Abstract
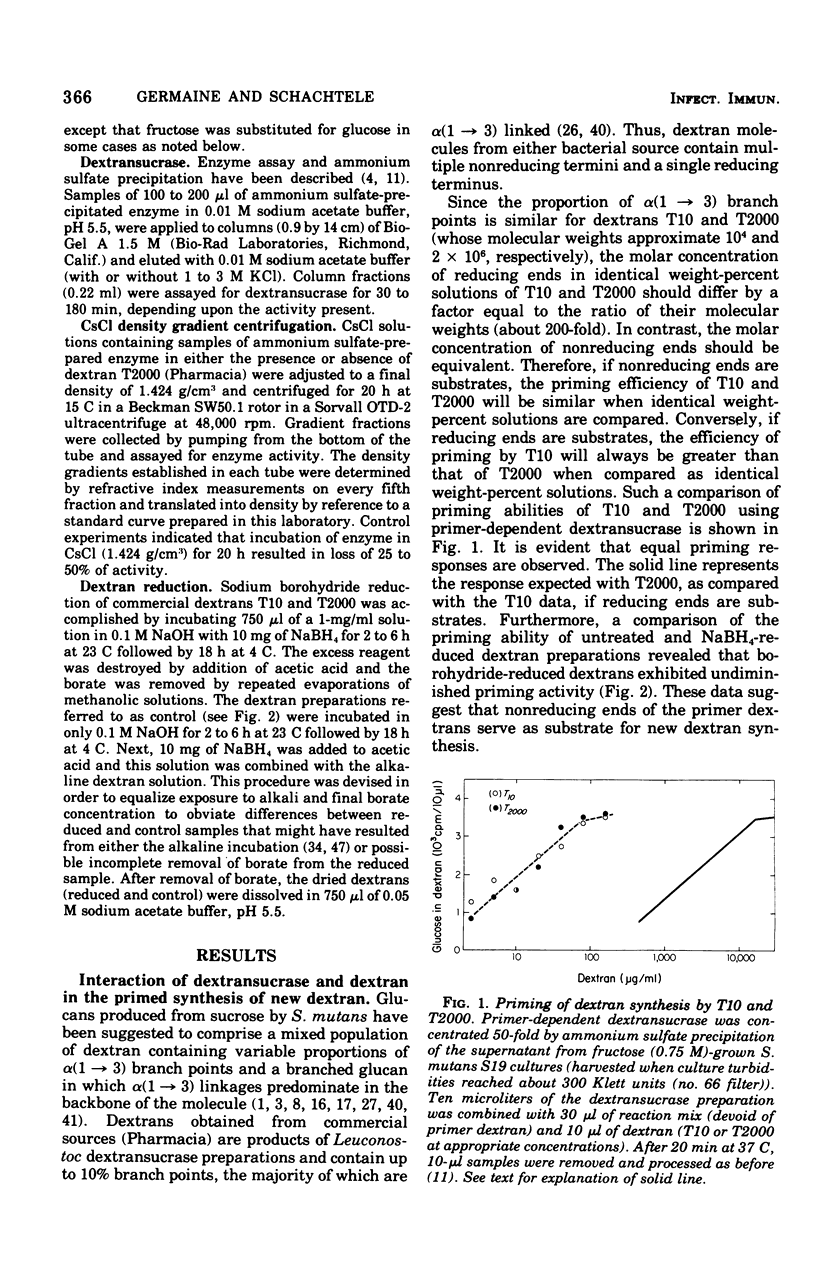
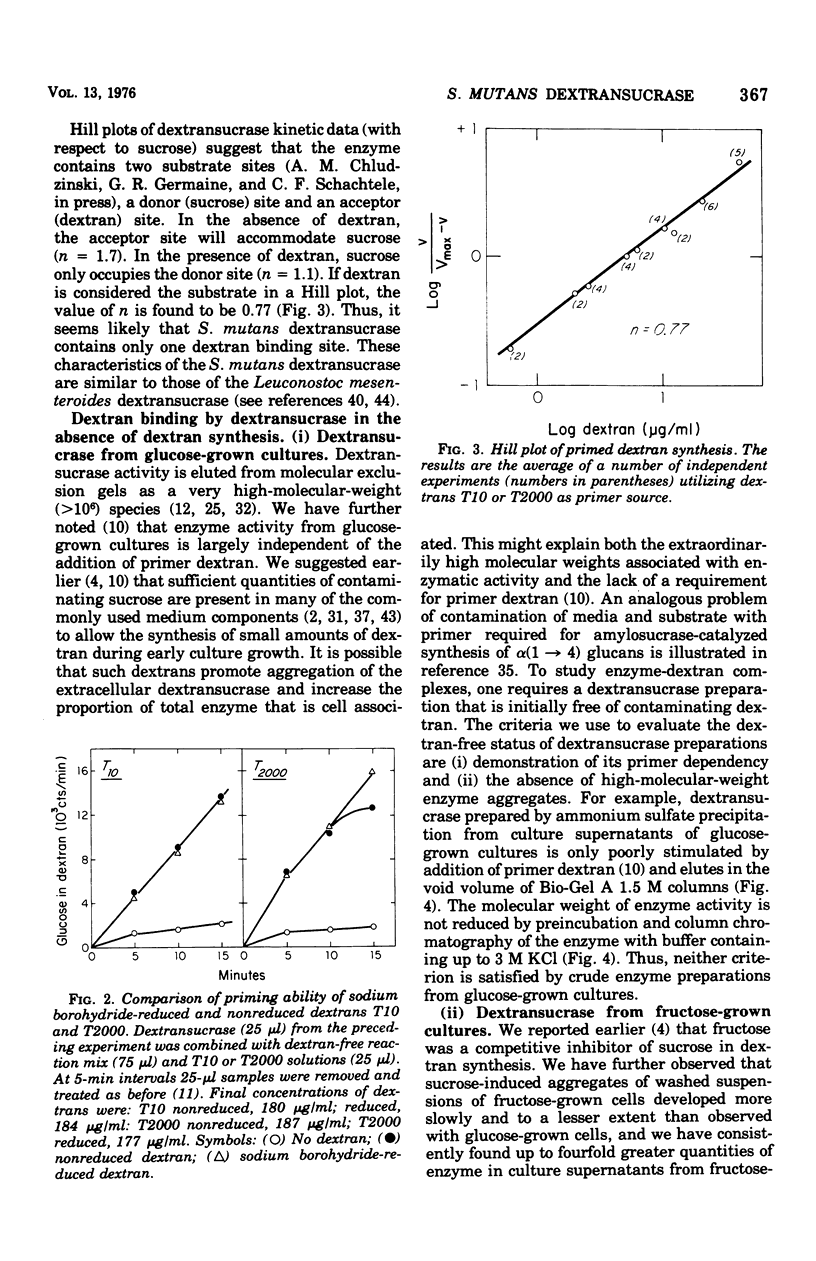
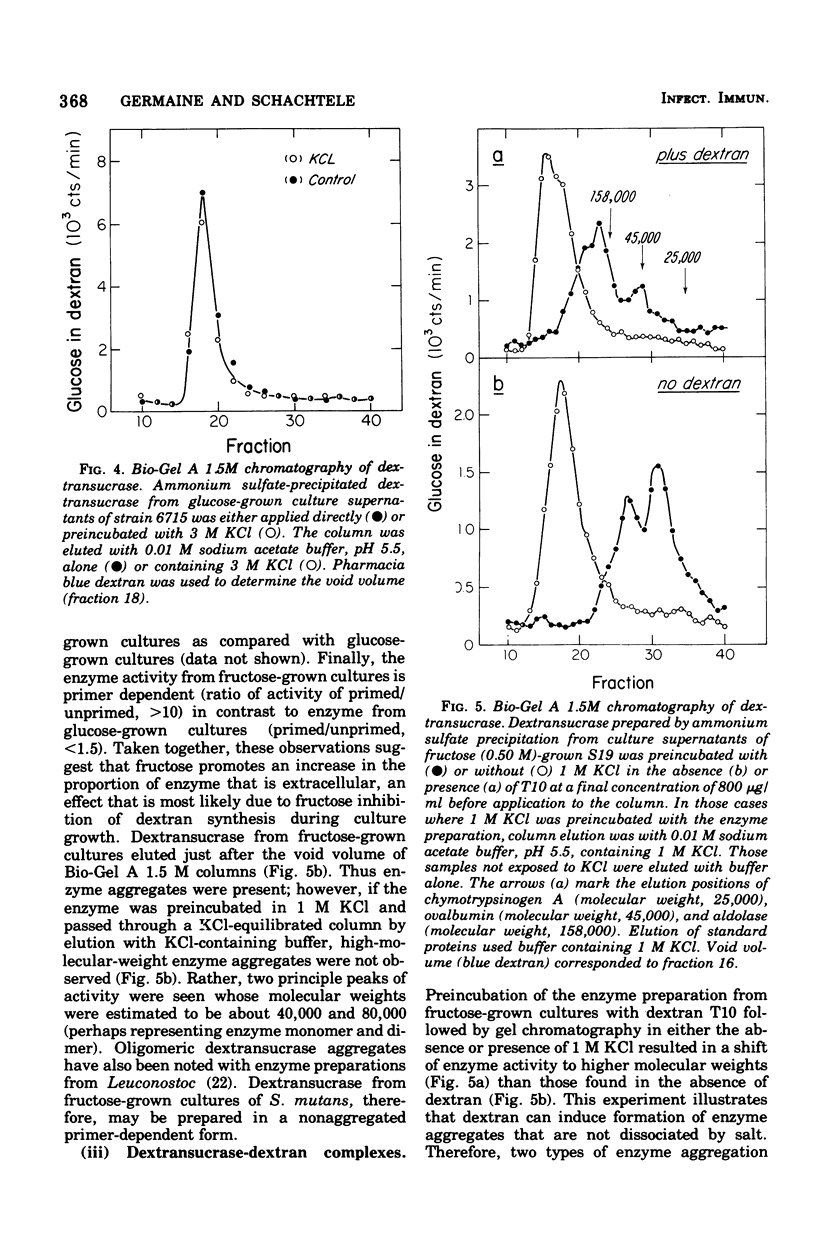
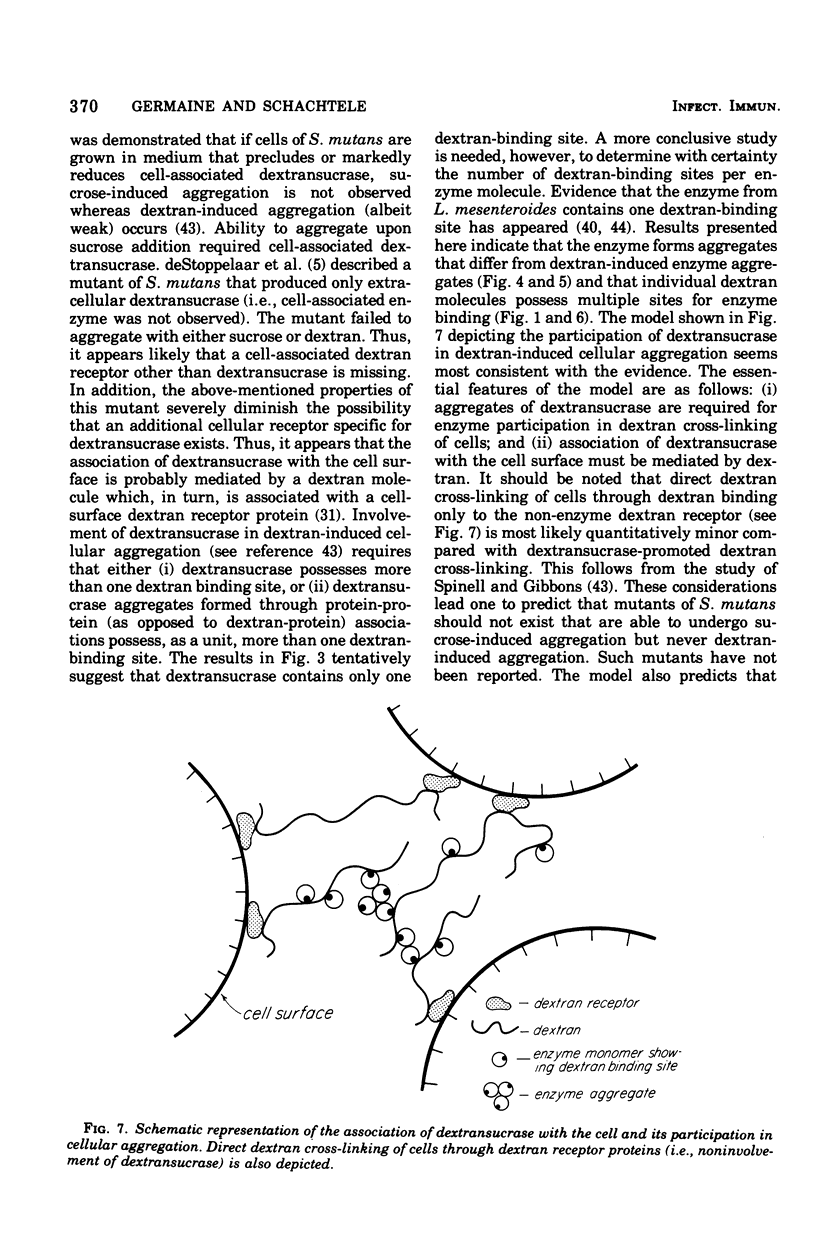
The interaction between Streptococcus mutans dextransucrase (EC 2.4.1.5) and high-molecular-weight dextran was studied in both the presence and absence of substrate sucrose. Equivalent weight-percent solutions of primer dextrans that differed 200-fold in molecular weight were found to be equally efficient in priming new dextran synthesis. Sodium borohydride reduction of dextran had no effect on its priming ability. These results suggest that dextran synthesis proceeds by addition of glucosyl residues to nonreducing termini of primer dextrans and that several enzyme molecules simultaneously bind to single high-molecular-weight dextran molecules. Kinetic data suggested that dextransucrase contains only one dextran binding site per enzyme molecule. The nature of the commonly observed highly aggregated state of dextransucrase was also studied. Two types of enzyme aggregates were distinguished: (i) oligomeric enzyme aggregates that formed in the absence of dextran and were dissociated by 1 M KCl; and (ii) dextran-induced enzyme aggregates that were stable to 3 M salt. Oligomeric enzyme aggregates were obtained from supernatants of fructose-grown cultures, whereas dextran-induced enzyme aggregates appeared to be present in glucose-grown cultures. The molecular weight of the smallest species of dextran-free detransucrase observed in solutions of 1 M KCl was estimated to be 40,000 by gel column chromatography. Addition of dextran to primer-dependent dextransucrase resulted in formation of complexes that were stable in CsCl density gradients and exhibited a buoyant density of 1.382 g/cm3 as compared with a buoyant density of 1.302 g/cm3 exhibited by dextransucrase. The enzyme-dextran complexes observed in CsCl density gradients contained about 25% dextran. This corresponded to 150 enzyme molecules (molecular weight, 40,000) per dextran molecule (molecular weight, 2 X 10(6)). The implication of these results to the mechanism of sucrose- and dextran-induced aggregation of S. mutans is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baird J. K., Longyear V. M., Ellwood D. C. Water insoluble and soluble glucans produced by extracellular glycosyltransferases from Streptococcus mutans. Microbios. 1973 Sep-Oct;8(30):143–150. [PubMed] [Google Scholar]
- Bratthall D. Immunodiffusion studies on the serological specificity of streptococci resembling Streptococcus mutans. Odontol Revy. 1969;20(3):231–243. [PubMed] [Google Scholar]
- Ceska M., Granath K., Norrman B., Guggenheim B. Structural and enzymatic studies on glucans synthesized with glucosyltransferases of some strains of oral streptococci. Acta Chem Scand. 1972;26(6):2223–2230. doi: 10.3891/acta.chem.scand.26-2223. [DOI] [PubMed] [Google Scholar]
- Chludzinski A. M., Germaine G. R., Schachtele C. F. Purification and properties of dextransucrase from Streptococcus mutans. J Bacteriol. 1974 Apr;118(1):1–7. doi: 10.1128/jb.118.1.1-7.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert K. H., Brosche M. Origin of branches in native dextrans. Biopolymers. 1967 Jun;5(5):423–429. doi: 10.1002/bip.1967.360050503. [DOI] [PubMed] [Google Scholar]
- Ebert K. H., Schenk G. Mechanisms of biopolymer growth: the formation of dextran and levan. Adv Enzymol Relat Areas Mol Biol. 1968;30:179–221. doi: 10.1002/9780470122754.ch4. [DOI] [PubMed] [Google Scholar]
- Ebisu S., Misaki A., Kato K., Kotani S. The structure of water-insoluble glucans of cariogenic Streptococcus mutans, formed in the absence and presence of dextranase. Carbohydr Res. 1974 Dec;38:374–381. doi: 10.1016/s0008-6215(00)82375-7. [DOI] [PubMed] [Google Scholar]
- Freedman M. L., Tanzer J. M. Dissociation of plaque formation from glucan-induced agglutination in mutants of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):189–196. doi: 10.1128/iai.10.1.189-196.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Chludzinski A. M., Schachtele C. F. Streptococcus mutans dextransucrase: requirement for primer dextran. J Bacteriol. 1974 Oct;120(1):287–294. doi: 10.1128/jb.120.1.287-294.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Schachtele C. F., Chludzinski A. M. Rapid filter paper assay for the dextransucrase activity from Streptococcus mutans. J Dent Res. 1974 Nov-Dec;53(6):1355–1360. doi: 10.1177/00220345740530061101. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Banghart S. B. Synthesis of extracellular dextran by cariogenic bacteria and its presence in human dental plaque. Arch Oral Biol. 1967 Jan;12(1):11–23. doi: 10.1016/0003-9969(67)90137-9. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Fitzgerald R. J. Dextran-induced agglutination of Streptococcus mutans, and its potential role in the formation of microbial dental plaques. J Bacteriol. 1969 May;98(2):341–346. doi: 10.1128/jb.98.2.341-346.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Synthesis of insoluble dextran and its significance in the formation of gelatinous deposits by plaque-forming streptococci. Arch Oral Biol. 1968 Oct;13(10):1249–1262. doi: 10.1016/0003-9969(68)90081-2. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J. Presence of an invertase-like enzyme and a sucrose permeation system in strains of Streptococcus mutans. Caries Res. 1972;6(2):122–131. doi: 10.1159/000259784. [DOI] [PubMed] [Google Scholar]
- Guggenheim B. Enzymatic hydrolysis and structure of water-insoluble glucan produced by glucosyltransferases from a strain of streptococcus mutans. Helv Odontol Acta. 1970 Nov;14(Suppl):89+–89+. [PubMed] [Google Scholar]
- Guggenheim B., Schroeder H. E. Biochemical and morphological aspects of extracellular polysaccharides produced by cariogenic streptococci. Helv Odontol Acta. 1967 Oct;11(2):131–152. [PubMed] [Google Scholar]
- KOEPSELL H. J., TSUCHIYA H. M., HELLMAN N. N., KAZENKO A., HOFFMAN C. A., SHARPE E. S., JACKSON R. W. Enzymatic synthesis of dextran; acceptor specificity and chain initiation. J Biol Chem. 1953 Feb;200(2):793–801. [PubMed] [Google Scholar]
- Kelstrup J., Funder-Nielsen T. D. Adhesion of dextran to Streptococcus mutans. J Gen Microbiol. 1974 Apr;81(2):485–489. doi: 10.1099/00221287-81-2-485. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Matsuda K. The dextransucrase isoenzymes of Leuconostoc mesenteroides NRRL B-1299. Biochim Biophys Acta. 1974 Dec 29;370(2):441–449. doi: 10.1016/0005-2744(74)90105-3. [DOI] [PubMed] [Google Scholar]
- Kuramitsu H. K. Adherence of Streptococcus mutans to dextran synthesized in the presence of extracellular dextransucrase. Infect Immun. 1974 Apr;9(4):764–765. doi: 10.1128/iai.9.4.764-765.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K. Characterization of cell-associated dextransucrase activity from glucose-grown cells of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):227–235. doi: 10.1128/iai.10.1.227-235.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larm O., Lindberg B., Svensson S. Studies on the length of the side chains of the dextran elaborated by Leuconostoc mesenteroides NRRL B-512. Carbohydr Res. 1971 Nov;20(1):39–48. doi: 10.1016/s0008-6215(00)84947-2. [DOI] [PubMed] [Google Scholar]
- Long L. W., Edwards J. R. Detailed structure of a dextran from a cariogenic bacterium. Carbohydr Res. 1972 Sep;24(1):216–217. doi: 10.1016/s0008-6215(00)82285-5. [DOI] [PubMed] [Google Scholar]
- McCabe M. M., Smith E. E. Origin of the cell-associated dextransucrase of Streptococcus mutans. Infect Immun. 1973 Jun;7(6):829–838. doi: 10.1128/iai.7.6.829-838.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. I. Roles of insoluble dextran-levan synthetase enzymes and cell wall polysaccharide antigen in plaque formation. Infect Immun. 1973 Oct;8(4):555–562. doi: 10.1128/iai.8.4.555-562.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. II. Nature of the binding site and the adsorption of dextran-levan synthetase enzymes on the cell-wall surface of the streptococcus. Infect Immun. 1974 Feb;9(2):419–429. doi: 10.1128/iai.9.2.419-429.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of the Adherence of Streptococcus mutans to Smooth Surfaces III. Purification and Properties of the Enzyme Complex Responsible for Adherence. Infect Immun. 1974 Nov;10(5):1135–1145. doi: 10.1128/iai.10.5.1135-1145.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbandian J., Freedman M. L., Tanzer J. M., Lovelace S. M. Ultrastructure of Mutants of Streptococcus mutans with Reference to Agglutination, Adhesion, and Extracellular Polysaccharide. Infect Immun. 1974 Nov;10(5):1170–1179. doi: 10.1128/iai.10.5.1170-1179.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada G., Hehre E. J. New studies on amylosucrase, a bacterial alpha-D-glucosylase that directly converts sucrose to a glycogen-like alpha-glucan. J Biol Chem. 1974 Jan 10;249(1):126–135. [PubMed] [Google Scholar]
- Olson G. A., Bleiweis A. S., Small P. A., Jr Adherence inhibition of Streptococcus mutans: an assay reflecting a possible role of antibody in dental caries prophylaxis. Infect Immun. 1972 Apr;5(4):419–427. doi: 10.1128/iai.5.4.419-427.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson G. A., Guggenheim B., Small P. A., Jr Antibody-mediated inhibition of dextran-sucrose-induced agglutination of Streptococcus mutans. Infect Immun. 1974 Feb;9(2):273–278. doi: 10.1128/iai.9.2.273-278.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robrish S. A., Reid W., Krichevsky M. I. Distribution of enzymes forming polysaccharide from sucrose and the composition of extracellular polysaccharide synthesized by Streptococcus mutans. Appl Microbiol. 1972 Aug;24(2):184–190. doi: 10.1128/am.24.2.184-190.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robyt J. F., Kimble B. K., Walseth T. F. The mechanism of dextransucrase action. Direction of dextran biosynthesis. Arch Biochem Biophys. 1974 Dec;165(2):634–640. doi: 10.1016/0003-9861(74)90291-4. [DOI] [PubMed] [Google Scholar]
- Schachtele C. F., Germaine G. R., Harlander S. K. Production of elevated levels of dextransucrase by a mutant of Streptococcus mutans. Infect Immun. 1975 Oct;12(4):934–937. doi: 10.1128/iai.12.4.934-937.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidebotham R. L. Dextrans. Adv Carbohydr Chem Biochem. 1974;30:371–444. doi: 10.1016/s0065-2318(08)60268-1. [DOI] [PubMed] [Google Scholar]
- Sidebotham R. L., Weigel H., Bowen W. H. Studies on dextrans and destranases. IX. Dextrans elaborated by cariogenic organisms. Carbohydr Res. 1971 Sep;19(2):151–159. doi: 10.1016/s0008-6215(00)81615-8. [DOI] [PubMed] [Google Scholar]
- Spinell D. M., Gibbons R. J. Influence of culture medium on the glucosyl transferase- and dextran-binding capacity of Streptococcus mutans 6715 cells. Infect Immun. 1974 Dec;10(6):1448–1451. doi: 10.1128/iai.10.6.1448-1451.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer J. M., Freedman M. L., Fitzgerald R. J., Larson R. H. Diminished virulence of glucan synthesis-defective mutants of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):197–203. doi: 10.1128/iai.10.1.197-203.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. J. Preparation of isomaltose oligosaccharides labelled with 14C in the non-reducing terminal unit, and their use in studies of dextranase activity. Carbohydr Res. 1973 Sep;30(1):1–10. doi: 10.1016/s0008-6215(00)82167-9. [DOI] [PubMed] [Google Scholar]
- de Stoppelaar J. D., König K. G., Plasschaert A. J., van der Hoeven J. S. Decreased cariogenicity of a mutant of Streptococcus mutans. Arch Oral Biol. 1971 Aug;16(8):971–975. doi: 10.1016/0003-9969(71)90186-5. [DOI] [PubMed] [Google Scholar]


