermate mice. Mice were anesthetized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg), shaved on the dorsum, and depilated with Nair cream (Church & Dwight Co. Inc., Princeton, NJ). A custom-made 220-g aluminum rod was heated in a 100°C water bath for 5 min. Two burns of 1.2-cm diameter each were produced on the dorsum of each mouse, which together represented approximately 10% of total body surface area. To assure burn uniformity, only the weight of the aluminum rod itself provided pressure to the skin surface. A 4-sec contact time measured with a metronome was chosen to produce a standardized full-thickness burn. Fluid resuscitation was performed according to twice the Parkland formula (4 ml/kg x percent body surface area wounded) by intraperitoneal injection of saline within 1 h after burning (i.e. 1.6 ml of saline was administered to a 20-g mouse with a burn covering 10% of body surface area). Buprenorphine (1 mg/kg) was administered by subcutaneous injection for analgesia during the first 24 h. With this regimen of anesthesia and resuscitation survival was 95%.
Protein isolation and immunoblot assays
Tissue samples were harvested from burned and control non-burned skin on the dorsum of the mice on day 2 using an 8-mm-diameter punch biopsy device. Two punches were taken from non-burned skin at least 1.5 cm cephalad of the burn wounds. Tissue was harvested from 4 quadrants of the burn wounds by four contiguous punch biopsies, with each punch including both burn wound tissue and adjacent skin margin. Approximately half of each 8-mm-diameter specimen consisted of the burn wound, and the other half was adjacent skin, with the healing margin included between these portions. Samples were frozen in liquid nitrogen. The samples were homogenized in lysis buffer containing 20 mM HEPES, 1.5 mM MgCl2, 0.2 mM EDTA, 0.1 M NaCl, 0.2 mM DTT, and protease inhibitors, followed by addition of NaCl to a final concentration of 0.45 M. The supernatant was collected after centrifugation and glycerol was added to a final concentration of 20%. Aliquots (250 μg) were fractioned by SDS-polyacrylamide gel electrophoresis and subjected to immunoblot assay using a rabbit polyclonal anti-HIF-1α antibody (Novus Biologicals, Littleton, CO) at 1:500 dilution, horseradish peroxidase-conjugated anti-rabbit immunoglobulin (GE Healthcare Bio-Sciences Corp., Piscataway, NJ) secondary antibody, and signal development with ECL reagents (GE Healthcare Bio-Sciences).
SDF-1 ELISA
Mouse blood was collected and clotted for 2 h at room temperature before centrifuging for 20 min at 2000 × g. The serum was collected and stored at −80°C before the ELISA assay was performed according to the manufacturer’s protocol (R&D Systems, Inc., Minneapolis, MN).
Short-term culture assay of CACs
Peripheral blood was assayed for CACs as described previously (11, 26). Mononuclear cells were isolated from peripheral blood by Histopaque-1083 (Sigma-Aldrich) density gradient centrifugation. Residual red blood cells were lysed with ammonium chloride. Mononuclear cells were seeded on 96-well plates coated with rat vitronectin at 1.5 × 106 cells/cm2 and cultured in endothelial basal medium 2 supplemented with EGM-2-MV SingleQuots (Lonza, Basel, Switzerland). After 4 d in culture, the cells were incubated in media with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine (DiI)-labeled acetylated low-density lipoprotein (LDL) (Invitrogen, Carlsbad, CA) at a concentration of 2.4 μg/ml at 37°C for 2 h. The cells were then fixed with 4% paraformaldehyde for 10 min, and incubated with fluorescein isothiocyanate (FITC) labeled Bandeira simplificifolia agglutinin-1 (BS-1 lectin; Sigma-Aldrich) at a concentration of 10 μg/ml for 1 h, counterstained with 4′,6-diamidino-2-phenylindole (Invitrogen), and cells staining positive for uptake of DiI-acLDL and binding of FITC-BS-1 were counted under a fluorescence microscope.
Flow cytometry
The number of CXCR4+/Sca1+ cells in peripheral blood was determined by fluorescence activated cell sorting (FACS; LSR II Becton Dickinson, San Jose, CA) as previously described (11). To 100-μl aliquots of whole blood, 1 μl of Fc block (CD16/CD32; BD Pharmingen) was added followed by 2 μl each of phycoerythrin (PE)-conjugated monoclonal antibody against CXCR4 and FITC-labeled monoclonal antibody against Sca1 (BD Pharmingen) and incubation for 25 min at 4°C in the dark. 1 ml of ammonium chloride solution (Stem Cell Technologies, Vancouver, BC) was added and incubated for 10 min at 4°C in the dark to lyse red blood cells. Mononuclear cells were pelleted, resuspended in staining buffer (0.5% bovine serum albumin/2 mM EDTA in PBS). The live cell population in the forward scatter vs side scatter window was gated, and data were recorded from the dot plot of FITC vs PE. 150,000 events were acquired for analysis.
Laser Doppler perfusion imaging (LDPI)
Blood flow in wound areas was measured using a 633-nm, He-Ne scanning laser Doppler imaging device (Moor Instruments, Devon, UK), which utilizes a near-infrared laser diode to measure subcutaneous blood flow as a function of light scattering by moving red blood cells (Doppler shift). For each measurement point, a signal was generated that scales linearly with tissue perfusion defined as the product of the blood cell velocity and concentration. This signal, termed the laser Doppler perfusion index, was represented as a 2-dimensional color image on a computer screen. A photographic image was produced simultaneously, which allowed for direct anatomical comparison of corresponding areas of burn. For each burn, the wound site was selected after exporting the image into the software package LDISOFT (Lisa Development AB, Linkoping, Sweden). Then, the mean laser Doppler perfusion index value within the area of interest was computed. The scanner was positioned 50 cm above each animal and serial scans were performed on days 0, 3, 7, and 14 to assess blood flow in the wound.
Immunohistochemistry
Mouse wound tissues were fixed with IHC zinc fixative (formalin-free; BD Pharmingen, San Diego, CA) for 24 h and 5-μm-thick paraffin sections were prepared. To prevent nonspecific binding, 100 μl of blocking solution containing 2% normal rabbit serum for CD31 or horse serum for α-smooth muscle actin (Jackson ImmunoResearch, West Grove, PA) was applied for 30 min, and then 100 μl of primary anti-CD31 antibody (1:50 dilution; BD Pharmingen, Franklin Lakes, NJ) or anti-α-smooth muscle actin antibody (1:200 dilution; Sigma-Aldrich, St. Louis, MO) was applied to the sections for 1 h at room temperature. The sections were further incubated with biotinylated secondary antibody (1:500 dilution; Vector Laboratories, Burlingame, CA). Streptavidin-biotin-horseradish peroxidase was used for signal amplification and diaminobenzidine was used for staining (Vector Laboratories). Counter-staining was performed with hematoxylin for 30 sec. 3% H2O2 (Fisher Scientific, Fair Lawn, NJ) was used for blocking endogenous peroxidase activity. All slides were examined in a blinded manner.
Wound area calculation
On days 0, 7, 14 and 21, the wound borders were traced in situ onto clear acetate paper. Images were digitized at 600 dpi (Paperport 6000, Visioneer, Fremont, CA) and wound areas (in pixels) were calculated using NIH Image software analysis (Scion Image, Frederick, MD).
Statistical analysis
Results are presented as mean ± standard error of the mean (SEM). Differences in means among groups were analyzed for significance by Student’s t-test or ANOVA as appropriate using Sigma Stat (SYSTAT Software Inc., Point Richmond, CA).
Results
Analysis of HIF-1α expression on day 2 after burn wounding
We have established a quantitative murine burn wound model in which uniform, graded severity of thermal injury can reliably be produced and we have shown by histological analysis that 4-sec burn contact time results in a reproducible full thickness burn (24). HIF-1α protein was extracted from burned and unburned tissue at 2 d after burn injury, and was evaluated by immunoblot assay. In WT mice, HIF-1α protein levels were below the level of detection in unburned tissue, whereas HIF-1α was easily detected in burned tissue (Figure 1). In contrast, there was no detectable increase in HIF-1α protein levels in the burned tissue of HET mice. The complete loss of HIF-1α induction has also been observed in the ischemic limb of HET mice 3 days after femoral artery ligation (11) and in the brains of HET mice subjected to 10 days of intermittent hypoxia (31). The complete loss of response at different time points in these other models of hypoxic-ischemic stress indicate that the failure to detect increased levels of HIF-1α protein in burn wounds of HET mice is unlikely to be due to delayed induction and provides a molecular basis for the impaired vascularization of burn wounds in HET mice that is described below.
Figure 1.
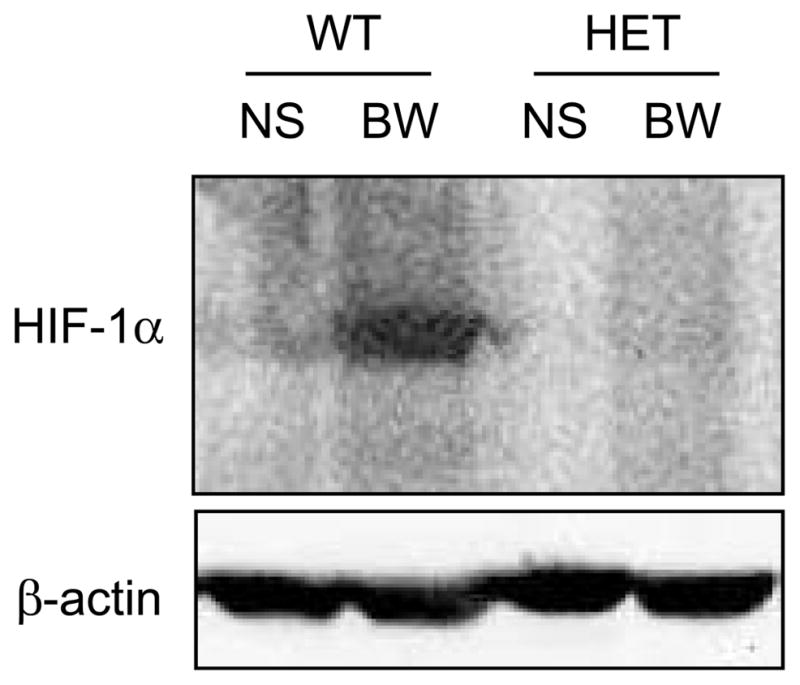
Analysis of HIF-1α protein levels in burn wounds. Tissue lysates were prepared from the non-burned skin (NS) and burn wound (BW) samples, which were harvested from wild type (WT) and HIF-1α heterozygous-null (HET) mice 2 days after burn wounding. Immunoblot assays were performed using antibodies against HIF-1α and β-actin, which was used as a loading control.
Analysis of SDF-1 expression on day 2 after burn wounding
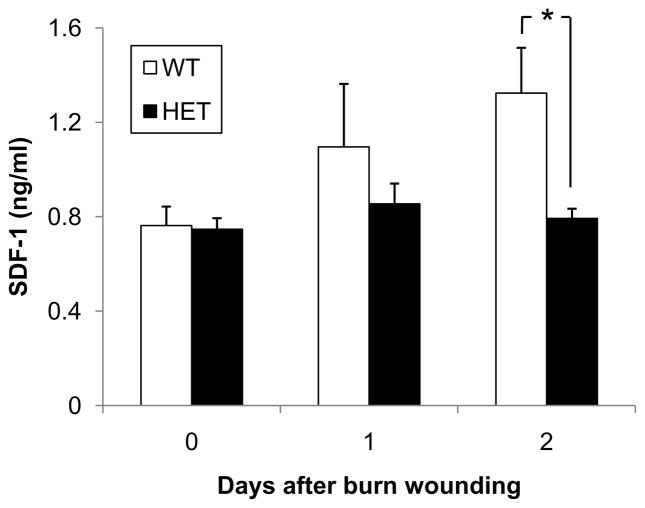
SDF-1 expression increases in a HIF-1-dependent manner in ischemic skin (9) and limb (11) tissue and in excisional wounds (22). Serum SDF-1 levels were significantly increased in WT mice on day 2 (Figure 2). In contrast, there was no increase in serum SDF-1 levels in HET mice after burn injury, resulting in significantly lower serum SDF-1 levels in HET mice than in WT littermates on day 2 (0.8 ± 0.1 vs 1.3 ± 0.2 ng/ml; P<0.05, ANOVA and Tukey post test). The temporal correlation between induction of HIF-1α and SDF-1 is consistent with the demonstration that HIF-1 binds directly to the promoter of the gene encoding SDF-1 and activates its transcription (9).
Figure 2.
Analysis of serum SDF-1 levels. Peripheral blood samples were collected from WT and HET mice 0, 1 and 2 days after burn wounding. An ELISA for SDF-1 was performed on serum samples (n = 4–9 for each condition). *P<0.05, ANOVA with Tukey Test.
Mobilization of circulating angiogenesis cells is impaired by HIF-1α deficiency
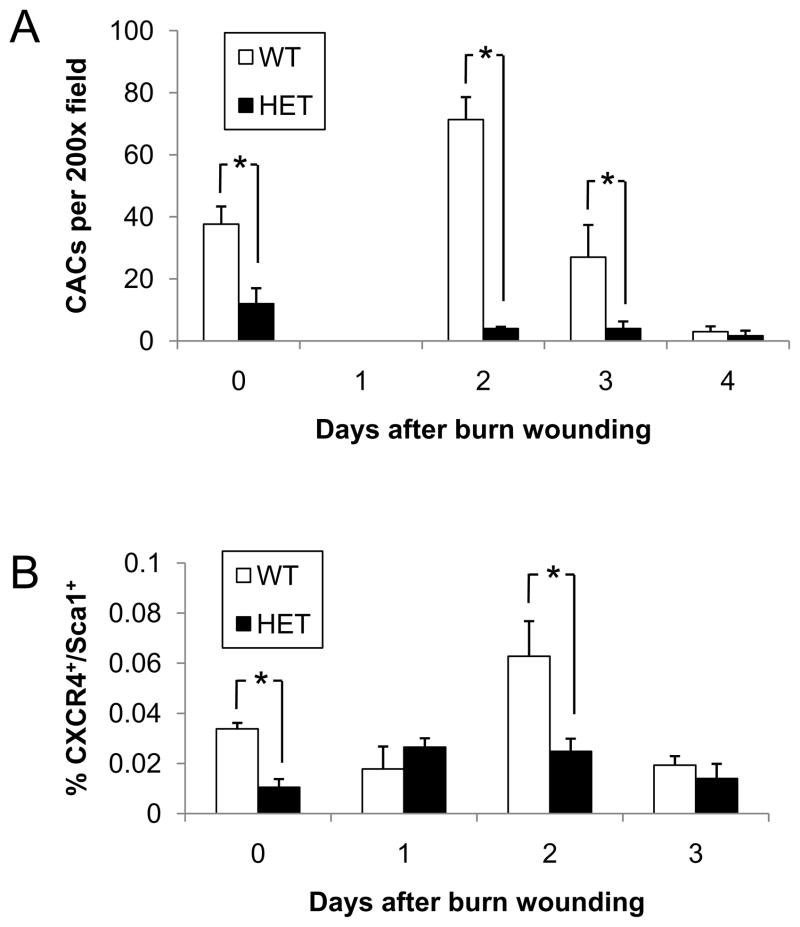
We hypothesized that an important consequence of increased serum SDF-1 levels would be increased mobilization of CACs into peripheral blood following burn injury. To test this hypothesis, peripheral blood mononuclear cells were isolated, cultured ex vivo under conditions favoring endothelial cell growth, and the number of cells which internalized DiI-labeled acetylated LDL (DiI-acLDL) and bound FITC-labeled BS-1 lectin was determined (Figure 3A). Although DiI-acLDL+/FITC-BS-1+ cells were originally proposed to be endothelial progenitor cells, more recent studies have indicated that the vast majority are pro-angiogenic myeloid cells (17, 27). Prior to burn injury, the CAC counts were significantly lower in HET mice than in WT littermates (12 ±5 vs 38 ± 6; P<0.05, ANOVA and Tukey post test). On day 1 after burn injury, the mean CAC count in both HET and WT mice dropped sharply to undetectable levels, which may reflect homing to the wound of cells that were in the circulation prior to burn wounding. In WT mice, CACs increased dramatically on day 2 and then gradually decreased to below basal levels by day 4, which may reflect homing of the newly mobilized cells to the wound. In contrast, no burn-induced change in the number of CACs was observed in HET mice on days 2–4. As a result, CACs were significantly lower in HET mice than in WT mice on days 2 and 3. The difference between genotypes was greatest on day 2, when the number of CACs was ~18-fold lower in HET mice than in WT littermates.
Figure 3.
Circulating angiogenic cells in WT and HET mice. (A) Peripheral blood samples were collected from WT and HET mice 0, 1, 2, 3, and 4 days after burn wounding. Blood samples from 3 mice per genotype were pooled for isolation of mononuclear cells, which were cultured in the presence of endothelial growth factors for 4 days, and the number of cells per 200x field which showed uptake of DiI-AcLDL and binding of FITC-BS-1 was determined. The mean number of CACs (± SEM, n = 3 pools for each condition) is shown; *P<0.05, ANOVA with Tukey test. (B) Peripheral blood samples were collected from WT and HET mice 0, 1, 2, and 3 days after burn wounding. The percentage of mononuclear cells that co-expressed CXCR4 and Sca1 was determined by flow cytometry (n = 4 for each condition); *P<0.05, ANOVA with Tukey test.
To analyze the mobilization of cells bearing specific cell surface receptors, flow cytometric analysis was performed on peripheral blood mononuclear cells from WT and HET mice before and after burn injury, focusing on cells that expressed the chemokine receptor CXCR4, which specifically binds SDF-1, and Sca1, a stem/progenitor cell antigen. The number of CXCR4+/Sca1+ cells (expressed as a percentage of the total mononuclear cell population) was significantly lower in HET mice as compared to WT mice at baseline (Figure 3B), which agrees with the data from the CAC culture assay described above. Also in agreement with these results, the number of CXCR4+/Sca1+ cells was increased in peripheral blood of WT mice but not in HET littermates, resulting in significantly lower numbers of circulating CXCR4+/Sca1+ cells in HET mice on day 2 (0.025 ± 0.005% [HET] vs. 0.063 ± 0.014% [WT]; P<0.05, ANOVA and Tukey post test). Taken together, these results show that HIF-1α deficiency is associated with impaired mobilization of CACs in response to burn wounding. It should be noted that, in agreement with our previous study of burn wound healing in 129S1/SvImJ mice (24), CAC mobilization in WT mice was transient, as an increased number of CACs (relative to unburned mice) was observed only on day 2 after burn wounding in both the culture (Figure 3A) and FACS (Figure 3B) assays. Taken together, the data in Figures 1–3 demonstrate that key angiogenic responses to burn wound healing occur on day 2 in WT mice but not in HET mice.
Effect of HIF-1α deficiency on wound vascularization and perfusion
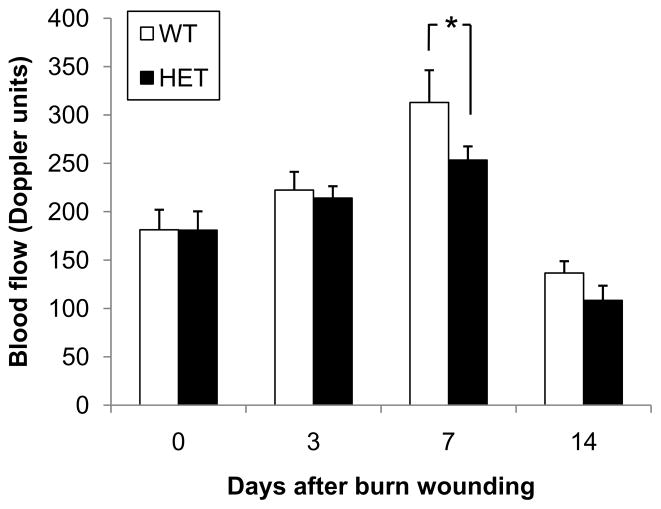
We hypothesized that the mobilization of CACs and their subsequent recruitment to the wound would stimulate angiogenesis, leading to increased perfusion of the burn wound tissue. To analyze the effect of HIF-1α deficiency on tissue perfusion after burn injury, dermal blood flow was measured by laser Doppler perfusion imaging (LDPI) on days 0, 3, 7, and 14 (Figure 4). Wound blood flow was significantly increased on day 7 after burn injury in WT mice as compared to HET mice (313 ± 33 vs 253 ± 14 LDPI units, P<0.05, ANOVA and Tukey post test).
Figure 4.
Burn wound blood flow in WT and HET mice. Laser Doppler perfusion imaging of burn wounds was performed on days 0, 3, 7 and 14 (mean ± SEM, n = 16 for each condition). *P<0.05, ANOVA with Tukey test.
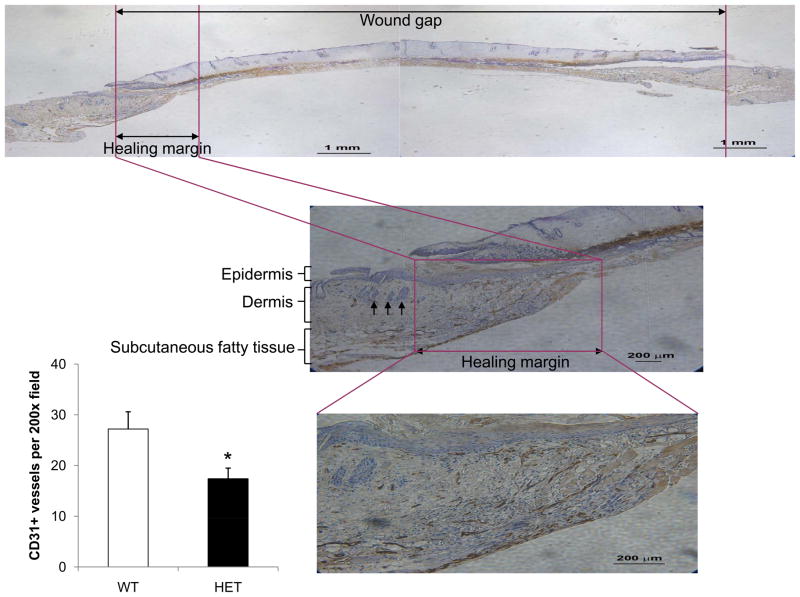
To examine the effect of HIF-1α deficiency on tissue vascularization, angiogenesis was assessed with quantitative immunohistochemistry. Burn wounds were excised on days 7 and 21 and immunohistochemical staining for PECAM-1 (CD31) and α-smooth muscle actin (SMA) was performed. On day 7, the number of CD31+ vessels within a 200x field at the healing margin of granulation tissue of each wound was counted (Figure 5). CD31+ vessel counts were significantly lower in HET mice as compared to WT mice (17 ± 2 vs 27 ± 3; P<0.05, ANOVA and Tukey post test). At day 7, SMA staining at the healing margin was negative (data not shown), as expected because immature vessels lack pericytes.
Figure 5.
Burn wound vascularization in WT and HET mice on day 7. Immunohistochemical staining for PECAM-1 (CD31) was performed at the healing margin of granulation tissue in each burn wound. In each panel, the healing margin with necrotic burn wound adjacent is outlined in purple. Arrows in the middle panel indicate hair follicles, which identify the normal dermis adjacent to the wound. The bar graph at lower left shows the mean number of CD31+ blood vessels per 200x field (bottom panel) in the healing margin (± SEM, n = 5 for each genotype). *P<0.05, ANOVA with Tukey test.
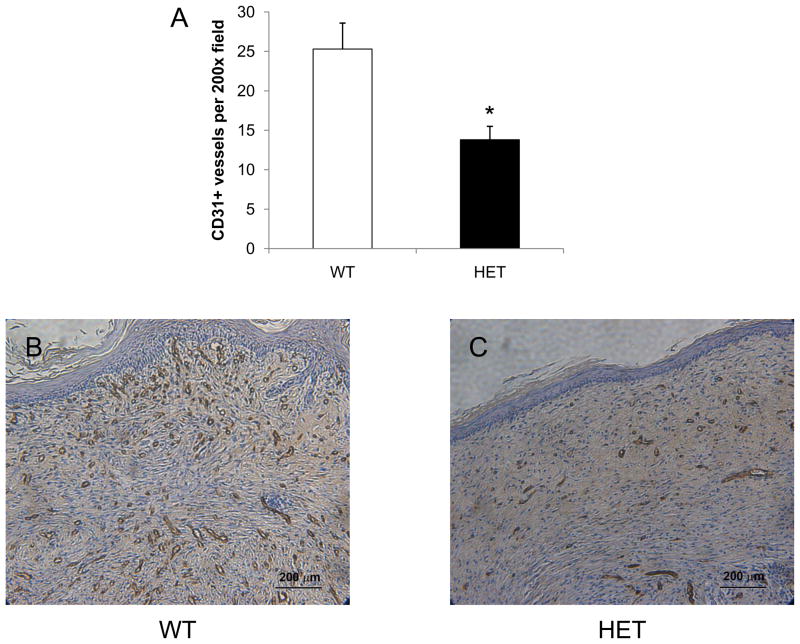
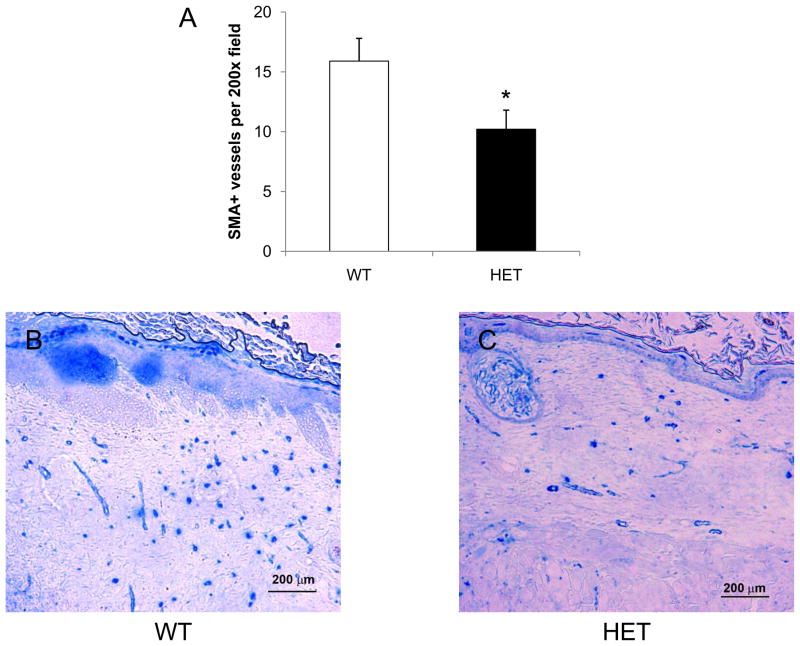
Vessel counts were also performed at the center of each wound on day 21 using antibodies against CD31 (Figure 6) and SMA (Figure 7). CD31+ vessel counts were significantly lower in HET mice as compared to WT mice (14 ± 2 vs 25 ± 3; P<0.05, ANOVA and Tukey post test). In HET mice, the number of SMA+ vessels within a 200x field at the center of each wound was also significantly decreased when compared to the wounds of WT mice (10 ± 2 vs 16 ± 2; P<0.05, ANOVA and Tukey post test). These results demonstrate that partial HIF-1α deficiency impairs vessel formation and maturation during burn wound healing, with differences in vascularization that persist on day 21 even after complete wound closure.
Figure 6.
Analysis of CD31+ vessels in burn wounds on day 21. Immunohistochemical staining for PECAM-1 (CD31) was performed at the healed center of each wound from WT (B) and HET (C) mice and the mean number of stained vessels (± SEM, n = 12 for each genotype) is shown (A). *P<0.05 vs WT, ANOVA with Tukey test.
Figure 7.
Analysis of SMA+ vessels in burn wounds on day 21. Immunohistochemical staining for SMA was performed at the healed center of each wound from WT (B) and HET (C) mice and the mean number of stained vessels (± SEM, n = 12 for each genotype) is shown (A). *P<0.05 vs WT, ANOVA with Tukey test.
Analysis of burn wound closure
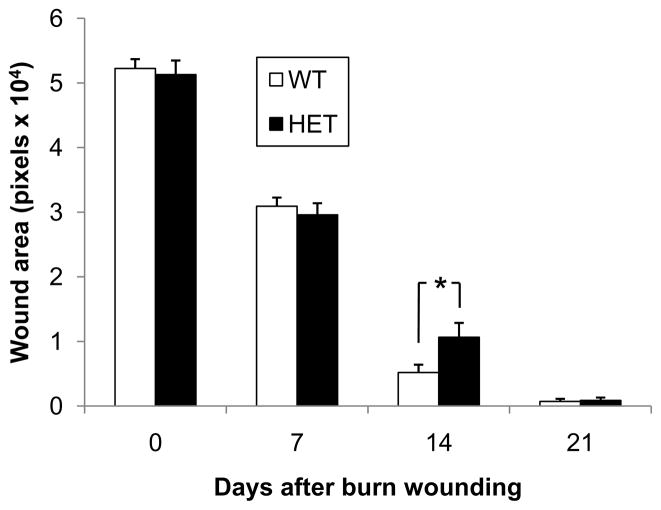
To investigate whether HIF-1α deficiency affects burn wound healing, we compared the kinetics of burn wound closure in HET mice and their WT littermates. The non-epithelialized wound areas were measured on days 0, 7, 14 and 21 by digital planimetry (Figure 8). The remaining wound area in the HET mice (10,700 ± 1,600 pixels) was 35% greater than that of their WT littermates on day 14 (Figure 8A), a difference that was statistically significant (P<0.05, ANOVA and Tukey post test). In addition, the percentage of wounds with >95% closure on day 14 (Figure 8B) was significantly decreased in HET mice (P<0.01, χ2 test).
Figure 8.
Kinetics of burn wound healing in WT and HET mice. The bar graph shows the wound area measured by computer-assisted planimetry on days 0, 7, 14 and 21 (mean ± SEM, n = 16 for each condition); *P<0.05 vs WT, ANOVA with Tukey test. Inset shows the percentage of wounds with >95% healing on day 14; *P<0.01 vs WT, χ2 test.
Discussion
This study demonstrates that compared to WT littermates, HET mice with reduced expression of HIF-1α have impaired burn wound vascularization. The apparent difference in the rate of wound healing between genotypes was less dramatic, which may be due to the fact that unlike humans, mice heal primarily by wound contracture and thus the effect of impaired vascularization on wound healing may be understated due to this major limitation of the mouse model. The importance of HIF-1α in wound healing has been suggested by other studies in which animals with conditions that impair excisional wound healing, such as aging and/or diabetes, were found to have impaired induction of HIF-1α as a secondary effect (21–23, 28, 29). Burn wounding has been shown to inhibit both the induction of HIF-1α and the healing of an excisional wound made concurrently at a distal site on the same animal (30). Conditional knockout mice with complete HIF-1α deficiency in endothelial cells manifest impaired healing of excisional skin wounds (10). However, the effect of primary HIF-1α deficiency on burn wound healing as studied here has not been addressed previously.
A significant impairment of burn wound angiogenesis was demonstrated in HET mice as documented physiologically by reduced blood flow during the early hyper-vascular phase of wound healing and histologically by reduced vascularity in the early phase that persisted even in the late phase of wound healing. The analysis of CACs suggests that impaired angiogenesis in HET mice is at least partially due to reduced mobilization of pro-angiogenic cells in response to burn wounding. The finding that induction of HIF-1α protein in burn wounds, induction of serum SDF-1 levels, and mobilization of CXCR4+/Sca1+ cells were all significantly impaired in HET mice on day 2 after burn wounding provides a molecular mechanism underlying the impairment of burn wound tissue perfusion and vascularization that was observed on day 7 in HET mice (Figure 9). This model is consistent with the role of HIF-1 → SDF-1 signaling in the vascularization of ischemic wound flap and excisional wounds that has been reported previously (9, 29).
Figure 9.
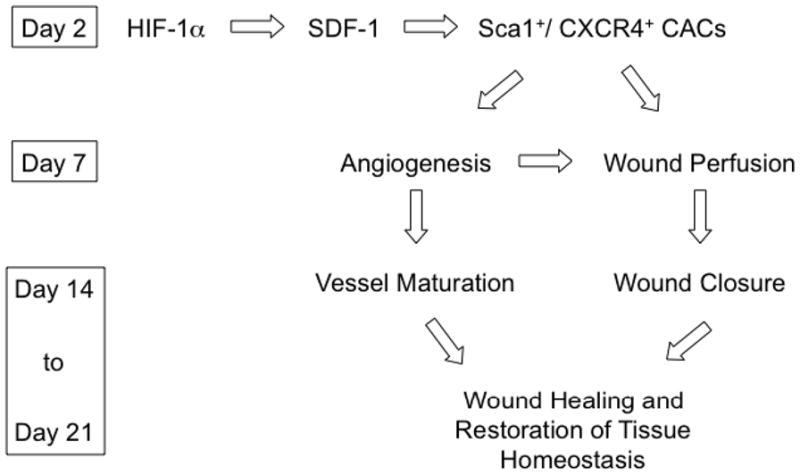
Role of HIF-1 in burn wound vascularization and healing.
It is remarkable that HIF-1α heterozygous knockout mice displayed significant impairment in wound healing and angiogenesis. A priori, the HET mice would be expected to produce ~50% less HIF-1α protein than WT littermates due to the inactivation of one out of two functional gene copies. However, in contrast to the marked increase in HIF-1α protein levels in burn wounds of WT mice, no significant induction of HIF-1α was observed in burn wounds of HET mice. This dramatic loss of HIF-1α induction also has been observed in limb muscle of HET mice subjected to femoral artery ligation (11) and the brains of HET mice subjected to chronic intermittent hypoxia (31). Data from those models suggest the existence of feed-forward mechanisms that amplify the HIF-1 response in WT mice and result in a greater than linear reduction in HIF-1α levels in HET mice.
The results of the present study have several important clinical implications. First, impaired HIF-1 activity in response to burn wounding may occur in patients as a result of genetic polymorphisms at the locus encoding HIF-1α, which have been shown to predict absence of coronary collaterals (32) and presentation with stable angina as opposed to myocardial infarction (33) in patients with coronary artery disease. Impaired induction of HIF-1 may also occur in patients with aging (11, 11, 24), diabetes (23; 28), or both (22). Second, genetic or pharmacologic strategies to increase HIF-1α levels have been shown to promote vascularization of ischemic limb muscle (11) and excisional wounds (21–23, 28) and may be useful for the treatment of burn wounds, especially in patients with known risk factors such as aging and diabetes. Major burn wounds are excised and grafted in humans. Further studies modeling these procedures in HET and WT mice may provide additional clinically relevant data regarding the role of HIF-1 in burn wound healing.
Acknowledgments
Funding for this project was provided by Public Health Service grant P20-GM78494 from the National Institutes of Health, the Hendrix Burn Fund, Johns Hopkins Bayview, Beijing Nova Star Plan Grant No. 2004B29, China Scholarship Council, and the Johns Hopkins Institute for Cell Engineering.
Abbreviations
- HIF-1
hypoxia-inducible factor 1
- HET
heterozygote
- WT
wild type
- VEGF
vascular endothelial growth factor
- SDF-1
stromal-derived factor 1
- CAC
circulating angiogenic cell
- VEGFR2
VEGF receptor 2
- FITC
fluorescein isothiocyanate
- BS-1
Bandeira simplificifolia agglutinin-1
- DiI
1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine
- acLDL
acetylated low-density lipoprotein
- PE
phycoerythrin
- SMA
α-smooth muscle actin
Footnotes
Conflict of Interest
The authors state no conflict of interest.
References
- 1.Brigham PA, McLoughlin E. Burn incidence and medical care use in the United States: estimates, trends, and data sources. J Burn Care Rehabil. 1996;17:95–107. doi: 10.1097/00004630-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Kerby JD, McGwin G, Jr, George RL, Cross JA, Chaudry IH, Rue LW., 3rd Sex differences in mortality after burn injury: results of analysis of the National Burn Repository of the American Burn Association. J Burn Care Res. 2006;27:452–6. doi: 10.1097/01.BCR.0000225957.01854.EE. [DOI] [PubMed] [Google Scholar]
- 3.Tredget EE. The basis of fibrosis and wound healing disorders following thermal injury. J Trauma. 2007;62:S69. doi: 10.1097/TA.0b013e318065ae84. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J. Is angiogenesis an organizing principle in biology and medicine? J Pediatr Surg. 2007;42:1–11. doi: 10.1016/j.jpedsurg.2006.09.048. [DOI] [PubMed] [Google Scholar]
- 5.Semenza GL. Vasculogenesis, angiogenesis, and arteriogenesis: mechanisms of blood vessel formation and remodeling. J Cell Biochem. 2007;102:840–7. doi: 10.1002/jcb.21523. [DOI] [PubMed] [Google Scholar]
- 6.Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci U S A. 1995;92:5510–4. doi: 10.1073/pnas.92.12.5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–13. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelly BD, Hackett SF, Hirota K, Oshima Y, Cai Z, Berg-Dixon S, Rowan A, Yan Z, Campochiaro PA, Semenza GL. Cell type-specific regulation of angiogenic growth factor gene expression and induction of angiogenesis in nonischemic tissue by a constitutively active form of hypoxia-inducible factor 1. Circ Res. 2003;93:1074–81. doi: 10.1161/01.RES.0000102937.50486.1B. [DOI] [PubMed] [Google Scholar]
- 9.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–64. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 10.Tang N, Wang L, Esko J, Giordano FJ, Huang Y, Gerber HP, Ferrara N, Johnson RS. Loss of HIF-1α in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell. 2004;6:485–95. doi: 10.1016/j.ccr.2004.09.026. [DOI] [PubMed] [Google Scholar]
- 11.Bosch-Marce M, Okuyama H, Wesley JB, Sarkar K, Kimura H, Liu YV, Zhang H, Strazza M, Rey S, Savino L, Zhou YF, McDonald KR, Na Y, Vandiver S, Rabi A, Shaked Y, Kerbel R, Lavallee T, Semenza GL. Effects of aging and hypoxia-inducible factor 1 activity on angiogenic cell mobilization and recovery of perfusion following limb ischemia. Circ Res. 2007;101:1310–8. doi: 10.1161/CIRCRESAHA.107.153346. [DOI] [PubMed] [Google Scholar]
- 12.Simon MP, Tournaire R, Pouyssegur J. The angiopoietin-2 gene of endothelial cells is up-regulated in hypoxia by a HIF binding site located in its first intron and by the central factors GATA-2 and Ets-1. J Cell Physiol. 2008;217:809–18. doi: 10.1002/jcp.21558. [DOI] [PubMed] [Google Scholar]
- 13.Elson DA, Thurston G, Huang LE, Ginzinger DG, McDonald DM, Johnson RS, Arbeit JM. Induction of hypervascularity without leakage or inflammation in transgenic mice overexpressing hypoxia-inducible factor-1alpha. Genes Dev. 2001;15:2520–32. doi: 10.1101/gad.914801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Leakage-resistant blood vessels in mice transgenically overexpressing angiopoietin-1. Science. 1999;286:2511–4. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- 15.Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:5302, 964–7. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 16.Grant MB, May WS, Caballero S, Brown GA, Guthrie SM, Mames RN, Byrne BJ, Vaught T, Spoerri PE, Peck AB, Scott EW. Adult hematopoietic stem cells provide functional hemangioblast activity during retinal neovascularization. Nat Med. 2002;8:607–12. doi: 10.1038/nm0602-607. [DOI] [PubMed] [Google Scholar]
- 17.Rehman J, Li J, Orschell CM, March KL. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation. 2003;107:1164–9. doi: 10.1161/01.cir.0000058702.69484.a0. [DOI] [PubMed] [Google Scholar]
- 18.Kinnaird T, Stabile E, Burnett MS, Lee CW, Barr S, Fuchs S, Epstein SE. Marrow-derived stromal cells express genes encoding a broad spectrum of arteriogenic cytokines and promote in vitro and in vivo arteriogenesis through paracrine mechanisms. Circ Res. 2004;94:678–85. doi: 10.1161/01.RES.0000118601.37875.AC. [DOI] [PubMed] [Google Scholar]
- 19.Gill M, Dias S, Hattori K, Rivera ML, Hicklin D, Witte L, Girardi L, Yurt R, Himel Rafii S. Vascular trauma induces rapid but transient mobilization of VEGFR2(+)AC133(+) endothelial precursor cells. Circ Res. 2001;88(2):167–174. doi: 10.1161/01.res.88.2.167. [DOI] [PubMed] [Google Scholar]
- 20.Fox A, Smythe J, Fisher N, Tyler MP, McGrouther DA, Watt SM, Harris AL. Mobilization of endothelial progenitor cells into the circulation in burned patients. Br J Surg. 2008;95:244–51. doi: 10.1002/bjs.5913. [DOI] [PubMed] [Google Scholar]
- 21.Chang EI, Loh SA, Ceradini DJ, Chang EI, Lin SE, Bastidas N, Aarabi S, Chan DA, Freedman ML, Giaccia AJ, Gurtner GC. Age decreases endothelial progenitor cell recruitment through decreases in hypoxia-inducible factor 1α stabilization during ischemia. Circulation. 2007;116:2818–29. doi: 10.1161/CIRCULATIONAHA.107.715847. [DOI] [PubMed] [Google Scholar]
- 22.Liu L, Marti GP, Wei X, Zhang X, Zhang H, Liu YV, Nastai M, Semenza GL, Harmon JW. Age-dependent impairment of HIF-1α expression in diabetic mice: Correction with electroporation-facilitated gene therapy increases wound healing, angiogenesis, and circulating angiogenic cells. J Cell Physiol. 2008;217:319–27. doi: 10.1002/jcp.21503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mace KA, Yu DH, Paydar KZ, Boudreau N, Young DM. Sustained expression of HIF-1α in the diabetic environment promotes angiogenesis and cutaneous wound repair. Wound Repair Regen. 2007;15:636–45. doi: 10.1111/j.1524-475X.2007.00278.x. [DOI] [PubMed] [Google Scholar]
- 24.Zhang X, Wei X, Liu L, Marti GP, Ghanamah MS, Arshad MJ, Strom L, Spence R, James Jeng, Milner S, Harmon JW, Semenza GL. Increasing burn severity in mice is associated with delayed mobilization of circulating angiogenic cells. Arch Surg. doi: 10.1001/archsurg.2009.285. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iyer NV, Kotch LE, Agani F, Leung SW, Laughner E, Wenger RH, Gassmann M, Gearhart JD, Lawler AM, Yu AY, Semenza GL. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes Dev. 1998;12:149–62. doi: 10.1101/gad.12.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 27.Yoder MC, Mead LE, Prater D, Krier TR, Mroueh KN, Li F, Krasich R, Temm CJ, Prchal JT, Ingram DA. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–9. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Botusan IR, Sunkari VG, Savu O, Catrina AI, Grünler J, Lindberg S, Pereira T, Ylä-Herttuala S, Poellinger L, Brismar K, Catrina SB. Stabilization of HIF-1α is critical to improve wound healing in diabetic mice. Proc Natl Acad Sci U S A. 2008;105:19426–31. doi: 10.1073/pnas.0805230105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Loh SA, Chang EI, Galvez MG, Thangarajah H, El-ftesi S, Vial IN, Lin DA, Gurtner GC. SDF-1α expression during wound healing in the aged is HIF dependent. Plast Reconstr Surg. 2009;123:65S–75S. doi: 10.1097/PRS.0b013e318191bdf4. [DOI] [PubMed] [Google Scholar]
- 30.Schwacha MG, Nickel E, Daniel T. Burn injury-induced alterations in wound inflammation and healing are associated with suppressed hypoxia inducible factor-1α expression. Mol Med. 2008;14:628–33. doi: 10.2119/2008-00069.Schwacha. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, Semenza GL, Prabhakar NR. Heterozygous HIF-1α deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol. 2006;577:705–16. doi: 10.1113/jphysiol.2006.114033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Resar JR, Roguin A, Voner J, Nasir K, Hennebry TA, Miller JM, Ingersoll R, Kasch LM, Semenza GL. Hypoxia-inducible factor 1α polymorphism and coronary collaterals in patients with ischemic heart disease. Chest. 2005;128:787–91. doi: 10.1378/chest.128.2.787. [DOI] [PubMed] [Google Scholar]
- 33.Hlatky MA, Quertermous T, Boothroyd DB, Priest JR, Glassford AJ, Myers RM, Fortmann SP, Iribarren C, Tabor HK, Assimes TL, Tibshirani RJ, Go AS. Polymorphisms in hypoxia inducible factor 1 and the initial clinical presentation of coronary disease. Am Heart J. 2007;154:1035–42. doi: 10.1016/j.ahj.2007.07.042. [DOI] [PubMed] [Google Scholar]
- 34.Sarkar K, Fox-Talbot K, Steenbergen C, Bosch-Marce M, Semenza GL. Adenoviral transfer to HIF-1a enhances vascular responses to critical limb ischemia in diabetic mice. Proc Natl Acad Sci USA. 2009;106:18769–74. doi: 10.1073/pnas.0910561106. [DOI] [PMC free article] [PubMed] [Google Scholar]