Abstract
The dorsal striatum plays a critical role in procedural learning and memory. Current models of basal ganglia assume that striatal neurons and circuitry are critical for the execution of over-learned, habitual sequences of action. However, less is known about how the striatum encodes task information that guides the performance of actions in procedural tasks. To explore the striatal encoding of task information, we compared the behavioral correlates of striatal neurons tested in two tasks: a Multiple-T-maze task in which reward delivery was entirely predictable based on spatial cues (the Multiple-T task), and a task in which rats ran on a rectangular track, but food delivery depended on the distance traveled on the track and was not dependent solely on spatial location (the Take-5 task). Striatal cells recorded on these tasks were divisible into three cell types: phasic-firing neurons (PFNs), tonically firing neurons (TFNs), and high-firing neurons (HFNs) and similar proportions of each cell type were found in each task. However, the behavioral correlates of each cell type were differentially sensitive to the type of task rats were performing. PFNs were responsive to specific task-parameters on each task. TFNs showed reliable burst-and-pause responses following food delivery and other events that were consistent with tonically active-neurons (TANs) on the Take-5 (non-spatial) task but not on the Multiple-T (spatial) task. HFNs showed spatial oscillations on the Multiple-T (spatial) task but not the Take-5 (non-spatial) task. Reconstruction of the rats’ position on the maze was highly accurate when using striatal ensembles recorded on the Multiple-T (spatial) task, but not when using ensembles recorded on the Take-5 (non-spatial) task. In contrast, reconstruction of time following food delivery was successful in both tasks. The results indicated a strong task dependency of the quality of the spatial, but not the reward-related, striatal representations on these tasks. These results suggest that striatal spatial representations depend on the degree to which spatial task-parameters can be unambiguously associated with goals.
Keywords: basal ganglia, TANs, striatum, procedural learning, tonically active neurons
Introduction
The basal ganglia are believed to play an important role in behavioral control, including the learning of habitual and goal-directed behaviors (Graybiel 1995; Bailey and Mair 2006; Yin and Knowlton 2006). An important question is then: how does neural activity in the basal ganglia support the learning and performance of any given behavior? By examining the responses of single neurons in awake, behaving animals, numerous studies have shown that neurons in the basal ganglia respond to a wide variety of task parameters. However, it is still unclear how neural activity in the striatum is organized into useful representations, and how this activity depends on the types of tasks that are being performed.
The basal ganglia, and in particular the dorsal striatum, have been implicated in the acquisition and performance of overlearned, automatic behaviors (Miyachi, Hikosaka et al. 1997; Jog, Kubota et al. 1999; Matsumoto, Hanakawa et al. 1999; Graybiel 2000; Bailey and Mair 2006; Yin and Knowlton 2006). These observations have led to the hypothesis that at least some regions of the dorsal striatum are important for the learning and performance of stimulus-response behaviors in which the actions are selected for performance on the basis of available sensory cues (Houk, Davis et al. 1995; Doya 1999; Swanson 2000; Samejima, Ueda et al. 2005; Hikosaka, Nakamura et al. 2006). Consistent with such hypotheses, striatal neurons have been observed to be well-tuned to many task-relevant parameters, such as visual cues (Boussaoud and Kermadi 1997), auditory cues (Gardiner and Kitai 1992; White and Rebec 1993; Jog, Kubota et al. 1999), orientation (Wiener 1993; Ragozzino, Leutgeb et al. 2001) or location (Wiener 1993; Schmitzer-Torbert and Redish 2004; Yeshenko, Guazzelli et al. 2004; Gill and Mizumori 2006) of the animal, as well as specific muscular movements (Alexander and DeLong 1985; Schultz and Romo 1988; West, Carelli et al. 1990; Gardiner and Kitai 1992).
Rather than simply encoding sensory information or muscle contractions, striatal neural responses are highly context-dependent. Striatal neurons have been reported to demonstrate context and sequence specific firing in rodents during highly stereotyped grooming behaviors (Aldridge and Berridge 1998), lever pressing (Carelli, Wolske et al. 1997), for auditory cues and movements (Gardiner and Kitai 1992) in visuomotor sequences in primates (Kermadi, Jurquet et al. 1993; Kermadi and Joseph 1995; Tolkunov, Orlov et al. 1998) and in navigation-related activity in rats (Schmitzer-Torbert and Redish 2004). In tasks in which the same stimulus can be associated with different movements depending on context, striatal neurons differentiate their responses based on the particular stimuli presented, the particular movements required, and the reward available (Boussaoud and Kermadi 1997; Hollerman, Tremblay et al. 1998; Watanabe, Lauwereyns et al. 2003; Kawagoe, Takikawa et al. 2004; Schmitzer-Torbert and Redish 2004; Samejima, Ueda et al. 2005; Hikosaka, Nakamura et al. 2006; Kobayashi, Kawagoe et al. 2006).
Studies from cued-reaction tasks in which animals (usually primates) have to respond to cues which predict reward with precise timing have found striatal cells to have precisely timed responses to cue-delivery, motor actions, and other task components (Schultz and Romo 1988; Aosaki, Kimura et al. 1995; Schultz, Apicella et al. 1995; Shimo and Hikosaka 2001; Itoh, Nakahara et al. 2003; Watanabe, Lauwereyns et al. 2003; Hikosaka, Nakamura et al. 2006). In contrast, however, studies from freely-behaving rats have found striatal cells with spatial tuning dependent on the actions taken at specific spatial locations of the animal (Wiener 1993; Ragozzino, Leutgeb et al. 2001; Ragozzino, Ragozzino et al. 2002; Schmitzer-Torbert and Redish 2004; Yeshenko, Guazzelli et al. 2004; Barnes, Kubota et al. 2005).
Given that the responses of striatal neurons, and particularly the projection neurons of the striatum, are highly context-dependent, the function of these responses is a critical question for understanding striatal information processing. Are these context-dependent responses of striatal neurons obligatory (i.e. will they be observed in any situation where there are reliable stimulus-action combinations) or are they informative (i.e. do they indicate something about the information that striatal neurons use during complex behavior)? For instance, we have previously shown that many putative striatal projection neurons respond as rats run through a multiple T-maze, while others respond following the receipt of reward (Schmitzer-Torbert and Redish 2004). Responses during navigation through the maze depended on combinations of spatial location, the actions performed at those locations, and the global sequence of actions rats performed as they ran through the maze. But, will these maze-responsive neurons encode spatial and sequence information in other tasks, where space may not be as informative about what an animal should do? To answer this question, we examined the responses of individual striatal neurons and striatal ensemble activity during navigation tasks. In these experiments, we compared the responses of striatal neurons collected on the Multiple-T task to data collected on a second task (the Take-5) where spatial information was available, but provided ambiguous information about where rewards would be obtained.
Experimental Procedures
Behavioral Tasks
Rats were trained to perform one or two behavioral tasks (the Multiple-T and Take-5 tasks, described below). Some rats were trained on both tasks, but each rat was tested in any given recording session while performing only one task (either the Multiple-T or the Take-5).
Multiple-T
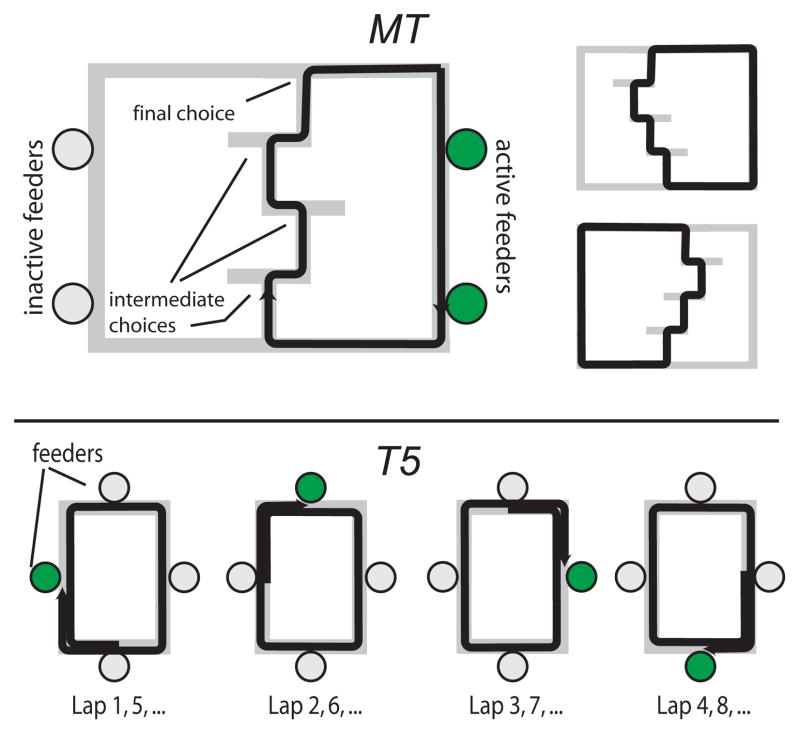
Details of the Multiple-T task are given in Schmitzer-Torbert and Redish (2004). Neural data was collected as rats ran through a complex maze composed of 3–4 sequentially ordered T maze choices (see schematic in Figure 1). After correctly navigating the sequence of turns, rats received food rewards (two 45 mg pellets, Research Diets, New Brunswick, NJ) delivered at two food delivery sites using automatic pellet dispensers (Med-Associates, St. Albans VT). After receiving their food rewards, rats returned to the turn sequence by a return path, thus running the task as a self-paced, continuous loop. The majority of the data was obtained from five rats running on mazes composed of 4 Ts. Several additional sessions using 3 T mazes were obtained from three rats trained on the Multiple-T after completing their recordings on the Take-5 task (described below).
Figure 1.
Schematic of the Multiple-T (top) and Take-5 (bottom) tasks.
Take-5
Rats were trained to run on an elevated, rectangular track for food. The track measured 61 cm by 92 cm, and was created out of plywood boards measuring 15 cm wide, covered with carpet. One automatic pellet dispenser was located at the center of each side of the track. Rats were trained to run clockwise around the track, and were rewarded with 4 pellets after making one complete journey around the track and then advancing to the next pellet dispenser (thus traveling 5/4 the total length of the track, see schematic in Figure 1). The completion of a trial was signaled by a 100 ms tone, the onset of which preceded food delivery by approximately 250 ms.
Surgery
After pre-training on either the Multiple-T, Take-5, or Nosepoke task, rats were chronically implanted with 14-tetrode hyperdrives (12 tetrodes used for recording, 2 electrodes used as references, Kopf, Tujunga, CA) targeting the striatum (+ 0.5mm AP, ± 3.0mm ML, relative to bregma). Details of the surgery have been described previously (Schmitzer-Torbert and Redish 2004). Rats were anesthetized with Nembutal (sodium pentobarbital, 40–50 mg/kg, Abbott Laboratories, North Chicago, IL) and maintained on isoflurane during the implantation (0.5–2% isoflurane vaporized in medical grade O2). Rats were then placed on a stereotaxic apparatus (Kopf) and received 0.1 cc Dualcillin (Phoenix Pharmaceutical Inc., St. Joseph, MI) intramuscularly in each hindlimb. The area over the implantation was shaved, disinfected with alcohol (70% isopropyl) and Betadine (Purdue Frederick, Norwalk, CT), and the skin overlying the scalp was removed. Up to eight jewelers screws were used to anchor the hyperdrive to the skull, with one screw serving as the recording ground. A craniotomy was opened using a surgical trephine, and the craniotomy around the hyperdrive was protected with silastic. Dental acrylic (Perm Reline and Repair Resin, The Hygenic Corp., Akron, OH) secured the hyperdrive to the skull. Following surgery, rats received 5–10 cc sterile saline subcutaneously, and 0.8 cc Tylenol orally after becoming ambulatory. All tetrodes were advanced 1 mm immediately following surgery. To prevent post-surgical infections, treatment regimens of topically applied Neosporin and subcutaneous Baytril (enrofloxacin, 1.1 mg/kg, Bayer, Shawnee Mission KS) were used in some rats.
Data collection
Over a period of a week following surgery, tetrodes were advanced 340–680 μm per day until reaching the striatum. The striatum was differentiated from the cortex by the observation of corpus callosum, which with our recording techniques is electrophysiologically quiet relative to the overlying cortex and underlying striatum. The striatum was further identified by the observation of extremely slow firing cells (< 1 action potential per minute) which we have not observed in cortex.
Neural activity was recorded using a 64 channel Cheetah recording system (Neuralynx, Tucson, AZ). A 72 channel motorized commutator (AirFlyte, Bayonne, NJ; Dragonfly, Ridgeley, WV; Neuralynx, Tucson, AZ) allowed the rats to run the task without twisting the tether cables which connected the hyperdrive to the recording system. Tetrode channels were sampled at 32 kHz. Signals were filtered between 600 Hz to 6 kHz (Multiple-T task, 6 animals) or between 300 Hz to 9 kHz (Take-5 or Multiple-T task, 5 animals). The filtering ranges used were changed midway through these experiments (from 0.6–6 kHz to 0.3–9 kHz) in an attempt to capture slow and fast waveform features observed in the initial recordings.
When the voltage on any of the four channels of a single tetrode exceeded a threshold set by the experimenter, the spike waveform on each of the four channels on the tetrode was recorded and timestamped with microsecond resolution. For five animals running the Multiple-T and Nosepoke tasks, a positive voltage threshold was used and 1 ms (32 samples) spike waveforms were recorded. For five animals running the Take-5 or Multiple-T tasks, the filtered electrical potentials were written directly to disk, and spikes were triggered in these recordings offline using both positive and negative voltage thresholds to trigger spikes and 2 ms (64 sample) waveforms were used for clustering.
Spikes were clustered offline into putative cells on the basis of their waveform properties using MClust 3.0 or MClust 3.4 (A. D. Redish, current software available at http://umn.edu/~redish/mclust), with automatic pre-clustering using KlustaKwik 1.0 or KlustaKwik 1.5 (K. Harris, available at http://klustakwik.sourceforge.net) to create a set of spike trains, each of which was a list of the times at which action potentials occurred for one putative neuron. The quality of each neuron was quantified using two cluster quality measures, Lratio and Isolation Distance (Schmitzer-Torbert, Jackson et al. 2005). Following recommendations in Schmitzer-Torbert et al. (2005), cluster quality was calculated for each tetrode using eight waveform measurements (energy and first principal component coefficients of the energy normalized waveform, calculated for each tetrode channel). Spike trains with values of Lratio greater than 0.10 or values of Isolation Distance less than 20 were not included in these analyses. Inclusion of all spike trains did not qualitatively change the results presented here.
During the recording session, the position of the rat was monitored using LEDs on the headstage and an LED backpack constructed in the lab and secured using an elastic wrap and Velcro. The position of the LED was observed by an overhead camera, and was recorded using a video input to the Cheetah recording system, which also timestamped the position samples. Experimental control was performed using Matlab and a computer interface designed in the lab. Events such as food delivery and the presentation of tones were also recorded and timestamped by the Cheetah recording system.
Histology
Following the completion of all experiments, the final locations of each tetrode were marked with small lesions by passing a small amount of anodal current (5μA for 5 seconds) through each tetrode. Two days later, rats were deeply anesthetized with Nembutal and perfused transcardially with saline followed by 10% formalin. Brains were stored in formalin followed by 30% sucrose formalin until slicing. Slices were made either coronally or horizontally through the area of the implantation and stained with ethidium bromide or cresyl violet to visualize electrode tracks.
Data analysis
Post-spike suppression
After firing an action potential, the amount of time that passed before a cell returned to its average firing rate was taken to be an indirect measure of the neuron’s refractory period. Post-spike suppression was calculated for each cell by measuring the length of time that a cell’s firing rate was suppressed following an action potential. Using an autocorrelation calculated over 1 second using 1 ms bins and smoothed with a 25 ms hamming window, the number of bins following an action potential until the firing rate of the cell met or exceeded the cell’s average firing rate was taken to be the duration of post-spike suppression.
PropISIs > 2 seconds
To separate phasic and non-phasic neurons, the proportion of time spent in long interspike-intervals (ISIs) was calculated for each spike train by finding all ISIs which exceeded a criterion (X), summing those ISIs, and dividing by the total session time (Schmitzer-Torbert and Redish 2004). The measure, PropISI>X, takes values between 0 and 1, and gives a measure of what proportion of the session was spent in ISIs equal to or longer than X. For these analyses, a criterion of X = 2 seconds was used.
HFN oscillations
Power spectra for each striatal cell were calculated and the average spectrum was found for each cell in a 6.4 second window (25 ms bins, 256 bins total) either selected before food delivery (On-maze) or after food delivery (At-feeder). Oscillation scores were then derived by finding the difference in average power in the observed oscillatory band (2–3 Hz) relative to the average power in an adjacent control band (3–4 Hz).
Reconstruction
Reconstruction was done using standard Bayesian techniques (Rieke, Warland et al. 1997; Zhang, Ginzburg et al. 1998). The goal of the reconstructions was to identify a behavioral variable (the position of the rat on the maze, or time following food delivery) using the firing pattern of the neural ensemble and the known tuning curves of each neuron. The goal of the reconstruction was to estimate P(X|F) using the firing rate of the neural ensemble (the firing rate vector F) and the tuning curves of each neuron in the ensemble (P(F|X)). Firing rates of neurons in the ensemble were assumed to be independent. The reconstructed X of the rat at any time was taken to be the location with the highest probability (i.e. argmaxX P(X|F)). To assess the quality of the reconstruction in each recording session, the proportion of the variance in X explained by the reconstructed parameter X̂ was measured using R2.
For reconstructions of the position of the rat on the maze, each 2-dimensional position sample was mapped to the closest point on an idealized path (the typical path taken through the maze by the rat, see Schmitzer-Torbert and Redish 2004 for an example) to create 1-dimensional representation of the route rats took through the Multiple-T maze and the Take-5 maze. The linearized position was then binned at a resolution of ~5 cm.
Results
Behavior
All rats were able to learn the Multiple-T maze, as previously reported (Schmitzer-Torbert and Redish 2002; Schmitzer-Torbert and Redish 2004). The data set used in these analyses included the previous rats used in Schmitzer-Torbert and Redish (2004) as well as new rats (total n = 7 rats). Across 115 sessions on the Multiple-T maze (2–24 sessions per rat), rats completed 55 ± 22 (SD) laps per 40 minute session.
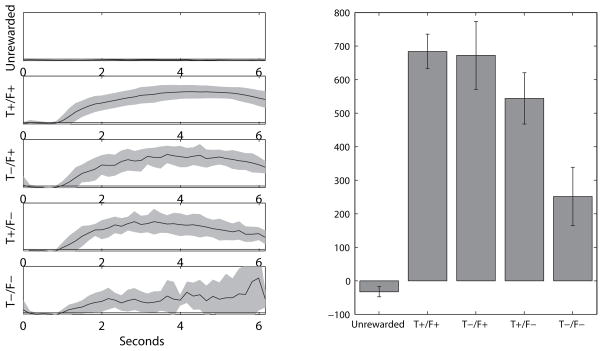
All rats were also able to learn the Take-5 task successfully. Across 51 sessions on the Take 5 task (7–15 sessions per rat), rats completed 50 ± 16 trials per 40 minute session. Although rats were able to earn a large number of rewards on the task, this performance alone did not indicate the nature of the behavioral strategy rats employed in order to complete trials on the task. For instance, rats could be completing Take 5 trials on the basis of sensory cues (the sounds of food delivery and the tone which signaled food delivery) or rats could have developed a strategy to predict the location of the next reward. To determine if rats had indeed learned to predict where the next food delivery would occur, probe trials were included in which there was an omission of either the tone predicting food delivery or food delivery itself, or both. On normal trials, rats typically only sampled the rewarded food port (Figure 2B, Tone+/Food+, in which both the tone predicting food delivery and food delivery itself occurred), but rats were unlikely to sample other food ports that they ran past on each trial on their way to the rewarded food port (Figure 2, Unrewarded). In probe trials, rats were biased to sample the food port in which reward was expected: on every type of probe trial, rats were biased to sample the food port at which food should have been expected. This food port sampling bias was observed even for probe trials in which all sensory cues were eliminated (Figure 2, Tone−/Food−, in which the food prediction tone and food delivery were both omitted) demonstrating that rats were performing this task using a more complex strategy than simply listening for tone or feeder-click to determine where each food delivery was expected. Sensory cues did play a role in the performance of rats on the Take 5 task: when all sensory cues were eliminated (Tone−/Food− probe trials), rats were less likely to sample the correct food port than in normal trials (Tone+/Food+ trials), although they were still more likely to sample the correct than unrewarded food ports.
Figure 2.
Sampling behavior on the Take 5 task. A: Average sampling traces from food ports on the Take 5 task in the 6 seconds following arrival at the food delivery locations. When no reward was delivered (Unrewarded), rats did not sample the food ports, while on normal trials (Tone+/Food+), rats began sampling the food ports approximately 1 second after arrival in the vicinity of the appropriate food delivery location. On probe trials, sampling behavior was similar to normal trials when either the tone (Tone−/Food+) or food delivery (Tone+/Food−) was omitted. Critically, rats also were likely to sample the food port when both sensory cues were omitted (Tone−/Food−), though sampling behavior on these probe trials was more variable. All sampling plots are to the same scale. B: Comparison of each trial type (averaged across the 6 second window shown in A). Robust sampling was seen for normal trials (Tone+/Food+) and probe trials in which at least one sensory cue was presented (Tone−/Food+ and Tone+/Food− probe trials). Weaker sampling was seen for probe trials in which both sensory cues were omitted (Tone−/Food−), but rat still sampled the food ports more than for Unrewarded arrivals. Error bars represent mean and 95% confidence intervals calculated across all Take 5 sessions.
Neurophysiology
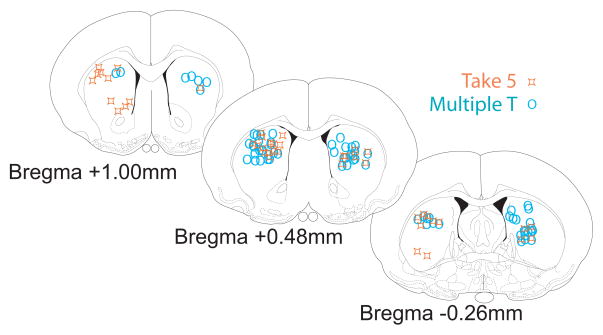
A total of 2,274 spike trains recorded extracellularly from the dorsal striatum in 10 rats (7 rats on Multiple-T, 5 rats on Take-5, including two rats recorded on both tasks) were analyzed. Cells were recorded from leaving corpus callosum (identified as a transiently quiet zone (corpus callosum) followed by the appearance of very slow spiking cells (<1 spike/second, striatum)). Final recording locations in Figure 3. Thus the recording zone entailed the striatal region dorsal to the final position marked in Figure 3. Tetrodes were generally found to end in dorsolateral striatum1
Figure 3.
Recording locations verified histologically. Shown separately are final tetrode locations from ten animals implanted with hyperdrives over the dorsal striatum. Final tetrode positions are marked by x’s, and all tetrode locations have been mapped to the nearest of the three coronal sections shown. Tetrodes were observed in a region extending approximately −0.5 to 1.5 mm anterior/posterior relative to bregma. Diagrams adapted from (Paxinos and Watson 1998). The final recording marks for one Take-5 rat included some ventral striatal locations. Removing this rat from our analyses does not qualitatively change our results. We have included this rat in the analyses because most of the cells recorded from this rat were recorded early (i.e. in the more dorsal zone).
Cells recorded were divisible into three discernable categories, phasic-firing cells (PFNs) that paused for long intervals and fired bouts of activity lasting approximately 0.5 sec (Kimura, Kato et al. 1990, tonically firing neurons (TFNs) with long refractory periods and firing rates reliably limited to 2–15 Hz (Kimura, Rajkowski et al. 1984), and high-firing neurons (HFNs), with highly variable, high firing rates reaching to >50 Hz (Redish, Schmitzer-Torbert et al. 2002; Berke, Okatan et al. 2004; Barnes, Kubota et al. 2005).
Classification
Neurons were separated into three categories (PFNs: phasic-firing neurons, TFNs: tonic firing neurons, and HFNs: high-firing neurons) based on the proportion of time spent in long interspike-intervals and the length of the post-spike suppression (see Figure 4A). The distribution of PropISI > 2 seconds was strongly bimodal (see Figure 4A), with a large proportion of neurons (which we term phasic neurons) that spent the majority of the recording session in inter-spike intervals greater than 2 seconds in duration (i.e. quiescent) except for brief periods of rapid firing. A smaller proportion of cells (which we term non-phasic neurons) were also observed which rarely paused for more than 2 seconds. Neurons with values of PropISIs > 2 seconds greater than 0.4 were classified as phasic (PFNs), while neurons with PropISIs > 2 seconds less than 0.4 were classified as non-phasic (HFNs, TFNs). Strong bimodal separation of non-phasic and phasic neurons were seen for separation critera ranging from 0.5 to 10 seconds, and were also obtained using other measures that have been used to classify neurons into basic types (time-local variation, see Shinomoto, Shima et al. 2003; Shinomoto, Miyazaki et al. 2005).
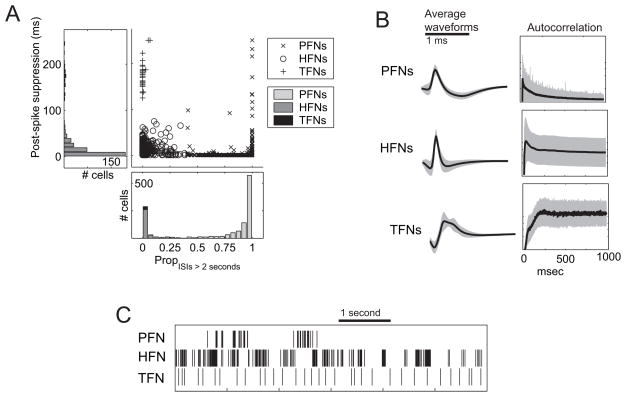
Figure 4.
Classification of striatal firing patterns. A: Measuring PropISIs > 2 seconds and postspike suppresion, striatal spike trains separate into three discernable categories: phasic firing neurons (PFNs), tonic firing neurons (TFNs), and high firing neurons (HFNs). B: The average waveforms of each category differ, with PFNs being preferentially biphasic, HFNs preferentially triphasic, and TFNs preferentially inverted. Scalebar shows 1 ms. C: The autocorrelation function, showing the strong post-spike suppression of TFNs. D: Sample spike trains from each category, showing the bouts and silence of PFNs, the variability with high-firing times of HFNs, and the steady firing of TFNs. Scalebar shows 1 second.
Non-phasic neurons with long post-spike suppression (greater than 100 ms) were classified as TFNs, while non-phasic neurons with short post-spike suppression (less than 100 ms) were classified as HFNs. The choice of 100 ms to divide HFNs and TFNs was motivated by the strong bimodality observed in the post-spike suppressions of non-phasic neurons (see Figure 4A) and by reports in the literature indicating that cholinergic neurons have long afterhyperpolarizations (80–120 ms) which prevent fast firing rates (Kawaguchi 1993; Aosaki, Kimura et al. 1995).
Average waveforms and autocorrelations for each neuron type are also shown in Figure 4B, as well as examples of spike trains from each type of neuron (Figure 4C). Across all animals, PFNs were the most frequently recorded neuron (n = 1,624, 71.4%), followed by HFNs (n = 611, 26.9%) and TFNs (n = 39, 1.7%). PFNs fired at very low rates throughout the session (Mean firing rate ± SD, 0.50 ± 0.75 Hz), and when active, frequently fired in bouts of activity (Maximum firing rate ± SD, 19 ± 13 Hz). HFNs and TFNs rarely paused, however, and fired at high rates throughout the recording session (Mean firing rate ± SD, HFNs: 16.1 ± 10.5 Hz; TFNs: 5.4 ± 1.6 Hz). There was a significant difference between the average firing rates of these cell types (Kruskal-Wallis, χ2(2, N = 1, 337) = 800.2, p < 0.0001). Post-hoc tests (Tukey-Kramer HSD) revealed that HFNs and TFNs had significantly higher mean firing rates than PFNs. HFNs tended to fire at high rates throughout the task, and showed bursting, reaching maximal firing rates greater than 100 Hz in some cells. Thus, HFNs showed maximum firing rates (Max FR ± SD, 58 ± 26 Hz) much higher than PFNs (Max FR ± SD, 19 ± 13 Hz) or TFNs (Max FR ± SD, 14 ± 4 Hz). These differences were significant by Kruskal-Wallis (χ2(2, N = 1329) = 539, p < 0.0001). Post-hoc tests (Tukey-Kramer HSD) revealed that HFNs had significantly higher maximum firing rates than PFNs and TFNs.
Within a single session, cell type classifications on the basis of firing patterns remained stable between sitting in a terra cotta pot (during five minute recording periods that were done immediately preceding and following each recording session) and running the behavioral task. When classifications made using the data from the pre- and post-run recording window were compared to classifications made using the data from when the animal was running on the task in the same session, striatal neurons predominantly retained their classification in both behavioral states (running on the task and sitting in the pot). The classifications of PFNs and HFNs were highly stable: 98.9% of PFNs and 95.3% of HFNs obtained the same classification in both the Rest and Task periods. Of the 30 TFNs identified during the behavioral task, 20 (2/3) retained their classification during the pre- and post-run Rest periods. The remaining 10 cells switched from TFN to HFN classifications, due to small early peaks in their autocorrelations (thus shrinking their post-spike suppression). As the behavior of the rat in these two conditions (sitting quietly in a terra cotta pot versus running for food rewards) differed greatly these striatal classifications represent firing patterns that are relatively independent of the behavior of the rat over the temporal windows examined (up to 40 minutes)2. When corrected for experimental recording methodology3, the proportions of cell type recorded in each task were not significantly different (Pearson’s χ2(4) = 7.5, p = 0.113), further supporting the claim that the classification used here did not depend strongly on the behavior that rats were performing (rest versus running, Multiple-T versus Take-5).
Phasic firing neurons
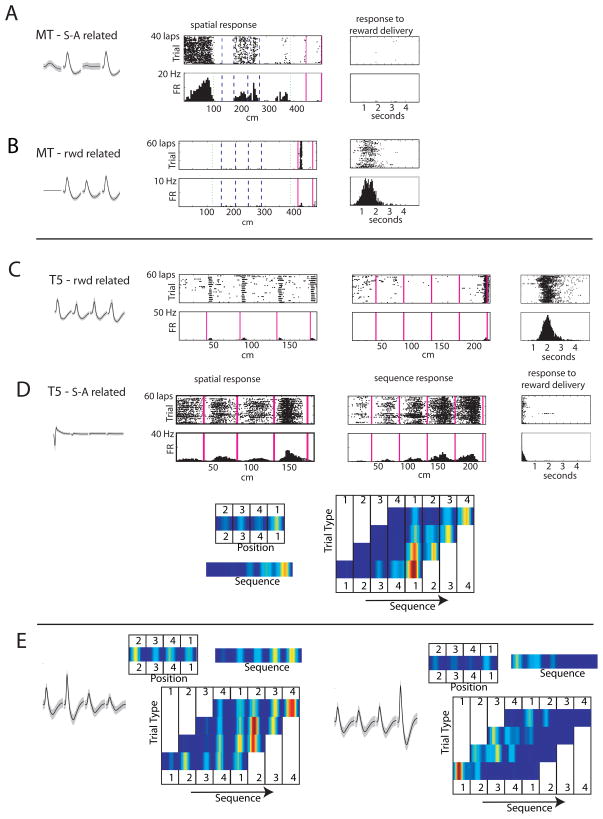
Similar proportions of PFNs were found on each task (Multiple-T: 1674/2271 (74%), Take-5: 643/850 (76%)). PFNs were generally responsive while rats ran on the maze portions of the task and during reward receipt. On the Multiple-T, PFNs responsive on the maze-portion of the task typically responded to a spatial location on the maze (Schmitzer-Torbert and Redish 2004). On the Take-5, some of these cells showed consistent responses to the spatial location of the animal, others showed more consistent responses to the sequence, and others responded to an interaction between space and sequence. Examples of cellular responses in each task are shown in Figure 5. On each task, a different set of PFNs responded temporally to delivery of the food-reward. Significantly fewer cells responded to both spatial location and food delivery than expected, given the proportion active during the running on the maze and the proportion active during reward-receipt (Multiple-T: χ2(1) = 11, p < 0.001; Take-5: χ2(1) = 35, p < 0.001). This indicates that the running-related and reward-related cells consist of non-overlapping populations of phasic neurons, consistent with our previous report for the Multiple-T (Schmitzer-Torbert and Redish 2004).
Figure 5.
PFN responses. A, B: example cells from the Multiple-T task. (A: a neuron turned to the spatial/sequence component of the task; B: a neuron tuned to the reward component of the task). C–E: Example cells from the Take-5 task. (C: a reward-related neuron. D: a neuron tuned to the spatial/sequence component of the task. Note that this neuron shows tuning both to spatial location as well as the sequential component.) Note: the apparent spatial/sequence tuning of the neurons in A&C is due to the firing of these neurons following reward-delivery. These neurons did not continue to fire at high rates while the rats sat at these locations, and the neuron in C from the Take-5 task did not fire in a given spatial location if the rat ran past a pellet dispenser that was not rewarded. E: two tuning curves showing tuning to both space, sequence, and the cross-product (space × sequence). In the bottom panel, the response of each neuron is shown to three conditions (top) the spatial journey to each feeder (1–4), (middle) the sequence around the five feeder journeys (1–5), and (bottom) the cross product. In the cross-product, each column shows spatial location (1–4, repeated) and each row shows a sequence (12341, 23412, etc.). Each PFN showed tuning to both spatial location and sequence.
Tonic firing neurons
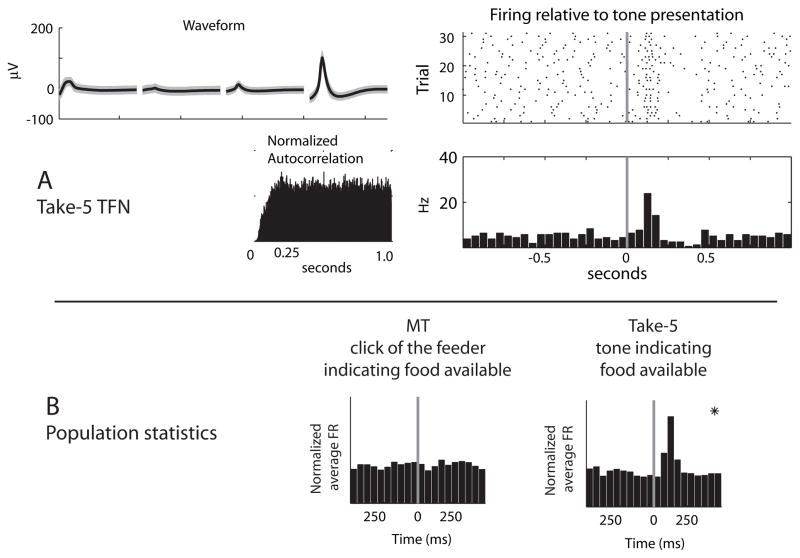
Similar proportions of TFNs were found on each task (Multiple-T: 20/2271, 1%; Take-5: 20/850, 2%). Although neurons with firing characteristics putting them in the TFN category were found in both tasks, the behavioral correlates of these cells were task dependent. (See Figure 6). On Take-5, which included tone cue-signals indicating the availability of food, TFN cells showed reliable burst and pause responses consistent with reports of primate tonically-active-neuron (TAN) responses (Kimura, Rajkowski et al. 1984; Aosaki, Graybiel et al. 1994; Aosaki, Kimura et al. 1995; Raz, Feingold et al. 1996; Matsumoto, Minamimoto et al. 2001) (Take-5 population response to tone: F(9, 108) = 14.08, p < 0.00001.). However, no such correlates were seen on Multiple-T (population response to feeder click: F(9, 108) = 1.2, p = 0.29). Post-hoc tests (Tukey HSD, α = 0.05) were used to test for any significant changes in firing rate from the first bin following food delivery/tone presentation. These post-hoc tests revealed a significant population response on Take-5 but not Multiple-T.
Figure 6.
TFN responses. A: TFN with short latency excitations following the presentation of the food-predictive tone on the Take 5 task. The left plot shows the average waveform, interspike-interval histogram and autocorrelation, while the right plot shows the raster-plot and firing rate histogram in a ± 1 second window surrounding cue/food delivery onset. B: Population responses of TFNs to reward-predictive cues. Average population responses of TFNs aligned to either food-delivery on the Multiple-T task (left) and a tone predicting food delivery in the Take-5 task (right). Panels in which there was a significant population response in the 500 ms following event onset are marked with asterisks.
High firing neurons
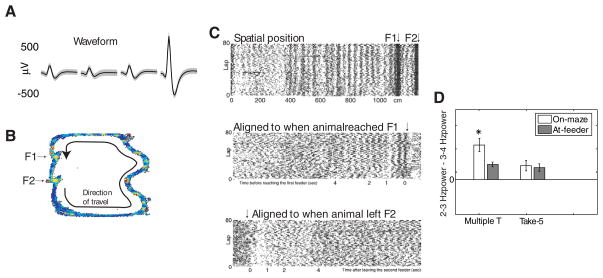
Similar proportions of high-firing neurons (HFNs) were found on each task (Multiple-T: 577/2271, 25%; Take-5: 187/850, 22%). However, in contrast to the lack of a spatial correlate observed in TFNs, HFNs showed a consistent spatial response, but only on the Multiple-T task. As shown in Figure 7, HFNs on the Multiple-T showed multiple narrow spatial activations of approximately constant spatial frequency. (In the example shown in Figure 7A, the rat ran at different speeds on each lap. Thus, we describe these as “spatial” rather than “temporal”.) These oscillations were transitory, were only observed as rats were navigating the Multiple-T maze, and ceased once rats reached the food delivery location. These oscillations were examined by calculating power spectra for each cell using a Fourier transform applied to the binned firing rate of the cell immediately preceding and following reward delivery. If a transient oscillation was present in some cells specifically when rats ran on the maze, we would expect to see enrichment in the oscillatory frequency band in the time window preceding food delivery compared to following food delivery. A repeated-measures ANOVA revealed a significant interaction of cell type, task, and the On-maze/At-Feeder parameter (Figure 7B, F(2, 1070) = 6.56, p = 0.001). Post-hoc comparisons (Tukey HSD, α = 0.05) revealed that HFNs had increased power in the 2–3 Hz band while rats were running on the maze compared to food delivery on Multiple-T task, but not Take-5. These oscillations were cell type and task specific, with only HFNs demonstrating these oscillations, and only on the Multiple-T task.
Figure 7.
HFN responses. A–C: Example HFN from the Multiple-T task. A: Average waveform. B: Spatial firing rate map. Color panel indicates firing rate as a function of position, with blue representing low firing rates and red representing high firing rates. C: Rastergrams. Top: linearized spatial rastergram. Note the spatial consistency of the oscillation (the rat ran at different speeds on each lap). These oscillations were not well-aligned temporally across all trials when arranged relative to when the rat arrived at the first food delivery site (Middle) or to when the rat started the trial (by leaving the second food delivery site, Bottom). There was a 3 Hz frequency in the temporal autocorrelation, but which was reset spatially on each lap. D: Population statistics. A significant increase at 3 Hz power was seen on Multiple-T but not the Take-5.
Reconstruction from neural ensembles
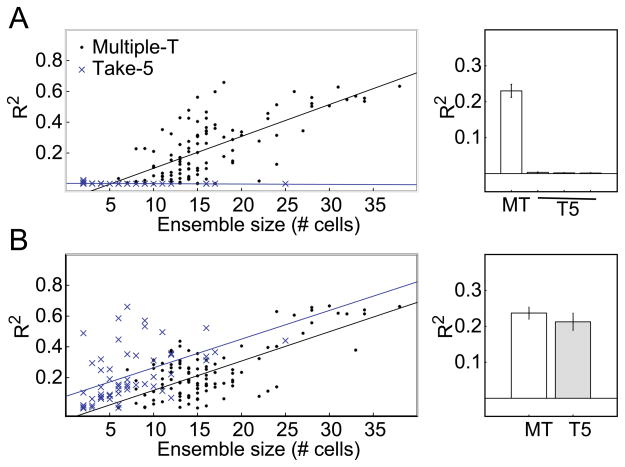
In order to directly measure the information encoded by these parameters in the recorded striatal neural ensembles, we attempted to reconstruct behavioral parameters from striatal neural ensembles. In the reconstruction analyses, all neurons were used (including both task-responsive and unresponsive neurons). The reconstruction quality for estimating spatial/sequence location in both tasks is shown in Figure 8A. On the Multiple-T, striatal neural ensembles provided a robust representation of the location of the rat on the maze (average reconstruction quality: R2 = 0.23, SD = 0.19). However, on the Take-5 task, the quality of the reconstructed position and sequence location was much lower (for spatial position, mean R2 = 0.003, SD = 0.006; for sequence location, mean R2 = 0.002, SD = 0.003; for space X sequence, mean R2 = 0.001, SD = 0.004). Compared to the reconstruction quality for ensembles recorded on the Multiple-T task, reconstruction quality for spatial location, sequence location and the cross-product of spatial location X sequence location was significantly lower on the Take-5 task (all t(162) > 8.9, all ps < 0.0001). The differences in reconstruction quality for the Multiple-T and Take-5 tasks were especially striking as the size of the recorded ensemble increased: As shown in Figure 8A reconstruction quality increased strongly on the Multiple-T as ensemble size increased, but qualities remained low for even the largest ensembles recorded on the Take-5 task.
Figure 8.
Ensemble reconstruction. A: Spatial reconstruction accuracy as a function of ensemble size of PFNs. Only on the Multiple-T, was there a significant increase in accuracy with increasing ensemble size. B: Reward-delivery reconstruction accuracy as a function of ensemble size. Striatal ensembles showed reliable representations of reward-delivery on both tasks.
In contrast to reconstructions of spatial information, reconstructions of time following food delivery were successful using striatal ensembles from both the Multiple-T and Take-5 tasks (see Figure 8B). Average reconstruction qualities of the six seconds following food delivery did not differ between tasks (Multiple-T: average reconstruction quality, R2 = 0.24, SD = 0.17; Take-5: R2 = 0.21, SD = 0.18, t(162) = 0.85, p = 0.40).
Discussion
We compared the behavioral correlates of dorsal striatal neural ensembles on two tasks, one in which spatial cues provided information about reward (Multiple-T), and one in which locomotor actions were taken, but in which spatial cues were dissociated from reward (Take-5). These results indicate a strong task dependency of the quality of the spatial, but not the reward-related, striatal representations on these tasks. An alluring hypothesis that explains the difference in the encoding of task-parameters between tasks is that the striatal representation may depend on the degree to which these parameters can be unambiguously associated with goals. The location of the rat on the Multiple-T maze is well-related to the sequence of actions that must be completed in order to receive reward. However, on the Take-5 task, the location of the rat is only informative if the rat also maintains a representation of the previously rewarded pellet dispenser. Thus, there is a strong encoding of space itself on the Multiple-T, but no encoding of space without sequence on Take-5.
Behaviorally, animals did keep track of the next rewarded feeder site on the Take-5 task (Figure 2)—even in the absence of auditory stimuli and/or reward (the tone signaling food delivery or the sound of the pellet dispensers), rats were still biased to respond at the location where reward was expected. This demonstrates that at some level, rats had a cognitive representation of their progress through the sequence leading to reward delivery. This representation of sequence progress was also reflected in the tuning of maze-responsive PFNs in the Take-5 task to sequence progress. Although striatal PFNs had access to information related to spatial location and sequence progress (reflected by their tuning to these parameters), at the ensemble level there was no evidence for a coherent representation of either of these parameters separately.
On both the Multiple-T and Take-5 tasks, animals performed identical actions at multiple locations on the task. For example, on the Take-5 task, animals continuously performed right turns at each of the four locations, for five steps through a sequence (5/4 around the track). Although the actions performed were essentially identical, the cells differentiated these actions as a function of the spatial position of the animal and the progress through the sequence. Consistent with previous studies (Graybiel 1998; Jog, Kubota et al. 1999; Itoh, Nakahara et al. 2003; Schmitzer-Torbert and Redish 2004; Barnes, Kubota et al. 2005; Samejima, Ueda et al. 2005; Hikosaka, Nakamura et al. 2006), cells encoded neither actions nor cues, but rather a combination of the two.
In head-fixed primates performing cue-response tasks, striatal cells tend to encode combinations of cue-action sequences (Kermadi, Jurquet et al. 1993; Kermadi and Joseph 1995; Graybiel 1998; Hikosaka, Nakamura et al. 2006). Similarly, in operant tasks in rats, many striatal neurons encode cue-action combinations (Gardiner and Kitai 1992; Teagarden and Rebec 2007). In freely-behaving rodents running spatial tasks, the cells tend to encode combinations of spatial-action sequences (Jog, Kubota et al. 1999; Schmitzer-Torbert and Redish 2004; Barnes, Kubota et al. 2005). On the Take-5 task, which required rats to run around a maze with spatial-extent, but in which space provided ambiguous information about reward, striatal cells encoded combinations of cue-action sequences, but did not encode purely spatial information, and at the ensemble level, neither spatial nor sequence location could be reconstructed from the activity of striatal ensembles. This suggests that the dorsal striatal representation of task parameters may depend on the types of “events” that are salient to the identification of reward. Unlike hippocampal cells, which continue to show spatial responses even under conditions in which space carries no information about reward (Muller, Kubie et al. 1987; Redish 1999; Kentros, Agnihotri et al. 2004), dorsal striatal cells only showed spatial responses when the spatial location of the animal provided information about the availability of reward.
Abbreviations
- HFN
High firing neuron
- PFN
Phasic firing neuron
- TFN
Tonic firing neuron
Footnotes
5/60 tetrodes from the Take-5 datasets were found to lie in more ventral positions. All of these were from 1 rat. Removing that rat from the analyses did not qualitatively change the results. This rat is included in the database because the cells were primarily recorded shortly after leaving corpus callosum, implying they most likely came from dorsal striatum.
It is worth noting that during the five minute recordings taken before and after rats ran on the task, the animals were not observed to fall asleep in this time. Berke and colleagues (2004) have reported strong changes in the firing patterns of striatal neurons when rats transition between sleep and waking. It is thus likely that the classification system used here may not generalize to non-waking states.
On the Multiple-T task, only positive-going spikes were recorded, while on the Take-5 task, both positive-going and negative-going spikes were recorded.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Neil C. Schmitzer-Torbert, Department of Psychology, Wabash College
A. David Redish, Department of Neuroscience, University of Minnesota.
References
- Aldridge JW, Berridge KC. Coding of serial order by neostriatal neurons: A “natural action” approach to movement sequence. Journal of Neuroscience. 1998;18:2777–2787. doi: 10.1523/JNEUROSCI.18-07-02777.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR. Microstimulation of the primate neostriatum. II. Somatotopic organization of striatal microexcitable zones and their relation to neuronal response properties. Journal of Neurophysiology. 1985;53(6):1417–1430. doi: 10.1152/jn.1985.53.6.1417. [DOI] [PubMed] [Google Scholar]
- Aosaki T, Graybiel AM, et al. Effect of the nigrostriatal dopamine system on acquired neural responses in the striatum of behaving monkeys. Science. 1994;265(5170):412–5. doi: 10.1126/science.8023166. [DOI] [PubMed] [Google Scholar]
- Aosaki T, Kimura M, et al. Temporal and spatial characteristics of tonically active neurons of the primate’s striatum. J Neurophysiol. 1995;73(3):1234–52. doi: 10.1152/jn.1995.73.3.1234. [DOI] [PubMed] [Google Scholar]
- Bailey KR, Mair RG. The role of striatum in initiation and execution of learned action sequences in rats. J Neurosci. 2006;26(3):1016–25. doi: 10.1523/JNEUROSCI.3883-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes TD, Kubota Y, et al. Activity of striatal neurons reflects dynamic encoding and recoding of procedural memories. Nature. 2005;437(7062):1158–61. doi: 10.1038/nature04053. [DOI] [PubMed] [Google Scholar]
- Berke JD, Okatan M, et al. Oscillatory entrainment of striatal neurons in freely-moving rats. Neuron. 2004;43(6):883–896. doi: 10.1016/j.neuron.2004.08.035. [DOI] [PubMed] [Google Scholar]
- Boussaoud D, Kermadi I. The primate striatum: Neuronal activity in relation to spatial attention versus motor preparation. European Journal of Neuroscience. 1997;9:2152–2168. doi: 10.1111/j.1460-9568.1997.tb01382.x. [DOI] [PubMed] [Google Scholar]
- Carelli RM, Wolske M, et al. Loss of lever press-related firing of rat striatal forelimb neurons after repeated sessions in a lever pressing task. J Neurosci. 1997;17(5):1804–14. doi: 10.1523/JNEUROSCI.17-05-01804.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doya K. What are the computations of the cerebellum, the basal ganglia and the cerebral cortex? Neural Netw. 1999;12(7–8):961–974. doi: 10.1016/s0893-6080(99)00046-5. [DOI] [PubMed] [Google Scholar]
- Gardiner TW, Kitai ST. Single-unit activity in the globus pallidus and neostriatum of the rat during performance of a trained head movement. Exp Brain Res. 1992;88(3):517–30. doi: 10.1007/BF00228181. [DOI] [PubMed] [Google Scholar]
- Gill KM, Mizumori SJ. Context-dependent modulation by D(1) receptors: differential effects in hippocampus and striatum. Behav Neurosci. 2006;120(2):377–92. doi: 10.1037/0735-7044.120.2.377. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. Building action repertoires: memory and learning functions of the basal ganglia. Curr Opin Neurobiol. 1995;5(6):733–41. doi: 10.1016/0959-4388(95)80100-6. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia and chunking of action repertoires. Neurobiol Learn Mem. 1998;70(1–2):119–36. doi: 10.1006/nlme.1998.3843. [DOI] [PubMed] [Google Scholar]
- Graybiel AM. The basal ganglia. Curr Biol. 2000;10(14):R509–11. doi: 10.1016/s0960-9822(00)00593-5. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Nakamura K, et al. Basal ganglia orient eyes to reward. J Neurophysiol. 2006;95(2):567–84. doi: 10.1152/jn.00458.2005. [DOI] [PubMed] [Google Scholar]
- Hollerman JR, Tremblay L, et al. Influence of reward expectation on behavior-related neuronal activity in primate striatum. J Neurophysiol. 1998;80(2):947–63. doi: 10.1152/jn.1998.80.2.947. [DOI] [PubMed] [Google Scholar]
- Houk JC, Davis JL, et al., editors. Models of Information Processing in the Basal Ganglia. Cambridge, MA: MIT Press; 1995. [Google Scholar]
- Itoh H, Nakahara H, et al. Correlation of primate caudate neural activity and saccade parameters in reward-oriented behavior. J Neurophysiol. 2003;89(4):1774–83. doi: 10.1152/jn.00630.2002. [DOI] [PubMed] [Google Scholar]
- Jog MS, Kubota Y, et al. Building neural representations of habits. Science. 1999;286(5445):1745–9. doi: 10.1126/science.286.5445.1745. [DOI] [PubMed] [Google Scholar]
- Kawagoe R, Takikawa Y, et al. Reward-predicting activity of dopamine and caudate neurons--a possible mechanism of motivational control of saccadic eye movement. J Neurophysiol. 2004;91(2):1013–24. doi: 10.1152/jn.00721.2003. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y. Physiological, morphological, and histochemical characterization of three classes of interneurons in the rat neostriatum. Journal of Neuroscience. 1993;13(11):4908–4923. doi: 10.1523/JNEUROSCI.13-11-04908.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kentros CG, Agnihotri NT, et al. Increased attention to spatial context increases both place field stability and spatial memory. Neuron. 2004;42(2):283–95. doi: 10.1016/s0896-6273(04)00192-8. [DOI] [PubMed] [Google Scholar]
- Kermadi I, Joseph JP. Activity in the caudate nucleus of monkey during spatial sequencing. Journal of Neurophysiology. 1995;74:911–933. doi: 10.1152/jn.1995.74.3.911. [DOI] [PubMed] [Google Scholar]
- Kermadi I, Jurquet Y, et al. Neural activity in the caudate nucleus of monkeys during spatial sequencing. Experimental Brain Research. 1993;94:352–356. doi: 10.1007/BF00230305. [DOI] [PubMed] [Google Scholar]
- Kimura M, Kato M, et al. Physiological properties of projection neurons in the monkey striatum to globus pallidus. Experimental Brain Research. 1990;82(3):672–676. doi: 10.1007/BF00228811. [DOI] [PubMed] [Google Scholar]
- Kimura M, Rajkowski J, et al. Tonically discharging putamen neurons exhibit set-dependent responses. Proceedings of the National Academy of Sciences. 1984;81:4998–5001. doi: 10.1073/pnas.81.15.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi S, Kawagoe R, et al. Functional differences between macaque prefrontal cortex and caudate nucleus during eye movements with and without reward. Exp Brain Res. 2006 doi: 10.1007/s00221-006-0622-4. [DOI] [PubMed] [Google Scholar]
- Matsumoto N, Hanakawa T, et al. Role of nigrostriatal dopamine system in learning to perform sequential motor tasks in a predictive manner. Journal of Neurophysiology. 1999;82:978–998. doi: 10.1152/jn.1999.82.2.978. [DOI] [PubMed] [Google Scholar]
- Matsumoto N, Minamimoto T, et al. Neurons in the thalamic CM-Pf complex supply striatal neurons with information about behaviorally significant events. Journal of Neurophysiology. 2001;85:960–976. doi: 10.1152/jn.2001.85.2.960. [DOI] [PubMed] [Google Scholar]
- Miyachi S, Hikosaka O, et al. Differential roles of monkey striatum in learning of sequential hand movement. Experimental Brain Research. 1997;115:1–5. doi: 10.1007/pl00005669. [DOI] [PubMed] [Google Scholar]
- Muller RU, Kubie JL, et al. Spatial firing patterns of hippocampal complex-spike cells in a fixed environment. J Neurosci. 1987;7(7):1935–50. doi: 10.1523/JNEUROSCI.07-07-01935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1998. [Google Scholar]
- Ragozzino KE, Leutgeb S, et al. Dorsal striatal head direction and hippocampal place representations during spatial navigation. Exp Brain Res. 2001;139(3):372–6. doi: 10.1007/s002210100795. [DOI] [PubMed] [Google Scholar]
- Ragozzino ME, Ragozzino KE, et al. Role of the dorsomedial striatum in behavioral flexibility for response and visual cue discrimination learning. Behav Neurosci. 2002;116(1):105–15. doi: 10.1037//0735-7044.116.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz A, Feingold A, et al. Neuronal synchronization of tonically active neurons in the striatum of normal and parkinsonian primates. Journal of Neurophysiology. 1996;76(3):2082–2088. doi: 10.1152/jn.1996.76.3.2083. [DOI] [PubMed] [Google Scholar]
- Redish AD. Beyond the Cognitive Map. Cambridge, MA: MIT Press; 1999. [Google Scholar]
- Redish AD, Schmitzer-Torbert NC, et al. 2002 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2002. Classification of dorsal striatal neurons from extracellular recordings in awake behaving rats. [Google Scholar]
- Rieke F, Warland D, et al. Spikes. Cambridge, MA: MIT Press; 1997. [Google Scholar]
- Samejima K, Ueda Y, et al. Representation of action-specific reward values in the striatum. Science. 2005;310(5752):1337–40. doi: 10.1126/science.1115270. [DOI] [PubMed] [Google Scholar]
- Schmitzer-Torbert N, Jackson J, et al. Quantitative measures of cluster quality for use in extracellular recordings. Neuroscience. 2005;131(1):1–11. doi: 10.1016/j.neuroscience.2004.09.066. [DOI] [PubMed] [Google Scholar]
- Schmitzer-Torbert N, Redish AD. Development of path stereotypy in a single day in rats on a multiple-T maze. Arch Ital Biol. 2002;140(4):295–301. [PubMed] [Google Scholar]
- Schmitzer-Torbert N, Redish AD. Neuronal activity in the rodent dorsal striatum in sequential navigation: separation of spatial and reward responses on the multiple T task. J Neurophysiol. 2004;91(5):2259–72. doi: 10.1152/jn.00687.2003. [DOI] [PubMed] [Google Scholar]
- Schultz W, Apicella P, et al., editors. Models of Information Processing in the Basal Ganglia. Cambridge, MA: MIT Press; 1995. [Google Scholar]
- Schultz W, Romo R. Neuronal activity in the monkey striatum during the initiation of movements. Exp Brain Res. 1988;71(2):431–6. doi: 10.1007/BF00247503. [DOI] [PubMed] [Google Scholar]
- Shimo Y, Hikosaka O. Role of tonically active neurons in primate caudate in reward-oriented saccadic eye movement. J Neurosci. 2001;21(19):7804–14. doi: 10.1523/JNEUROSCI.21-19-07804.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinomoto S, Miyazaki Y, et al. Regional and laminar differences in in vivo firing patterns of primate cortical neurons. Journal of Neurophysiology. 2005;94:567–575. doi: 10.1152/jn.00896.2004. [DOI] [PubMed] [Google Scholar]
- Shinomoto S, Shima K, et al. Differences in spiking patterns among cortical neurons. Neural Computation. 2003;15:2823–2842. doi: 10.1162/089976603322518759. [DOI] [PubMed] [Google Scholar]
- Swanson LW. Cerebral hemisphere regulation of motivated behavior. Brain Res. 2000;886(1–2):113–164. doi: 10.1016/s0006-8993(00)02905-x. [DOI] [PubMed] [Google Scholar]
- Teagarden MA, Rebec GV. Subthalamic and striatal neurons concurrently process motor, limbic, and associative information in rats performing an operant task. J Neurophysiol. 2007;97(3):2042–58. doi: 10.1152/jn.00368.2006. [DOI] [PubMed] [Google Scholar]
- Tolkunov BF, Orlov AA, et al. Involvement of striatum (putamen) neurons in motor and nonmotor behavior fragments in monkeys. Neurosci Behav Physiol. 1998;28(3):224–30. doi: 10.1007/BF02462950. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Lauwereyns J, et al. Neural correlates of rewarded and unrewarded eye movements in the primate caudate nucleus. J Neurosci. 2003;23(31):10052–7. doi: 10.1523/JNEUROSCI.23-31-10052.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MO, Carelli RM, et al. A region in the dorsolateral striatum of the rat exhibiting single-unit correlations with specific locomotor limb movements. J Neurophysiol. 1990;64(4):1233–46. doi: 10.1152/jn.1990.64.4.1233. [DOI] [PubMed] [Google Scholar]
- White IM, Rebec GV. Responses of rat striatal neurons during performance of a lever-release version of the conditioned avoidance response task. Brain Res. 1993;616(1–2):71–82. doi: 10.1016/0006-8993(93)90194-r. [DOI] [PubMed] [Google Scholar]
- Wiener SI. Spatial and behavioral correlates of striatal neurons in rats performing a self-initiated navigation task. J Neurosci. 1993;13(9):3802–17. doi: 10.1523/JNEUROSCI.13-09-03802.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeshenko O, Guazzelli A, et al. Context-dependent reorganization of spatial and movement representations by simultaneously recorded hippocampal and striatal neurons during performance of allocentric and egocentric tasks. Behav Neurosci. 2004;118(4):751–69. doi: 10.1037/0735-7044.118.4.751. [DOI] [PubMed] [Google Scholar]
- Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nature Reviews Neuroscience. 2006;7:464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- Zhang K, Ginzburg I, et al. Interpreting neuronal population activity by reconstruction: Unified framework with application to hippocampal place cells. Journal of Neurophysiology. 1998;79:1017–1044. doi: 10.1152/jn.1998.79.2.1017. [DOI] [PubMed] [Google Scholar]