Abstract
Background
Combining bronchodilators with different mechanisms of action may improve efficacy and reduce risk of side effects compared to increasing the dose of a single agent in chronic obstructive pulmonary disease (COPD). We investigated this by combining two long-acting bronchodilators: once-daily muscarinic antagonist tiotropium and once-daily β2-agonist olodaterol.
Methods
Two replicate, double-blind, randomized, 12-week studies (ANHELTO 1 [NCT01694771] and ANHELTO 2 [NCT01696058]) evaluated the efficacy and safety of olodaterol 5 μg once daily (via Respimat®) combined with tiotropium 18 μg once daily (via HandiHaler®) versus tiotropium 18 μg once daily (via HandiHaler®) combined with placebo (via Respimat®) in patients with moderate to severe COPD. Primary efficacy end points were area under the curve from 0–3 hours of forced expiratory volume in 1 second (FEV1 AUC0–3) and trough FEV1 after 12 weeks (for the individual trials). A key secondary end point was health status by St George’s Respiratory Questionnaire (SGRQ) total score (combined data set).
Results
Olodaterol + tiotropium resulted in significant improvements over tiotropium + placebo in FEV1 AUC0–3 (treatment differences: 0.117 L [P<0.001], ANHELTO 1; 0.106 L [P<0.001], ANHELTO 2) and trough FEV1 (treatment differences: 0.062 L [P<0.001], ANHELTO 1; 0.040 L [P=0.0029], ANHELTO 2); these were supported by secondary end points. These effects translated to improvements in SGRQ total scores (treatment difference −1.85; P<0.0001). The tolerability profile of olodaterol + tiotropium was similar to tiotropium monotherapy.
Conclusion
These studies demonstrated that olodaterol (Respimat®) and tiotropium (HandiHaler®) provided bronchodilatory effects above tiotropium alone in patients with COPD. In general, both treatments were well tolerated.
Keywords: bronchodilator, long-acting beta2-agonist, long-acting muscarinic antagonist, olodaterol Respimat®, tiotropium HandiHaler®
Introduction
Chronic obstructive pulmonary disease (COPD) currently affects more than 5% of the adult population;1 it is the fourth leading cause of death in the US.2 Bronchodilators are central to symptom management in this disease, with long-acting muscarinic antagonists (LAMAs) and long-acting β2-agonists (LABAs) being established maintenance therapies, as supported by national and international recommendations.1,3
The once-daily inhaled LAMA tiotropium has been demonstrated to provide improvement in airflow limitation, reduce lung hyperinflation, and increase exercise tolerance in COPD. Furthermore, long-term data demonstrate benefits in reducing the rate of exacerbations and related hospitalizations.4–8 The once-daily LABA olodaterol has recently been evaluated in a Phase III clinical trial program; full results of this are yet to be communicated.9–12 In the clinical development program for olodaterol, the device system used was the Respimat® Soft Mist™ inhaler (Boehringer Ingelheim GmbH and Co. KG, Ingelheim, Germany). Tiotropium is also available in several countries via the Respimat® but in the US it is currently only approved for administration as a dry powder through the HandiHaler® dry powder inhaler device (Boehringer Ingelheim GmbH and Co. KG).
For patients with COPD who remain symptomatic despite monotherapy, international guidelines indicate that combining bronchodilators with different mechanisms of action may improve efficacy and reduce the risk of side effects compared to increasing the dose of the single agent.3,8,13 The two 12-week studies in our report, conducted across multiple centers in the US, evaluated the efficacy and safety of using olodaterol 5 μg once daily (via Respimat®) in combination with tiotropium 18 μg once daily (via HandiHaler®) compared to tiotropium 18 μg once daily (via HandiHaler®) in combination with placebo (via Respi-mat®) in patients with moderate to severe COPD. These are the first randomized, double-blind studies evaluating the regular use of this combination compared to the established monotherapy with tiotropium (HandiHaler®) in patients with COPD.
Methods
Study design
ANHELTO 1 (Study 1222.51)14 and ANHELTO 2 (Study 1222.52)15 were replicate, 12-week, randomized, double-blind, parallel-group, multicenter trials to assess the efficacy and safety of 12 weeks of once-daily coadministration of tiotropium 18 μg (via the HandiHaler®) and olodaterol 5 μg (via the Respimat® inhaler) compared to once-daily coadministration of tiotropium 18 μg (via the HandiHaler®) and placebo (via the Respimat® inhaler). The studies were registered with Clinical-Trials.gov, identifying numbers NCT01694771 (ANHELTO 1) and NCT01696058 (ANHELTO 2).
Randomization
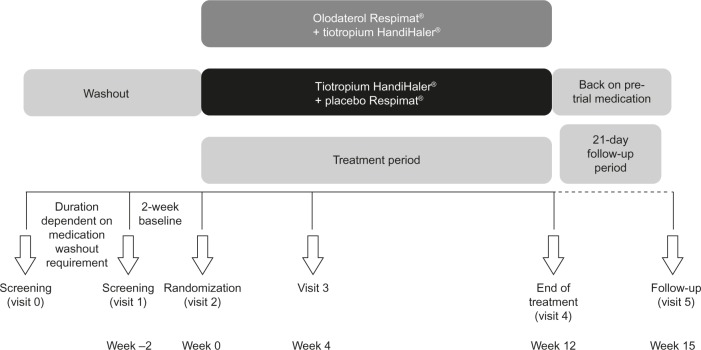
Patients who met the inclusion and exclusion criteria were randomized to one of the two treatment groups for 12 weeks (Figure 1) and followed up for 3 weeks after the last dose of study medication.
Figure 1.
Design of ANHELTO 1 and ANHELTO 2.
Each morning during the randomized treatment period, patients took two inhalations from the assigned Respimat® inhaler followed by two inhalations of one capsule of tiotropium dry powder via the HandiHaler®. Patients were requested to take study medication at the same time each morning between 7 am and 10 am.
Procedures
During the screening and 12-week treatment periods, patients were not permitted to take concurrent inhaled corticosteroids (ICS) in fixed combination with LABA, ICS/short-acting β-agonists, short-acting muscarinic antagonist/short-acting β-agonist combinations, or phosphodiesterase Type 4 inhibitors. ICS, oral (≤10 mg prednisone per day, or equivalent) and injected steroids, cromolyn sodium/nedocromil sodium, antihistamines, antileukotrienes, methylxanthines, mucolytics, and theophyllines were permitted. Albuterol was provided as rescue medication only.
The study was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Tripartite Guideline for Good Clinical Practice. Before the studies started, the protocol was reviewed and approved by the following Institutional Review Boards: Chesapeak Research Review, Inc, Columbia, MD; University of Nevada, Reno Office of Human Research Protection, Reno, NV; Christina Care, Newark, DE; Mercy St Vincent Medical Center Adult IRB, Toledo, OH; UCSD Human Research, La Jolla, CA; UCLA Torrence Danial Griffen School of Medicine, Torrence, CA; Baylor College of Medicine, Houston, TX; LSU Health Services IRB, New Orleans, LA; Saint Francis and Medical Center IRB, Hartford, CT, USA. All patients were required to provide written, informed consent.
Patients
Patients with COPD were eligible for the studies if they had post-bronchodilator forced expiratory volume in 1 second (FEV1) greater than or equal to 30% and less than 80% of predicted normal and post-bronchodilator FEV1/forced vital capacity (FVC) less than 70% (Global initiative for chronic Obstructive Lung Disease 2–3).3,13 Patients were at least 40 years of age and current or former smokers with a smoking history of more than 10 pack-years. The key exclusion criteria included significant disease other than COPD (ie, a disease that, in the opinion of the investigator, may put the patient at risk because of participation in the study, may influence the results of the study, or may affect the patient’s ability to participate in the study), a history of asthma, thyrotoxicosis, paroxysmal tachycardia, unstable or life-threatening cardiac arrhythmia, myocardial infarction within the previous year, or hospitalization for heart failure within the past year. Patients who regularly used daytime oxygen therapy for more than 1 hour per day and, in the investigator’s opinion, were unable to abstain from its use during clinic visits were excluded, as were those who were either currently on a pulmonary rehabilitation program or who had completed such a program in the previous 6 weeks.
End points
The co-primary efficacy end points were: area under the curve from 0–3 hours (AUC0–3) of FEV1; and trough FEV1 responses (ie, change from baseline) at 12 weeks of treatment. St George’s Respiratory Questionnaire (SGRQ) total score, a measure of respiratory-specific health status, at week 12 was identified as a key secondary efficacy end point for the combined data set. Other secondary efficacy end points were peak FEV1, FVC AUC0–3, peak and trough FVC responses at 12 weeks, and rescue medication use over the 12-week treatment period. The safety end points included adverse events (AEs), serious AEs, vital signs, blood chemistry, and electrocardiogram.
Assessments
Spirometry was conducted at study entry to determine patient eligibility. Testing was also performed at randomization and after 4 and 12 weeks of treatment at the following time points: 1 hour pre-dose, 10 minutes pre-dose, and at less than or equal to 3 hours post-dose (5, 15, and 30 minutes, and 1, 2, and 3 hours post-dose). Two additional measurements were conducted after 12 weeks (at 23:00 and 23:50 hours post-dose) in order to capture trough FEV1.
Vital signs were measured in conjunction with pulmonary function tests pre-dose and 1 hour post-dose at all visits during the randomized treatment period. SGRQ was administered at randomization and at the completion of 12 weeks of treatment (or at early discontinuation).
At each visit, all AEs reported by the patient were recorded, irrespective of causality. Blood pressure and pulse rate were measured prior to spirometry at all visits. Clinical laboratory testing (hematology, blood chemistry, and urinalysis) was performed at screening, after the treatment period, and in case of premature withdrawal from the study. A standard 12-lead electrocardiogram was performed at screening for all patients and at the end of randomized treatment.
Statistical analysis
It was estimated that with 509 treated patients, each study would be able to detect treatment differences of 0.046 L for FEV1 AUC0–3 response (standard deviation 0.226 L) with 90% power, and 0.046 L for trough FEV1 response (standard deviation 0.225 L) with 92% power. In each study, 560 patients were to be randomized for each treatment group, with a total of 1,120 patients. Such a sample size would provide 92% power.
FEV1 AUC0–3 and trough FEV1 responses (ie, change from baseline) at week 12 for each study were analyzed using a restricted maximum likelihood-based mixed model for repeated measurements (MMRM). Analyses included the fixed, categorical effects of treatment, visit, and treatment-by-visit interaction, as well as the continuous, fixed covariates of baseline and baseline-by-visit interaction. An unstructured (co)variance structure was used to model the within-patient errors.
SGRQ total score at week 12 was analyzed using MMRM as described in the primary analysis. The SGRQ analysis was based on the combined data from both studies. Descriptive statistics were provided for the other secondary end points. No formal statistical analysis was planned for the safety comparisons; summary statistics are presented.
All efficacy and safety analyses were based on the treated set (ie, all randomized patients who received at least one dose of double-blind study treatment).
Results
Patient disposition and baseline characteristics
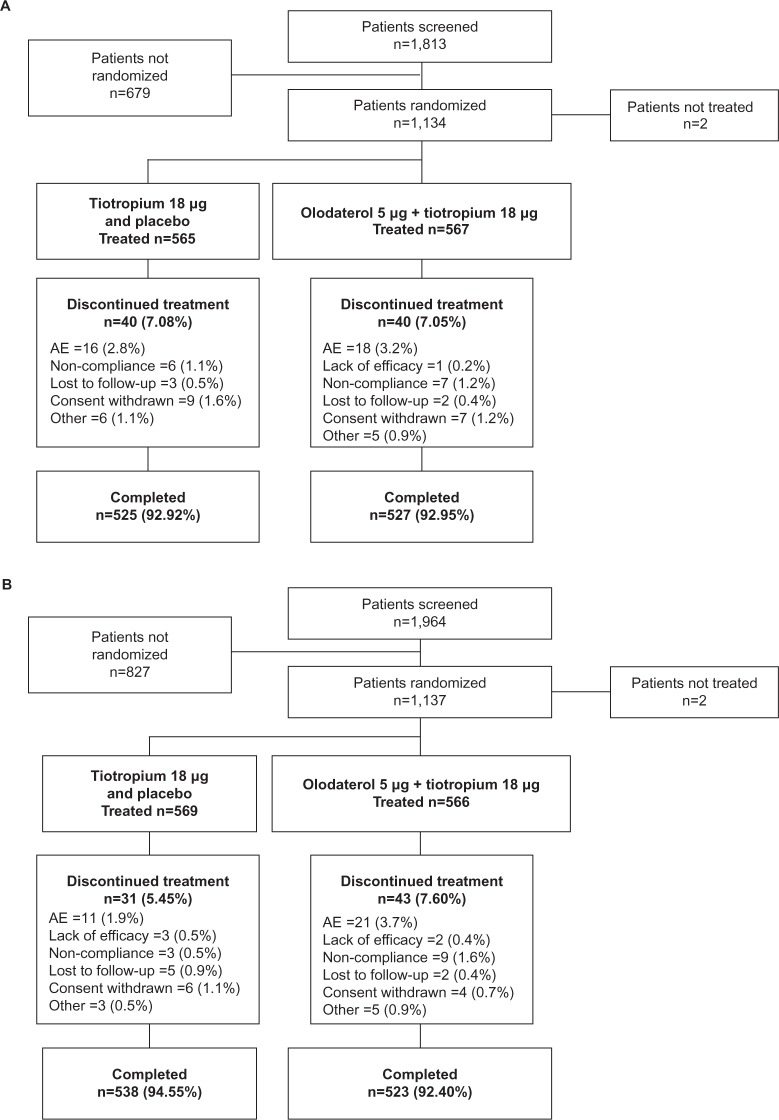
A total of 2,267 patients were randomized and received treatment in the two studies: 1,132 in ANHELTO 1 and 1,135 in ANHELTO 2 (Figure 2). The majority of patients completed treatment (93% in both studies), with a similar incidence of discontinuation across the treatment groups. AEs were the most frequent reason for discontinuation.
Figure 2.
Patient disposition (A) ANHELTO 1; (B) ANHELTO 2.
Abbreviation: AE, adverse event.
The baseline demographic characteristics of patients in the two studies are shown in Table 1. The percentage change in pre- to post-FEV1 at baseline was 18.9% in ANHELTO 1 and 17.3% in ANHELTO 2; 50.2% and 46.4%, respectively, were female. The characteristics were generally well balanced across treatment groups, although mean duration of COPD was slightly longer in the olodaterol + tiotropium group and disease severity was slightly worse in the tiotropium + placebo group in both studies. The mean (standard deviation) post- bronchodilator percentage of predicted normal FEV1 was 54.0 (13.0) in ANHELTO 1 and 53.3 (13.8) in ANHELTO 2.
Table 1.
Baseline patient demographics
| ANHELTO 1
|
ANHELTO 2
|
|||
|---|---|---|---|---|
| Tiotropium + placebo (n=565) | Olodaterol + tiotropium (n=567) | Tiotropium + placebo (n=569) | Olodaterol + tiotropium (n=566) | |
| Male, n (%) | 285 (50.4) | 279 (49.2) | 303 (53.3) | 305 (53.9) |
| Age, mean (SD), years | 64.8 (9.1) | 64.3 (9.1) | 63.6 (8.9) | 64.6 (9.0) |
| COPD diagnosis, mean (SD), years | 7.9 (6.1) | 8.5 (7.5) | 7.1 (6.3) | 8.2 (7.2) |
| Pre-bronchodilator | ||||
| FEV1, mean (SD), L | 1.251 (0.502) | 1.248 (0.490) | 1.254 (0.514) | 1.266 (0.480) |
| Post-bronchodilator | ||||
| FEV1, mean (SD), L | 1.450 (0.528) | 1.453 (0.501) | 1.442 (0.528) | 1.451 (0.497) |
| FEV1 change from pre-bronchodilator, mean (SD), L | 0.200 (0.159) | 0.205 (0.152) | 0.188 (0.166) | 0.185 (0.188) |
| FEV1/FVC, mean (SD), % | 50.1 (10.5) | 50.0 (10.7) | 50.0 (10.9) | 50.2 (10.6) |
| % of predicted normal FEV1, mean (SD) | 53.9 (13.0) | 54.2 (13.0) | 53.0 (13.9) | 53.6 (13.6) |
| GOLD, n (%) | ||||
| 1 | 0 | 0 | 0 | 0 |
| 2 | 338 (59.8) | 343 (60.5) | 317 (55.7) | 338 (59.7) |
| 3 | 227 (40.2) | 223 (39.3) | 252 (44.3) | 227 (40.1) |
| 4 | 0 | 1 (0.2) | 0 | 1 (0.2) |
| Body mass index, mean (SD), kg/m2 | 28.1 (6.8) | 28.7 (6.4) | 28.5 (6.7) | 28.3 (6.4) |
| Current smoker, n (%) | 295 (52.2) | 282 (49.7) | 274 (48.2) | 259 (45.8) |
| Smoking history, mean (SD), pack-years | 52.7 (27.1) | 54.0 (25.4) | 51.4 (26.8) | 53.9 (28.1) |
| Baseline pulmonary medications, n (%) | ||||
| Any pulmonary medications | 406 (71.9) | 399 (70.4) | 401 (70.5) | 421 (74.4) |
| SAMAa | 53 (9.4) | 49 (8.6) | 36 (6.3) | 45 (8.0) |
| LAMAa,b | 129 (22.8) | 134 (23.6) | 173 (30.4) | 158 (27.9) |
| LABAa,b | 179 (31.7) | 152 (26.8) | 183 (32.2) | 170 (30.0) |
| β-adrenergics (oral)a | 3 (0.5) | 1 (0.2) | 3 (0.5) | 1 (0.2) |
| SABAc | 257 (45.5) | 255 (45.0) | 272 (47.8) | 275 (48.6) |
| Leukotriene receptor antagonistsd | 16 (2.8) | 21 (3.7) | 18 (3.2) | 21 (3.7) |
| Mucolyticsd | 1 (0.2) | 1 (0.2) | 0 | 0 |
| Oxygen | 38 (6.7) | 42 (7.4) | 32 (5.6) | 40 (7.1) |
| Steroidsd | ||||
| Inhaled | 214 (37.9) | 203 (35.8) | 215 (37.8) | 213 (37.6) |
| Oral | 11 (1.9) | 9 (1.6) | 9 (1.6) | 6 (1.1) |
| Xanthinesd | 4 (0.7) | 1 (0.2) | 1 (0.2) | 10 (1.8) |
Notes:
Not permitted during treatment period
patients switched to study medication during treatment period
all patients were provided with SABA as rescue medication during this study
permitted during treatment period.
Abbreviations: SD, standard deviation; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GOLD, Global initiative for chronic Obstructive Lung Disease; SAMA, short-acting muscarinic antagonist; LAMA, long-acting muscarinic antagonist; LABA, long-acting β2-agonist; SABA, short-acting β2-agonist.
Efficacy
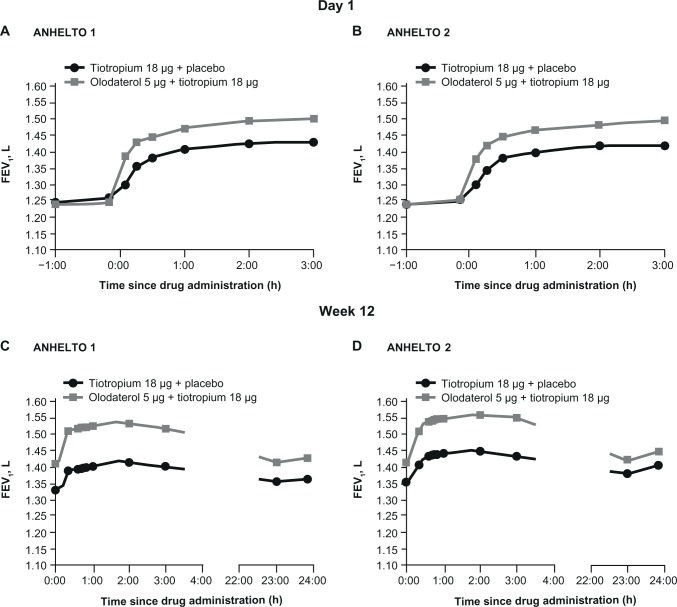
The FEV1 profiles at day 1 and week 12 are presented in Figure 3 for each study.
Figure 3.
FEV1 on day 1 in (A) ANHELTO 1; (B) ANHELTO 2, and at week 12 in (C) ANHELTO 1; (D) ANHELTO 2.
Abbreviations: FEV1, forced expiratory volume in 1 second; h, hours.
Primary end points
Improvements in FEV1 AUC0–3 and trough FEV1 responses were greater with olodaterol + tiotropium than tiotropium + placebo at week 12 in each study (Table 2; Figures 4 and 5). Both studies reached statistical significance (P<0.01) for each of the primary end points.
Table 2.
FEV1 AUC0–3 and trough FEV1 responses (L) after 12 weeks
| Trial/treatment | Change from baseline
|
Mean difference from tiotropium + placebo
|
|||
|---|---|---|---|---|---|
| n | Mean (SE), L | Mean (SE), L | P-value | 95% CI | |
| FEV1AUC0–3 | |||||
| ANHELTO 1 | |||||
| Tiotropium + placebo | 564 | 0.196 (0.010) | |||
| Olodaterol + tiotropium | 563 | 0.313 (0.010) | 0.117 (0.014) | <0.0001 | 0.090, 0.144 |
| ANHELTO 2 | |||||
| Tiotropium + placebo | 566 | 0.191 (0.010) | |||
| Olodaterol + tiotropium | 563 | 0.297 (0.010) | 0.106 (0.014) | <0.0001 | 0.078, 0.135 |
| Trough FEV1 | |||||
| ANHELTO 1 | |||||
| Tiotropium + placebo | 551 | 0.133 (0.009) | |||
| Olodaterol + tiotropium | 548 | 0.195 (0.009) | 0.062 (0.013) | <0.0001 | 0.037, 0.088 |
| ANHELTO 2 | |||||
| Tiotropium + placebo | 555 | 0.135 (0.009) | |||
| Olodaterol + tiotropium | 550 | 0.175 (0.009) | 0.040 (0.013) | 0.0029 | 0.014, 0.065 |
Abbreviations: FEV1, forced expiratory volume in 1 second; AUC0–3, area under the curve from 0–3 hours; SE, standard error; CI, confidence interval.
Figure 4.
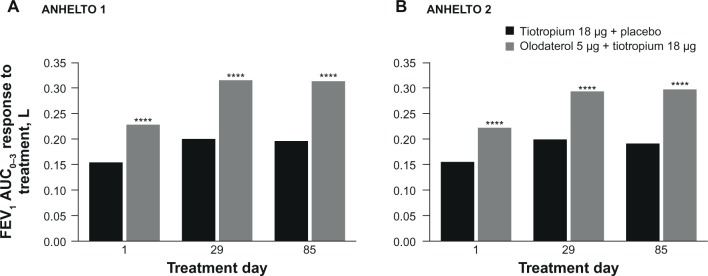
FEV1 AUC0–3 response to treatment in (A) ANHELTO 1; (B) ANHELTO 2.
Notes: ****P<0.0001 (olodaterol + tiotropium versus tiotropium + placebo); n=1,132 (ANHELTO 1), n=1,135 (ANHELTO 2).
Abbreviation: FEV1 AUC0–3, area under the curve from 0–3 hours of forced expiratory volume in 1 second.
Figure 5.
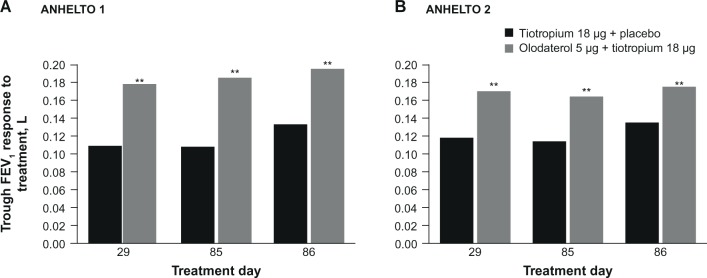
Trough FEV1 response to treatment in (A) ANHELTO 1; (B) ANHELTO 2.
Notes: **P<0.01 (olodaterol + tiotropium versus tiotropium + placebo); n=1,132 (ANHELTO 1), n=1,135 (ANHELTO 2).
Abbreviation: FEV1, forced expiratory volume in 1 second.
Key secondary end point: SGRQ pooled analysis
At week 12, SGRQ total scores were statistically significantly lower (ie, improved) with olodaterol + tiotropium than with tiotropium + placebo; mean (95% confidence interval) difference between groups was –1.85 (–2.757, –0.951; P<0.0001). This improvement did not meet the threshold (ie, 4 units) established as a clinically meaningful change with this instrument.16 Results of the SGRQ scores are presented in Table 3.
Table 3.
SGRQ sub-scale scores at 12 weeks
| Treatment | Treatment
|
Mean difference from tiotropium + placeboa
|
||
|---|---|---|---|---|
| n | Mean (SE) | Mean (SE) | 95% CI | |
| SGRQ total scoreb | ||||
| Tiotropium + placebo | 1,055 | 43.1 (0.33)c | ||
| Olodaterol + tiotropium | 1,039 | 41.2 (0.33)c | −1.9 (0.46)**** | −2.8, −1.0 |
| SGRQ symptom score | ||||
| Tiotropium + placebo | 1,059 | 51.4 (0.53) | ||
| Olodaterol + tiotropium | 1,043 | 47.6 (0.53) | −3.8 (0.75) | −5.2, –2.3 |
| SGRQ activity score | ||||
| Tiotropium + placebo | 1,061 | 60.5 (0.42) | ||
| Olodaterol + tiotropium | 1,046 | 58.7 (0.42) | −1.8 (0.59) | −2.9, −0.6 |
| SGRQ impact score | ||||
| Tiotropium + placebo | 1,060 | 30.5 (0.35) | ||
| Olodaterol + tiotropium | 1,041 | 29.3 (0.36) | −1.1 (0.50) | −2.1, −0.2 |
Notes:
A decrease in score reflects an improvement in health status
baseline SGRQ scores were 46.97 for tiotropium + placebo (n=1,123) and 47.68 for olodaterol + tiotropium (n=1,123)
the SGRQ total score change from baseline to week 12 was –4.128 units for tiotropium + placebo and –5.982 units for tiotropium + olodaterol.
P<0.0001.
Abbreviations: SGRQ, St George’s Respiratory Questionnaire; SE, standard error; CI, confidence interval.
A post hoc responder analysis was conducted on the SGRQ data after 12 weeks. 556 (49.3%) patients receiving olodaterol + tiotropium and 480 (42.5%) patients receiving tiotropium + placebo had an SGRQ improvement of ≥4 units.
Other secondary end points
Results of the other secondary end points are presented in Table 4. These results support results of the primary end points, with improvements in peak FEV1, FVC AUC0–3, and peak and trough FVC at week 12. Responses were numerically higher in ANHELTO 1 than ANHELTO 2.
Table 4.
Secondary efficacy variable responses (L) after 12 weeks
| Trial/treatment | Change from baseline
|
Mean difference from tiotropium + placebo
|
||
|---|---|---|---|---|
| n | Mean (SE), L | Mean (SE), L | 95% CI | |
| Peak FEV1 | ||||
| ANHELTO 1 | ||||
| Tiotropium + placebo | 564 | 0.270 (0.010) | ||
| Olodaterol + tiotropium | 563 | 0.389 (0.010) | 0.119 (0.014) | 0.090, 0.147 |
| ANHELTO 2 | ||||
| Tiotropium + placebo | 566 | 0.271 (0.011) | ||
| Olodaterol + tiotropium | 563 | 0.371 (0.011) | 0.100 (0.015) | 0.071, 0.129 |
| FVC AUC0–3 | ||||
| ANHELTO 1 | ||||
| Tiotropium + placebo | 564 | 0.292 (0.017) | ||
| Olodaterol + tiotropium | 563 | 0.438 (0.017) | 0.146 (0.024) | 0.100, 0.192 |
| ANHELTO 2 | ||||
| Tiotropium + placebo | 566 | 0.306 (0.017) | ||
| Olodaterol + tiotropium | 563 | 0.424 (0.017) | 0.118 (0.023) | 0.072, 0.164 |
| Peak FVC | ||||
| ANHELTO 1 | ||||
| Tiotropium + placebo | 564 | 0.433 (0.017) | ||
| Olodaterol + tiotropium | 563 | 0.586 (0.017) | 0.153 (0.024) | 0.106, 0.201 |
| ANHELTO 2 | ||||
| Tiotropium + placebo | 566 | 0.457 (0.017) | ||
| Olodaterol + tiotropium | 563 | 0.562 (0.017) | 0.104 (0.024) | 0.057, 0.152 |
| Trough FVC | ||||
| ANHELTO 1 | ||||
| Tiotropium + placebo | 551 | 0.213 (0.016) | ||
| Olodaterol + tiotropium | 548 | 0.276 (0.016) | 0.063 (0.022) | 0.019, 0.106 |
| ANHELTO 2 | ||||
| Tiotropium + placebo | 555 | 0.235 (0.015) | ||
| Olodaterol + tiotropium | 550 | 0.269 (0.015) | 0.034 (0.022) | −0.008, 0.076 |
Abbreviations: CI, confidence interval; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; AUC0–3, area under the curve from 0–3 hours; SE, standard error.
Rescue medication usage was lower with olodaterol + tiotropium than with tiotropium + placebo, with a greater number of days free of rescue medication during treatment with the combination (Table 5).
Table 5.
Mean percentage rescue medication-free days at week 12
| Trial/treatment | Treatment
|
Mean difference from tiotropium + placebo
|
||
|---|---|---|---|---|
| n | Mean (SE) | Mean (SE) | 95% CI | |
| ANHELTO 1 | ||||
| Tiotropium + placebo | 561 | 54.9 (1.55) | ||
| Olodaterol + tiotropium | 557 | 63.4 (1.55) | 8.5 (2.19) | 4.2, 12.8 |
| ANHELTO 2 | ||||
| Tiotropium + placebo | 559 | 55.1 (1.51) | ||
| Olodaterol + tiotropium | 552 | 62.3 (1.53) | 7.2 (2.15) | 3.0, 11.4 |
Abbreviations: SE, standard error; CI, confidence interval.
Safety
Incidence of AEs was similar across treatment groups in both studies (Table 6). The most frequent events across the two studies were worsening of COPD (10.7%) and dry mouth (2.7%). Most events were mild to moderate in intensity and not considered related to study treatment. Incidence of AEs was considered to be low in both studies. In ANHELTO 1, there were more serious AEs with olodaterol + tiotropium (7.1%) than tiotropium + placebo (4.6%); however, in ANHELTO 2, the incidence of serious AEs was well balanced across treatments (4.2% and 4.7%, respectively).
Table 6.
Summary of AEs
| ANHELTO 1
|
ANHELTO 2
|
|||
|---|---|---|---|---|
| Tiotropium + placebo (n=565) | Olodaterol + tiotropium (n=567) | Tiotropium + placebo (n=569) | Olodaterol + tiotropium (n=566) | |
| Any AE | 242 (42.8) | 257 (45.3) | 246 (43.2) | 227 (40.1) |
| Serious AE | 26 (4.6) | 40 (7.1) | 27 (4.7) | 24 (4.2) |
| AE leading to death | ||||
| During treatment | 0 | 2 (0.4)a | 1 (0.2) | 1 (0.2) |
| During 21-day follow-up | 1 (0.2) | 5 (0.9)a,b | 1 (0.2) | 2 (0.4) |
| After 21-day follow-up | 1 (0.2) | 0 | 0 | 0 |
| AE preferred term occurring with an incidence of ≥1% in any group | ||||
| Upper respiratory tract infection | 10 (1.8) | 6 (1.1) | 13 (2.3) | 10 (1.8) |
| Bronchitis | 7 (1.2) | 11 (1.9) | 11 (1.9) | 4 (0.7) |
| Sinusitis | 7 (1.2) | 12 (2.1) | 9 (1.6) | 6 (1.1) |
| Nasopharyngitis | 14 (2.5) | 14 (2.5) | 8 (1.4) | 4 (0.7) |
| Influenza | 5 (0.9) | 7 (1.2) | 2 (0.4) | 3 (0.5) |
| Urinary tract infection | 7 (1.2) | 6 (1.1) | 8 (1.4) | 4 (0.7) |
| Candidiasis | 1 (0.2) | 3 (0.5) | 6 (1.1) | 4 (0.7) |
| Anemia | 2 (0.4) | 0 | 6 (1.1) | 1 (0.2) |
| Headache | 10 (1.8) | 8 (1.4) | 7 (1.2) | 6 (1.1) |
| Dizziness | 1 (0.2) | 7 (1.2) | 6 (1.1) | 3 (0.5) |
| Hypertension | 2 (0.4) | 5 (0.9) | 4 (0.7) | 6 (1.1) |
| COPD | 61 (10.8) | 74 (13.1) | 55 (9.7) | 52 (9.2) |
| Cough | 17 (3.0) | 9 (1.6) | 11 (1.9) | 8 (1.4) |
| Dysphonia | 5 (0.9) | 3 (0.5) | 7 (1.2) | 2 (0.4) |
| Dyspnea | 11 (1.9) | 6 (1.1) | 6 (1.1) | 4 (0.7) |
| Dry mouth | 15 (2.7) | 16 (2.8) | 14 (2.5) | 16 (2.8) |
| Diarrhea | 10 (1.8) | 7 (1.2) | 10 (1.8) | 4 (0.7) |
| Constipation | 6 (1.1) | 8 (1.4) | 7 (1.2) | 3 (0.5) |
| Vomiting | 3 (0.5) | 8 (1.4) | 5 (0.9) | 5 (0.9) |
| Nausea | 3 (0.5) | 5 (0.9) | 3 (0.5) | 6 (1.1) |
| Arthralgia | 2 (0.4) | 5 (0.9) | 7 (1.2) | 1 (0.2) |
| Back pain | 2 (0.4) | 6 (1.1) | 7 (1.2) | 6 (1.1) |
| Muscle spasms | 3 (0.5) | 5 (0.9) | 4 (0.7) | 6 (1.1) |
| Chest pain | 4 (0.7) | 4 (0.7) | 7 (1.2) | 4 (0.7) |
Notes:
One patient had two AEs identified as leading to death: COPD exacerbation was cited during treatment and acute respiratory failure during 21-day washout
one patient had two AES leading to death: myocardial infarction and arteriosclerosis.
Abbreviations: AE, adverse event; COPD, chronic obstructive pulmonary disease.
The incidence of AEs leading to death is presented in Table 6. A total of 12 deaths were reported in the two studies: four with tiotropium + placebo and eight with olodaterol + tiotropium. Three occurred during the randomized treatment period: one in the tiotropium + placebo group (cause not defined by treating physician) and two in the olodaterol + tiotropium group (myocardial ischemia and toxicity to cocaine with contributory oxycodone toxicity). The remaining deaths occurred after discontinuation of study treatment: eight occurred during the 21-day post-treatment follow-up (two with tiotropium + placebo [myocardial infarction and convulsions] and six with olodaterol + tiotropium [hemorrhagic cerebral infarction, myocardial infarction/arteriosclerosis, acute respiratory failure (COPD exacerbation was also cited that started during randomized treatment), COPD, cardiac arrest, and sudden death]); one death occurred after the 21-day post-treatment follow-up (tiotropium + placebo [subarachnoid hemorrhage]).
No changes in laboratory parameters, vital signs, or electrocardiogram were indicative of a safety signal.
Discussion
The results of these studies demonstrate that combining olodaterol Respimat® and tiotropium HandiHaler® provided additional improvements in lung function greater than with tiotropium alone in patients with COPD without an increased incidence of AEs. Multiple trials have evaluated the impact of combining the short-acting muscarinic antagonist ipratropium and β2-agonist albuterol, which led to the development of the fixed-dose combination of these agents.17 The evolution of treatment options brought the discovery of new long-acting compounds that also improve lung function and provide sustained bronchodilation when administered once daily. Tiotropium has become one of the most widely studied and used drugs in COPD, and, accordingly, new treatment options have been tested in comparison, and in combination, with this molecule.18–20 Olodaterol has been shown to provide lung-function improvements consistent with those of tiotropium.10 ANHELTO 114 and ANHELTO 215 demonstrate that combining olodaterol Respimat® and tiotropium HandiHaler® resulted in improvements in lung function greater than those achieved by the well-known and established LAMA tiotropium alone. The additional benefit of the olodaterol Respimat® and tiotropium HandiHaler® once-daily LABA and LAMA combination was indicated by effects on the co-primary end points (FEV1 AUC0–3 and trough FEV1). The results of the primary efficacy end points were supported by the secondary spirometry end points, including peak FEV1, FVC AUC0–3, and peak and trough FVC. These beneficial effects were achieved without a concomitant increase in incidence of AEs.
Previously, in a similar set of two 12-week studies, patients with moderate to severe COPD were randomized to treatment with indacaterol (150 μg once daily) or matching placebo; all patients received concurrent open-label tiotropium 18 μg once daily.21 These trials also demonstrated the benefit of combining tiotropium with a LABA in terms of lung function. However, the assessment of lung function (FEV1 AUC from 5 minutes to 8 hours post-dose at week 12) cannot be compared directly with the outcome of the ANHELTO trials due to the difference of the time interval assessed and the study design (these were placebo-controlled studies with open-label use of tiotropium in both arms).
Measuring both the trough FEV1 and the FEV1 AUC0–3 is a reasonable way to assess once-daily bronchodilator effectiveness: the trough measurement of FEV1 determines the extent of bronchodilation at the end of the 24-hour dosing interval while FEV1 AUC0–3 provides a close description of the peak effects.
The within-group changes from baseline in trough FEV1 exceeded the threshold of 100 mL with both olodaterol + tiotropium and tiotropium + placebo, which is generally considered as a clinically relevant difference.16 However, the between-treatment group difference in change from baseline in trough FEV1 was below the 100 mL threshold; this suggests that the incremental improvement from adding a second treatment on top of an effective monotherapy cannot be expected to be as large as the difference between the active treatment and placebo.16 Overall, these results, together with the FEV1 time profiles, show that significant additional bronchodilation can be consistently achieved throughout a once-daily dosing interval with olodaterol + tiotropium. Since symptoms of COPD are often worse in the morning, the sustained bronchodilation over the 24-hour period should be beneficial in this respect.22
The beneficial effects on lung function appeared to be translated into benefits for the respiratory-specific health status of the patients assessed in these studies, with statistically significant improvements in SGRQ total scores on top of improvements achieved with tiotropium alone. These studies may not have had sufficient duration to see the full effects on respiratory-specific health status; the literature reports that the largest effects in SGRQ typically occur after approximately 6 months of treatment,16 possibly due in part to a reduction in the frequency of exacerbations that may be observed over this time period. Previous trials of tiotropium in combination with another LABA (indacaterol) did not report on SGRQ.21
Some differences were observed in the magnitude of responses in the two studies, which may be due to differences between individuals as well as in the demographic characteristics of populations on entry to the studies. Given that they were designed as replicate studies, it is likely that these reflect random observed phenomena.
Studies have recently been conducted testing other combinations of LAMAs and LABAs, both in free combination21 and as a fixed-dose combination.23–25 These studies have shown benefits of the treatment combinations on lung function and other parameters; however, due to differences in patient populations and study designs, direct comparisons in terms of measurement of airflow improvements are difficult to undertake.
While the treatments used in the ANHELTO studies were generally well tolerated, there appear to be small, inexplicable imbalances in the incidence of serious AEs and deaths with olodaterol + tiotropium compared to tiotropium + placebo in ANHELTO 1.14 These effects were not seen in ANHELTO 215 and no such imbalances have been observed in any previous clinical trials with olodaterol. This includes data from a large clinical program with olodaterol within which a proportion of patients continued to receive background therapy with tiotropium.9–12 A large-scale clinical program has recently been completed to evaluate the efficacy and safety of the fixed-dose combination of olodaterol and tiotropium via the Respimat® device; results to date have also not indicated any safety imbalances compared with placebo (Boehringer Ingelheim; data on file 2013, 2014).
The timing of the deaths could also be an important consideration. In this regard, it is important to point out that most deaths (nine of 12) occurred after patients returned to treatment regimens they were receiving prior to entering the trials. Assumptions on causal relationships could be speculative and are difficult, especially when patients with COPD tend to have multiple comorbidities and receive several medications to control or treat these conditions. The fact that each patient is different with respect to their health status and that specific conditions can change over time are important considerations in determining the clinical view of these fatalities. The idea that comorbidity is a substantial driver of morbidity and mortality is an important concept in the management of COPD.26
It is also important to note that the incidence of COPD exacerbations was similar between treatment arms. Given the relatively short duration of the studies and the fact that they were not powered for statistical comparison of exacerbations between treatment arms, these results are not surprising. Further analyses in long-term, randomized clinical trials would be of interest to determine whether a greater reduction in exacerbations occurs with the combination of olodaterol + tiotropium on top of that previously reported with tiotropium.
Conclusion
These studies demonstrated that combining treatment with the LABA olodaterol (Respimat®) and the LAMA tiotropium (HandiHaler®) provided further bronchodilation and greater health-related quality of life effects than those achieved with tiotropium alone in the treatment of COPD. The combination provided effective and enhanced bronchodilation over the full 24-hour dosing period and was well tolerated.
Footnotes
Disclosure
Richard ZuWallack and Roger Abrahams received no compensation related to the development of the manuscript. Lisa Allen, Gemzel Hernandez, and Naitee Ting are employees of Boehringer Ingelheim Pharmaceuticals Inc. This work was supported by Boehringer Ingelheim Pharmaceuticals Inc. Medical writing assistance was provided by Rob Kite, BSc, of Complete HealthVizion, which was contracted and compensated by Boehringer Ingelheim Pharmaceuticals Inc. The authors declare no other conflicts of interest in this work.
References
- 1.Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med. 2011;155(3):179–191. doi: 10.7326/0003-4819-155-3-201108020-00008. [DOI] [PubMed] [Google Scholar]
- 2.National Heart, Lung, and Blood Institute [homepage on the Internet] NHLBI Factbook. 4. Disease Statistics [updated 2006] [Accessed March 21, 2014]. Available from: http://www.nhlbi.nih.gov/about/factbook-06/chapter4data.htm.
- 3.Global Initiative for Chronic Obstructive Lung Disease [homepage on the Internet] Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. [Accessed March 5, 2014]. Updated 2013 [updated 2013]. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf.
- 4.Casaburi R, Mahler DA, Jones PW, et al. A long-term evaluation of once-daily inhaled tiotropium in chronic obstructive pulmonary disease. Eur Respir J. 2002;19(2):217–224. doi: 10.1183/09031936.02.00269802. [DOI] [PubMed] [Google Scholar]
- 5.O’Donnell DE, Flüge T, Gerken F, et al. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. Eur Respir J. 2004;23(6):832–840. doi: 10.1183/09031936.04.00116004. [DOI] [PubMed] [Google Scholar]
- 6.Maltais F, Hamilton A, Marciniuk D, et al. Improvements in symptom-limited exercise performance over 8 h with once-daily tiotropium in patients with COPD. Chest. 2005;128(3):1168–1178. doi: 10.1378/chest.128.3.1168. [DOI] [PubMed] [Google Scholar]
- 7.Keating GM. Tiotropium bromide inhalation powder: a review of its use in the management of chronic obstructive pulmonary disease. Drugs. 2012;72(2):273–300. doi: 10.2165/11208620-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8.Tashkin DP, Celli B, Senn S, et al. A 4-year trial of tiotropium in chronic obstructive pulmonary disease. N Engl J Med. 2008;359(15):1543–1554. doi: 10.1056/NEJMoa0805800. [DOI] [PubMed] [Google Scholar]
- 9.Feldman G, Bernstein JA, Hamilton A, Nivens C, LaForce C. The 24-hour FEV1 time profile of olodaterol once daily (QD) via Respimat® and formoterol twice daily (BID) via Aerolizer® in patients with COPD: results from two 6-week studies. Chest. 2013;144(4 Meeting Abstracts):749A. (abstract) [Google Scholar]
- 10.Lange P, Aumann J-L, Derom E, et al. The 24-h FEV1 time profile of olodaterol QD delivered via Respimat® in COPD: Results from two 6-week studies. Eur Respir J. 2013;42(Suppl 57):982s–4635. (abstract) [Google Scholar]
- 11.Koch A, Pizzichini E, Hamilton A, et al. Lung function efficacy of olodaterol QD delivered via Respimat® vs placebo and formoterol BID in patients with COPD: Two 48-week studies. Eur Respir J. 2013;42(Suppl 57):146s–P764. (abstract) [Google Scholar]
- 12.Ferguson G, Feldman G, Hofbauer P, et al. Lung function efficacy of olodaterol QD delivered via Respimat® in COPD patients: Results from two 48-week studies. Eur Respir J. 2013;42(Suppl 57):5s–187. (abstract) [Google Scholar]
- 13.Tashkin DP, Ferguson GT. Combination bronchodilator therapy in the management of chronic obstructive pulmonary disease. Respir Res. 2013;14:49. doi: 10.1186/1465-9921-14-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boehringer Ingelheim Co-administration of Olodaterol Respimat® and Tiotropium Handihaler®. [Accessed September 23, 2014]. Available from http://clinicaltrials.gov/show/NCT01694771. Identifier: NCT1694771.
- 15.Boehringer Ingelheim Co-administration of Olodaterol Respimat® and Tiotropium Handihaler®. [Accessed September 23, 2014]. Available from http://clinicaltrials.gov/show/NCT01696058. Identifier: NCT1696058.
- 16.Jones PW, Beeh KM, Chapman KR, Decramer M, Mahler DA, Wedzicha JA. Minimal clinically important differences in pharmacological trials. Am J Respir Crit Care Med. 2014;189(3):250–255. doi: 10.1164/rccm.201310-1863PP. [DOI] [PubMed] [Google Scholar]
- 17.COMBIVENT Inhalation Solution Study Group Routine nebulized ipratropium and albuterol together are better than either alone in COPD. Chest. 1997;112(6):1514–1521. doi: 10.1378/chest.112.6.1514. [DOI] [PubMed] [Google Scholar]
- 18.van Noord JA, Aumann J-L, Janssens E, et al. Comparison of tiotropium once daily, formoterol twice daily and both combined once daily in patients with COPD. Eur Respir J. 2005;26(2):214–222. doi: 10.1183/09031936.05.00140404. [DOI] [PubMed] [Google Scholar]
- 19.Decramer M, Wedzicha JA, Sandström T, et al. Safety and tolerability of QVA149, glycopyrronium and tiotropium in patients with severe to very severe COPD: the SPARK study; Abstract presented at the American Thoracic Society Annual Meeting; Philadelphia PA, USA. May 17–21, 2013. [Google Scholar]
- 20.Decramer M, Anzueto A, Kerwin E, et al. Efficacy and safety of umeclidinium plus vilanterol versus tiotropium, vilanterol, or umeclidinium monotherapies over 24 weeks in patients with chronic obstructive pulmonary disease: results from two multicentre, blinded, randomised controlled trials. Lancet Respir Med. 2014;2(6):472–486. doi: 10.1016/S2213-2600(14)70065-7. [DOI] [PubMed] [Google Scholar]
- 21.Mahler DA, D’Urzo A, Bateman ED, et al. Concurrent use of indacaterol plus tiotropium in patients with COPD provides superior bronchodilation compared with tiotropium alone: a randomised, double-blind comparison. Thorax. 2012;67:781–788. doi: 10.1136/thoraxjnl-2011-201140. [DOI] [PubMed] [Google Scholar]
- 22.Kessler R, Partridge MR, Miravitlles M, et al. Symptom variability in patients with severe COPD: a pan-European cross-sectional study. Eur Respir J. 2011;37(2):264–272. doi: 10.1183/09031936.00051110. [DOI] [PubMed] [Google Scholar]
- 23.Dahl R, Jadayel D, Alagappan VKT, Chen H, Banerji D. Efficacy and safety of QVA149 compared to the concurrent administration of its monocomponents indacaterol and glycopyrronium: the BEACON study. Int J Chron Obstruct Pulmon Dis. 2013;8:501–508. doi: 10.2147/COPD.S49615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bateman ED, Ferguson GT, Barnes N, et al. Dual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE study. Eur Respir J. 2013;42(6):1484–1494. doi: 10.1183/09031936.00200212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Donohue JF, Niewoehner D, Brooks J, O’Dell D, Church A. Safety and tolerability of once-daily umeclidinium/vilanterol 125/25 mcg and umeclidinium 125 mcg in patients with chronic obstructive pulmonary disease: results from a 52-week, randomized, double-blind, placebo-controlled study. Respir Res. 2014;15(1):78. doi: 10.1186/1465-9921-15-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barnes PJ, Celli BR. Systemic manifestations and comorbidities of COPD. Eur Respir J. 2009;33(5):1165–1185. doi: 10.1183/09031936.00128008. [DOI] [PubMed] [Google Scholar]