Abstract
Objective
Sun exposure is the main cause of melanoma in populations of European origin. No previous study has examined the effect of sun exposure on risk of multiple primary melanomas compared with people who have one melanoma.
Methods
We identified and enrolled 2,023 people with a first primary melanoma (controls) and 1,125 with multiple primary melanomas (cases) in seven centers in four countries, recorded their residential history to assign ambient UV and interviewed them about their sun exposure.
Results
Risk of multiple primary melanomas increased significantly (P < 0.05) to OR = 2.10 for the highest exposure quarter of ambient UV irradiance at birth and 10 years of age, to OR = 1.38 for lifetime recreational sun exposure, to OR = 1.85 for beach and waterside activities, to OR = 1.57 for vacations in a sunnier climate, to OR = 1.50 for sunburns. Occupational sun exposure did not increase risk (OR = 1.03 for highest exposure). Recreational exposure at any age increased risk and appeared to add to risk from ambient UV in early life.
Conclusions
People who have had a melanoma can expect to reduce their risk of a further melanoma by reducing recreational sun exposure whatever their age. The same is probably true for a person who has never had a melanoma.
Keywords: Melanoma, Multiple primary neoplasms, Sunlight, Case-control studies
Introduction
People who have had one melanoma have an important risk of a second, estimated at an average of 1% a year [1]. Studies of risk of multiple primary melanomas have been concerned mainly with influences of the previous history of melanoma, presence of dysplastic nevi, or possible genetic susceptibility [2–4]. While sun exposure is recognized as the major cause of melanoma in populations of European origin [5], studies of sun exposure and melanoma so far published have compared sun exposure in people with a first melanoma, or any melanoma, with that in people who have not had a melanoma. They have not specifically examined the effect of sun exposure on risk of multiple (second or higher order) primary melanomas.
The Genes, Environment and Melanoma Study (GEM) is the first study designed to be able to systematically evaluate the effects of sun exposure on risk of multiple primary melanomas in people who have already had a melanoma [6] and we report in this paper the first results of sun exposure analyses. We address ambient UV irradiance and reported sun exposure hours over life and in specific periods of life and their interactions in determining risk of multiple primary melanomas. Despite the many studies of melanoma risk factors in general populations, few have examined risk related to sun exposure in specific periods of life and their interactions.
Methods
GEM participants were ascertained from seven population-based cancer registries: two in Australia (New South Wales, Tasmania), two in Canada (British Columbia, Ontario) and three in the USA (Orange County and San Diego County in California, New Jersey, North Carolina); those from a Michigan center and an Italian center were excluded from these analyses because data on sun exposure and related covariates were incomplete. The study protocol was approved by the Institutional Review Board at Memorial Sloan-Kettering Cancer Center in New York, the study Coordinating Center, and those at each of the contributing centers. All participants provided written informed consent.
Subjects
Controls had a first invasive primary melanoma diagnosed in the first 6 or more months of 2000 and cases had a second or higher order invasive or in situ melanoma diagnosed in 2000–2003 in New South Wales, North Carolina and Ontario and in 1998–2003 in British Columbia, California, New Jersey and Tasmania [6].
People were considered ineligible if they had poor English language skills or illness or disability that prevented a 60 min interview. Of 2,075 people who were eligible as cases and invited to participate, 1,030 (50%) took part, as did 2,024 of 3,865 (52%) eligible as controls. More eligible females participated (54%) than did eligible males (50%); the same percentage of people younger than 50 (52%) and 50 years and older (52%) participated. Most who did not participate refused (30% each of eligible cases and controls who were approached) or could not be contacted (12% and 9%); few were barred by physician refusal (4% each of cases and controls) or had died (4% each of cases and controls). Ninety-four subjects were eligible and were included as both a case and a control [6].
Data collection
All participants completed a self-administered questionnaire and calendar before a telephone interview and gave a DNA sample. The questionnaire asked them to record their skin, hair and eye color, childhood freckling, current density of moles and to give a count of moles on their back. They reported their residential locations and job titles when held for a year or more in the calendar; residence history and job titles were used in the subsequent interview to aid recall of sun exposure.
Each participant was asked at interview for their sun exposure hours in each decade year of life (10, 20, 30, etc. – previously shown to be good predictors of total sun exposure to at least 40 years of age (R2 = 0.93 [7]) and to recall separately their lifetime recreational and occupational sun exposure from age 15. European ancestry was recorded for Caucasians (99% of participants) in seven categories and ability to tan on repeated exposure to sunlight in four categories from ‘go very brown and deeply tanned’ to ‘get no suntan or get freckled only’.
Sun exposure variables
Ambient UV irradiance
Individual life histories of annual erythemal UV irradiance were calculated for residential locations at birth and at each decade of age. The UV data were derived from a model based on satellite observations and supplied to GEM as j/m2 per month and annual totals in kJ/m2 at each residential location by the National Centre for Atmospheric Research (NCAR, Dr Julia M Lee Taylor). To calculate lifetime totals, the erythemal UV irradiance at birth (age 0) was assigned to each year from birth to age 4, that at age 10 to each year from age 5 to age 14, that at age 20 to the years from 15 to 24, and so on, except that the irradiance in the last completed decade year was used up to the exact age at diagnosis (e.g. UV irradiance at age 50 was assigned to each year between 45 and an age at diagnosis of 58 years). Average annual lifetime ambient UV irradiance was calculated as the lifetime total divided by age.
Reported sun exposure hours
The interview sought recreational sun exposure from age 15 by asking participants if they had done each of 12 common outdoor recreational activities between 9 a.m. and 5 p.m. on at least 10 days in any year since leaving school; if so, they were asked the years started and stopped and the usual outdoor hours per day by season. The interview also allowed for the same questions on up to three additional activities named by participants. Lifetime hours of recreational exposure were the sum of all reported daily exposure hours per activity weighted by frequency and duration. Occupational sun exposure from age 15 was assessed by eliciting a history of paid or unpaid jobs held for a year or more that usually entailed more than an hour outdoors between 9 a.m. and 5 p.m.; direct questions asked how many outdoor hours per day in each such job. Lifetime occupational sun exposure was summed across all jobs as daily exposure hours weighted by employment duration. Average annual recreational or occupational exposure hours were calculated as the respective lifetime totals divided by completed years from age 15 to diagnosis. Exposure hours were also summed within age intervals 15–24, 25–34, 35–44, 45–54, 55–64 and 65– 74 years for people who had completed each age interval.
The interview also asked about sun exposure hours during vacations and sunburns in each decade year from age 10 (i.e. 10, 20, 30, 40 etc.) up to their last completed decade of age. The questions about vacations asked how many days or weeks were taken in the warmer and cooler months and the outdoor hours between 9 a.m. and 5 p.m. on a typical vacation day, whether any vacation time was spent in a sunnier climate than the usual residence and if so, the numbers of days and outdoor hours a day there. To estimate lifetime vacation sun exposure hours from age 5, reported vacation sun exposure hours at age 10 was assigned to each year from age 5 to age 14, those at age 20 to the years from 15 to 24, and so on as described for ambient UV irradiance. The lifetime total was divided by age minus 5 to give the average annual lifetime vacation sun exposure hours. The same method was applied to vacation hours in a sunnier-than-usual climate.
We calculated the average annual lifetime number of sunburns. The lifetime number from age 5 was calculated from the number reported in each decade year as described above for vacation sun exposure hours. Analyses at each decade of age used only the numbers of sunburns reported for that decade of age.
Statistical analyses
Age at diagnosis was defined as age at first melanoma diagnosis for controls and age at most recent diagnosis for cases. Cases (87% were aged 50+) were substantially older than controls (65% aged 50+) because both were sampled from people with incident melanomas (see Begg et al. [6]). Comparison of analyses that adjusted for age in 5-year age groups, age in decades and age as a continuous variable showed that age as a continuous variable provided the best control of confounding by age. GEM-wide quantiles were used to categorize sun exposure variables (usually into quarters of exposure) using cut points based on the exposure distribution in cases and controls together. Participants eligible as both a case and a control (N = 94) were included as both in analyses.
Conventional methods for case-control studies were followed. Odds ratios (ORs) and accompanying 95% confidence intervals (CIs) were calculated in logistic regression models in SAS (SAS Institute, Cary NC., 1989) with study center as a covariate and adjustment for age (continuous), sex, ability to tan (deep tan, moderate tan, mild tan, no tan) and European ancestry (British, other northern European, southern European, eastern European, mixed, other or unknown), and an age–sex interaction term to account for the divergent trends in melanoma incidence with increasing age in women and men. Socioeconomic status, inferred from education, did not confound the association between sun exposure variables and multiple primary melanomas in our data.
We assessed heterogeneity of sun exposure effects across centers by including an interaction term with center for each sun exposure variable in the relevant model; none were significant at P < 0.05. Results reported here are from models without these interaction terms. We also tested interactions between exposure and sex for significance. All sun exposure-related variables were modeled as both nominal and ordinal categorical variables to test for significance of heterogeneity among and trend across categories; the tables present only the p for trend. We limited the presentation of risk estimates in age intervals by tabulating ORs only for the highest exposure level at each age interval.
The joint effects of early life ambient UV irradiance and personal sun exposure measurements (beach and waterside activities, sunnier vacations, sunburns) were evaluated on both additive and multiplicative scales. The variables were dichotomized according to the level of risk indicated by the main effect models: UV irradiance was categorized as quarter 1 versus quarters 2–4, beach and waterside exposure as none vs. any, and sunnier vacation exposure and sunburns as above vs. below the median, and odds ratios calculated with reference to the joint low exposure category in each case. We used Rothman's synergy index (SI) to explore departures from lack of interaction on an additive scale [8] and the ORs and 95% CIs for a standard interaction term included in logistic regression models containing the main effect terms to assess departure from a lack of interaction on a multiplicative scale. In each case the value 1 indicates lack of interaction on the relevant scale. All significance tests in this paper were two-sided tests and a P value of 0.05 was considered statistically significant.
Since the controls in GEM had incident not prevalent diagnoses of first primary melanoma (those with prevalent diagnoses are the population from which second and subsequent primary melanomas arise), we tested for survival bias by assessing the correlation between the interval from first primary to second primary and sun exposure in cases [9]. All Spearman correlation coefficients calculated were less than 0.1.
Results
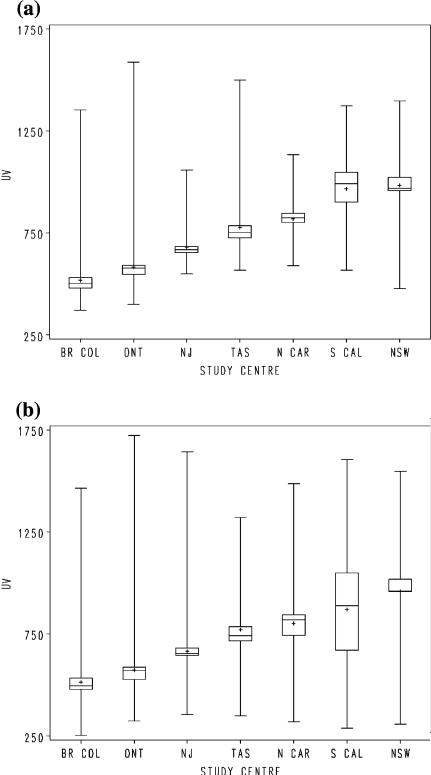
Ambient erythemal UV irradiance (Fig. 1a) was positively associated with multiple primary melanomas (Table 1). Risk increased with increasing average annual lifetime ambient erythemal UV irradiance to OR = 1.61 in the 4th exposure quarter (P for trend = 0.04). This trend was statistically significant for exposure at birth and age 10 and was not at all evident for decade years of age from 40 years onwards (Table 1). When adjusted for ambient UV irradiance at birth, the OR for the highest quarter of irradiance at age 10 increased from 1.92 to 2.04 (95% CI 1.18–3.58) and the ORs for the 2nd and 3rd quarters fell a little; the P for trend remained < 0.001. With similar adjustment the ORs in the highest exposure quarters of ambient UV at ages 20 and 30 fell: ORs 1.20 (95% CI 0.76–1.89) and 1.40 (95% CI 0.86–2.28) respectively (data not tabulated). The average of UV irradiance at birth and age 10 (Fig. 1b) was more strongly associated with multiple primary melanomas than that at birth or at age 10 alone (Table 1).
Fig. 1.
(a) Average annual lifetime ambient UV irradiance in kJ/m2 at place of residence by study center. (b) Average annual ambient UV irradiance in kJ/m2 at birth and age 10 averaged, by study center. Legend. Mean (+), median (—), interquartile range (sh=squ) and range (|); Br Col: British Columbia, Ont: Ontario, NJ: New Jersey, Tas: Tasmania, N Car: North Carolina, S Cal: Southern California, NSW: New South Wales
Table 1.
ORs for ambient UV irradiance in kJ/m2 over lifetime and at specified ages
| Erythemally weighted ambient UV irradiance (kJ/m2) | Cases N = 1067 | Controls N = 1886 | ORa (95% CI) | P for trend |
|---|---|---|---|---|
| Lifetime | ||||
| 370-652 | 194 | 530 | 1.00 | |
| 653-867 | 208 | 494 | 0.94 (0.62-1.44) | |
| 868-977 | 330 | 413 | 1.56 (0.93-2.61) | |
| 978-1,587 | 313 | 417 | 1.61 (0.96-2.69) | 0.04 |
| Birth year | ||||
| 252-593 | 195 | 552 | 1.00 | |
| 594-822 | 253 | 464 | 1.46 (1.03-2.07) | |
| 823-959 | 346 | 485 | 1.48 (1.05-2.10) | |
| 960-1,723 | 268 | 377 | 1.54 (1.09-2.18) | 0.04 |
| Age 10 | ||||
| 252-604 | 188 | 550 | 1.00 | |
| 605-838 | 250 | 469 | 1.35 (0.91-1.99) | |
| 839-959 | 306 | 448 | 1.47 (1.00-2.15) | |
| 960-1,736 | 323 | 419 | 1.92 (1.32-2.80) | <0.001 |
| Birth and age 10 (averaged) | ||||
| 252-608 | 183 | 555 | 1.00 | |
| 609-830 | 256 | 455 | 1.81 (1.22-2.67) | |
| 831-959 | 300 | 450 | 1.78 (1.21-2.62) | |
| 960-1,723 | 322 | 417 | 2.10 (1.43-3.08) | <0.001 |
| Age 20 | ||||
| 297-614 | 189 | 549 | 1.00 | |
| 615-847 | 246 | 473 | 1.38 (0.93-2.06) | |
| 848-960 | 325 | 425 | 1.24 (0.84-1.84) | |
| 961-1,828 | 307 | 433 | 1.44 (0.98-2.13) | 0.09 |
| Age 30 | ||||
| 223-638 | 180 | 530 | 1.00 | |
| 639-889 | 241 | 449 | 1.53 (0.93-2.51) | |
| 890-966 | 366 | 400 | 1.66 (1.03-2.68) | |
| 967-1,812 | 268 | 421 | 1.57 (0.98-2.49) | 0.26 |
| Age 40 | ||||
| 204-640 | 209 | 479 | 1.00 | |
| 641-957 | 235 | 392 | 1.02 (0.67-1.54) | |
| 958-971 | 326 | 340 | 0.80 (0.49-1.30) | |
| 972-1,808 | 265 | 400 | 0.80 (0.49-1.28) | 0.27 |
| Age 50 | ||||
| 348-640 | 185 | 364 | 1.00 | |
| 641-957 | 200 | 290 | 0.85 (0.51-1.40) | |
| 958-976 | 316 | 302 | 0.66 (0.36-1.21) | |
| 977-1,934 | 251 | 309 | 0.71 (0.39-1.30) | 0.49 |
| Age 60 | ||||
| 319-669 | 146 | 236 | 1.00 | |
| 670-957 | 171 | 192 | 1.25 (0.70-2.21) | |
| 958-976 | 262 | 207 | 1.04 (0.54-2.03) | |
| 977-1,715 | 217 | 206 | 1.09 (0.57-2.08) | 0.99 |
| Age 70 | ||||
| 332-684 | 115 | 134 | 1.00 | |
| 685-960 | 115 | 139 | 0.78 (0.37-1.65) | |
| 961-976 | 137 | 107 | 0.97 (0.41-2.31) | |
| 977-1,753 | 135 | 117 | 1.09 (0.46-2.59) | 0.31 |
ORs and 95% CIs are for approximate quarters of annual average ambient UV irradiance. They were calculated in logistic regression models with multivariable adjustment for: study centre (six centers), age (continuous), sex, ability to tan (deep tan, moderate tan, mild tan, no tan) European ancestry (British, other northern European, southern European, eastern European, mixed, other or unknown) and age by sex interaction
Risk of multiple primary melanomas did not increase with increasing hours of occupational sun exposure from 15 years of age to the age at diagnosis. The ORs in approximate thirds of exposure, with reference to no occupational exposure, were successively 1.08 (95% CI 0.84–1.38), 1.01 (95% CI 0.79–1.30) and 1.03 (95% CI 0.80–1.33) (P for trend = 0.82). There was a significant interaction of occupational exposure with sex (P = 0.03): risk in the highest of the three exposure levels was increased approximately twofold in women but was slightly below 1.0 in men, with little evidence of a trend in either sex (data not shown). The number of occupationally exposed women was small (71 cases, 168 controls).
Risk increased with increasing average annual hours of sun exposure in recreational activities from 15 years of age to OR = 1.38 (95% CI 1.08–1.75) in the highest quarter of exposure (P for trend = 0.01) (Table 2). Among specific activity categories, risk increased significantly only with sun exposure in beach or waterside activities (OR for highest quarter = 1.85, 95% CI 1.45–2.37; P < 0.001) (Table 2). The ORs for lifetime recreational exposure fell when we excluded beach and waterside activities from them and the P for trend was no longer significant (P = 0.21). The increasing risk with increasing exposure in beach and waterside activities was evident with exposure in all age groups, but was somewhat stronger in younger than older age groups (Table 2).
Table 2.
ORs for sun exposure in all recreational activities and in beach and waterside activities alone over lifetime and in specific age groups
| Sun exposurea in: | Cases N = 1090 | Controls N = 1926 | ORb (95% CI) | P for trend |
|---|---|---|---|---|
| All recreational activities from age 15 to diagnosis ORs for successive quarters of exposure | ||||
| 0-140 h | 212 | 511 | 1.00 | |
| 141-296 h | 263 | 473 | 1.24 (0.97-1.58) | |
| 297-538 h | 295 | 448 | 1.27 (1.00-1.62) | |
| 539-2,920 h | 295 | 453 | 1.38 (1.08-1.75) | 0.01 |
| Beach and waterside activities from age 15 to diagnosis ORs for successive quarters of exposure | ||||
| None | 201 | 504 | 1.00 | |
| 1-24 h | 315 | 451 | 1.62 (1.27-2.06) | |
| 25-76 h | 275 | 504 | 1.50 (1.17-1.92) | |
| 77-1,898 h | 299 | 467 | 1.85 (1.45-2.37) | <0.001 |
| Beach and waterside activities in specific age groups ORs for the highest quarter of exposure | ||||
| 15-24 years | 282 | 452 | 1.69 (1.34-2.13) | <0.001 |
| 25-34 years | 280 | 411 | 1.71 (1.35-2.16) | <0.001 |
| 35-44 years | 261 | 349 | 1.39 (1.09-1.77) | 0.007 |
| 45-54 years | 223 | 244 | 1.37 (1.05-1.80) | 0.02 |
| 55-64 years | 154 | 168 | 1.39 (0.98-1.95) | 0.02 |
| 65-74 years | 64 | 71 | 1.59 (0.86-2.96) | 0.04 |
Measured in each case as annual average hours over the period of life in question
ORs and 95% CIs were calculated in logistic regression models with multivariable adjustment for study centre (six centers), age (continuous), sex, ability to tan (deep tan, moderate tan, mild tan, no tan) European ancestry (British, other northern European, southern European, eastern European, mixed, other or unknown) and age by sex interaction
Vacation sun exposure as a whole was weakly positively associated with risk of multiple primary melanomas. It was more strongly associated with sun exposure during vacations in sunnier climates: ORs increased with increasing hours of exposure to 1.57 (95% CI 1.19–2.06; P for trend < 0.001) (Table 3). The ORs for vacation sun exposure as a whole fell to below 1.0 when we excluded from them vacations taken in a sunnier climate and the p for trend was high (P = 0.28). In age intervals, ORs for the highest category of sun exposure in vacations in sunnier climates ranged from 1.02 to 1.54 and there was little evidence of a trend in them with age (Table 3). There was a significant interaction of vacations in a sunnier climate with sex (P = 0.04); ORs were increased for all exposure quarters above the baseline in men (OR = 1.69, 95% CI 1.17–2.43 for the highest exposure category) but not in women.
Table 3.
ORs for sun exposure in all vacations and in vacations in sunnier climates over lifetime and in specific age groups
| Sun exposurea in: | Cases | Controls | ORb (95% CI) | P for trend |
|---|---|---|---|---|
| All vacations from age 5 to diagnosis ORs for successive quarters of exposure | ||||
| 0-91 h | 188 | 376 | 1.00 | |
| 92-129 h | 216 | 350 | 1.28 (0.98-1.68) | |
| 130-179 h | 179 | 380 | 1.25 (0.94-1.66) | |
| 180-928 h | 150 | 413 | 1.16 (0.86-1.57) | 0.37 |
| Vacations in sunnier climates from age 5 to diagnosis ORs for successive categories of exposure | ||||
| None | 157 | 421 | 1.00 | |
| 1-18 h | 170 | 382 | 1.11 (0.84-1.47) | |
| 19-42 h | 184 | 369 | 1.35 (1.02-1.78) | |
| 43-678 h | 215 | 352 | 1.57 (1.19-2.06) | <0.001 |
| Vacations in sunnier climates in specific age groups ORs for the highest category of exposure c | ||||
| Age 10 | 79 | 126 | 1.32 (0.95-1.82) | 0.16 |
| Age 20 | 65 | 133 | 1.24 (0.88-1.75) | 0.07 |
| Age 30 | 133 | 200 | 1.30 (1.00-1.69) | 0.02 |
| Age 40 | 175 | 207 | 1.54 (1.20-1.97) | <0.001 |
| Age 50 | 110 | 146 | 1.02 (0.76-1.37) | 0.37 |
| Age 60 | 105 | 103 | 1.36 (0.98-1.89) | 0.008 |
| Age 70 | 56 | 51 | 1.25 (0.80-1.97) | 0.48 |
| Average annual number of sunburns from age 5 to diagnosis ORs for successive categories of exposure | ||||
| None | 279 | 592 | 1.00 | |
| >0-0.3 sunburns | 224 | 312 | 1.17 (0.91-1.49) | |
| >0.3-0.75 sunburns | 197 | 339 | 1.44 (1.12-1.85) | |
| >0.75 sunburns | 160 | 367 | 1.50 (1.15-1.96) | <0.001 |
| Number of sunburns at each decade of age ORs for the highest category of exposure d | ||||
| Age 10 | 163 | 237 | 1.64 (1.27-2.12) | <0.001 |
| Age 20 | 73 | 137 | 1.15 (0.83-1.59) | 0.37 |
| Age 30 | 62 | 119 | 1.09 (0.77-1.55) | 0.69 |
| Age 40 | 32 | 64 | 0.88 (0.55-1.40) | 0.55 |
Table shows data for vacations: 1,062 cases, 1836 controls; sunny vacations: 1063 cases, 1890 controls; sunburns: 946 cases, 1718 controls
Measured in each case as annual average hours over the period of life in question
Adjusted for: study centre, age, sex, ability to tan (except sunburn), European ancestry, and age by sex interaction
Quarters of exposure for age 10, thirds with reference to no exposure for other ages
Thirds of exposure with reference to no exposure. There were few reported sunburns after 40 years of age
Increasing frequency of sunburn was associated with increasing risk of multiple primary melanomas: the OR for the highest category of average annual number of sunburns from 10 years of age to the age at diagnosis was 1.50 (95% CI 1.15–1.96; P for trend < 0.001) (Table 3). Within age categories, only number of sunburns at age 10 was positively associated with subsequent melanoma (OR = 1.64, 95% CI 1.27–2.12 for three or more sunburns; P for trend < 0.001) (Table 3). By age 40, few people reported any sunburns (10% of males, 8% of females) and we did not examine risk with sunburn at older ages.
To assess the independence of their effects, we included early life ambient UV irradiance together with each, separately, of sun exposure in beach and water-side activities, vacations in sunnier climates and frequency of sunburn in the logistic models in which their individual effects had been examined. The ORs for the sun exposure variables changed little if at all with adjustment for early life ambient UV, and vice versa; all remained statistically significant (data not tabulated).
The interacting effects of early life ambient UV and personal sun exposure were evaluated against additive and multiplicative models of joint action (Table 4). Synergy indexes close to 1.0 for exposure in beach and waterside activities and in vacations in a sunnier climate suggest lack of interaction on an additive scale between these variables and ambient UV in early life. The hypothesis of a multiplicative model of joint action for both could not be rejected however since the interaction P values were high. The odds ratio of 2.17 for high lifetime sunburns and high ambient UV in early life, although high, was not significantly different from the expected joint risk on a multiplicative scale (interaction OR 1.47, 95% CI 0.93–2.33) or on an additive scale (SI = 2.52, 95% CI 0.71–8.95).
Table 4.
Interactions between ambient UV irradiance in early life and lifetime personal sun exposure in increasing risk of multiple primary melanomas
| Early life UV dose (kJ/m2) | Sun exposure | Cases | Controls | Odds ratioa (95% CI) | Odds ratio (95% CI) for interaction | Synergy index (95% CI) |
|---|---|---|---|---|---|---|
| Beach and waterside activities | ||||||
| Low UV | None | 44 | 176 | 1 | ||
| <609 | Any | 137 | 367 | 1.99 (1.33-2.98) | ||
| High UV | None | 153 | 313 | 2.31 (1.41-3.78) | 0.73 (0.46-1.17) | 1.02 (0.70-1.49) |
| 609+ | Any | 724 | 1,008 | 3.35 (2.11-5.31) | P† = 0.19 | P = 0.90 |
| Vacations in sunnier climates | ||||||
| Low UV | None-Low | 66 | 274 | 1 | ||
| <609 | High | 82 | 202 | 1.52 (1.02-2.25) | ||
| High UV | None-Low | 251 | 506 | 1.75 (1.08-2.83) | 0.88 (0.56-1.39) | 1.06 (0.61-1.83) |
| 609+ | High | 302 | 498 | 2.34 (1.45-3.77) | P† = 0.59 | P = 0.84 |
| Sunburns | ||||||
| Low UV | None-Low | 100 | 273 | 1 | ||
| <609 | High | 49 | 191 | 1.03 (0.68-1.56) | ||
| High UV | None-Low | 390 | 611 | 1.43 (0.96-2.14) | 1.47 (0.93-2.33) | 2.52 (0.71-8.95) |
| 609+ | High | 298 | 488 | 2.17 (1.43-3.30) | P† = 0.10 | P = 0.15 |
Table shows data for vacations: 701 cases, 1480 controls; beach and waterside activities: 1058 cases, 1864 controls; sunburns: 837 cases, 1563 controls
Adjusted for study centre, age, sex, ability to tan (except sunburns), ancestry, and age by sex interaction
P for departure from multiplicative interaction of exposure effects in model with product term for the two exposures
To assess the impact on our results of including cases with in situ melanomas we repeated all models with them removed. The ORs fell for all quarters of average annual lifetime ambient UV irradiance: that for the highest exposure quarter was reduced by 25% to OR = 1.20 (95% CI 0.68–2.13) and the P for trend was no longer significant (P = 0.36). Within age intervals, however, the ORs for ambient UV changed only by about 6–12% and P values for trend retained their initial level of significance; the OR for the highest category of ambient UV at birth and age 10 was 2.2 (95% CI 1.43–3.44; P for trend 0.002). Changes in the ORs for personal sun exposure were small (<3%), with some falls (sunnier vacations, sunburns) and some increases (vacations, occupations). In the models to evaluate the presence of interaction, removing the in situ melanomas had little effect on the interaction ORs or the SIs.
Discussion
We found that risk of multiple primary melanomas increased with increasing ambient UV irradiance at places of residence and that this effect was largely due to ambient UV at birth and 10 years of age. Risk increased independently with lifetime recreational sun exposure, particularly in beach and waterside activities and vacations in a sunnier climate, and sunburn, but not occupational sun exposure. There was little evidence that effects of recreational exposure varied by the age at which it occurred but risk with frequent sunburns at age 10 was greater than that at any later decade of age. Our analysis of interactions was most consistent with simple addition of the independent effects of ambient UV in early life and lifetime recreational sun exposure.
The strengths of the GEM study are its population-based case ascertainment and its use of cancer regis tries to identify cases and controls the same way in all 7 populations in this analysis. While overall participation was around 50%, it was nearly identical in cases (50%) and controls (52%), thus minimizing the probability of bias due to non-participation. Interview procedures were standardized and based on substantial experience of previous melanoma and skin cancer studies [7]. GEM is also unique among melanoma studies in its geographical coverage and comprehensive, quantitative approach to measuring ambient UV irradiance and sun exposure in different activities and at different times of life. For practical reasons, GEM used incident cases rather than prevalent survivors of invasive melanoma as controls and inclusion of patients with in situ melanoma as cases. Despite this design there was minimal correlation between interval from first to second primary melanoma and the exposure measures we analyzed, thus ruling out appreciable correlation between them and survival time in this data set, and exclusion of in situ cases from the analysis had little impact on the results.
There are no previous studies of multiple primary melanomas with which we can compare our results. Therefore, we will discuss them in the context of studies of first or any primary melanoma. We found ambient UV irradiance in early life (birth and 10 years of age) to be the strongest sun-related risk factor for multiple primary melanomas. While ambient UV at ages 20 and 30 was also associated with multiple primary melanomas, this was due, at least in part, to confounding with exposure earlier in life. One study of actual ambient UV irradiance at places of residence in individuals with first primary melanoma found no consistent uptrend in risk for increasing UV in any age group [10]. A meta-analysis of risk of any primary melanoma with all measures of residential ambient UV in case-control and cohort studies reported an OR of 1.86 (95% CI 1.41–2.46) [11] in the highest categories of exposure, which is similar to what we observed, 1.61 (95% CI 0.96–2.69). Our results are also very consistent with the evidence from studies of individual risk of any primary melanoma in relation to place of birth and age at migration from or to areas of high ambient UV irradiance, which show up to threefold gradients in risk and that arrival in an area of high ambient UV before about 10 years of age confers the same high risk of melanoma as being born there [12, 13].
While other effects are possible, for example on the development of pigmented nevi [14], the exclusivity to early life of the effects of ambient UV might simply reflect a high degree of uniformity of outdoor exposure in children from one environment to another. Correspondingly, the lack of any substantial effect of ambient UV in later life on melanoma risk might be due mainly to much greater individual variation in sun-related behavior than in average annual ambient UV at their places of residence. The individual variability in ambient UV in this analysis has been constrained by the inevitable matching of controls to cases by study center and the necessary statistical control of this variable in analysis.
The odds ratio for multiple primary melanomas in our highest category of occupational sun exposure, 1.03 (95% CI 0.80–1.33), is similar to that reported in the meta-analysis referred to above for chronic (mainly occupational) sun exposure, 0.95 (95% CI 0.87–1.04) [15]. Likewise, our odds ratios for the highest categories of exposure in beach and waterside activities (1.85, 95% CI 1.45–2.37) and vacations in sunnier climates (1.57, 1.19–2.06) encompass the corresponding meta-analysis estimate of 1.61 (95% CI 1.31–1.99) for all measures of intermittent (mainly recreational) sun exposure [15]. Our estimate for the risk of multiple primary melanomas in the highest category of lifetime average annual sunburns (OR 1.50, 95% CI 1.15–1.96) was also similar to that for any primary melanoma in the meta-analysis (OR 1.73, 95% CI 1.47–2.04, corrected for suspected publication bias) [15].
With respect to effects of age at exposure, we calculated pooled estimates from results in relevant other studies [16]: for general outdoor exposure (four studies) the summary ORs were 1.1 (95% CI 0.8–1.4) for childhood exposure and 1.6 (95% CI 1.1–2.4) for adult exposure, and for beach exposure (six studies) 1.3 (95% CI 1.0–1.7) for childhood exposure and 1.4 (95% CI 1.1– 1.9) for adult exposure. Whiteman et al.'s meta-analysis showed summary ORs for sunburn (10 studies) of 1.8 (95% CI 1.6–2.2) for childhood exposure and 1.5 (95% CI 1.3–1.8) for adult exposure [16]. These results concur completely with ours in suggesting that recreational sun exposure in later life increases risk of melanoma to a similar degree to that in earlier life. Only for sunburn might risk of melanoma be greater with exposure in childhood than exposure in adulthood (Table 3).
While the relative risks of the major measures of personal sun exposure for multiple primary melanomas are similar to those for a first or any melanoma, the absolute risks conferred by equivalent levels of exposure will be quite different, because the absolute risk of a subsequent melanoma in a person who has already had a melanoma is some 2–4 times greater than that of a first melanoma in an otherwise similar person [1, 17]. Minimizing sun exposure might, therefore, be of particular value in reducing risk of multiple primary melanomas. That this is so is supported by our evidence that high recreational sun exposure confers an increased risk of multiple primary melanomas at whatever age it is received.
The great similarity between our results for the main effects of ambient UV and occupational and recreational sun exposure on risk of multiple melanomas and those for a first or any melanoma in the general population, which was predicted on theoretical grounds [18], suggest that these results can be taken to apply to risk of all melanomas.
The associations with multiple primary melanomas of ambient UV in early life and of recreational sun exposure and sunburn throughout life beg the question: How do early life and lifetime exposure effects interact? While our study has limited power to answer this question, the synergy indexes for early life UV and exposure in beach and waterside activities and in vacations in sunnier climates were very close to 1.0 (Table 4), suggesting simple addition of their effects. Somewhat in contrast, the synergy index for the interaction of ambient UV with sunburn was high at 2.52, but its 95% CI (0.71–8.95) included 1.0. One of us has previously advanced the hypothesis “that the lifetime potential for skin cancer is determined to a substantial degree by sun exposure in the first 10 years of life and the extent to which this potential is realized is determined by sun exposure in later life” [14]; that is, that the joint effects of the two would be multiplicative. A small European case-control study that directly examined the interaction between childhood and adult sun exposure in causing any primary melanoma reported that their results suggested multiplication of the two effects [19]. The meta-analysis of Gandini et al. [15], however, suggests the possibility of an additive model of joint action for ambient UV and sunburn. If it was multiplicative, the ORs would be the same at different latitudes but the pooled OR for sunburn was higher when UV was lower (pooled OR at 50°+ was 2.54, 95% CI 1.99–3.24) and lower when UV was higher (at <50° OR = 1.91, 95% CI 1.58–2.31).
It is important to know more certainly what the model of joint action of early life and later life sun exposure on melanoma risk is, because it determines the relative weights put on sun protection in children and adults in public communication and investment in sun protection programs. This certainly will only come through the conduct of additional large studies of melanoma, pooled analyses of the results of existing studies or both.
Sun exposure confers a similar relative risk of multiple primary melanomas in people who have had a melanoma as it does of a first or any melanoma in the whole population. Since the absolute risk of a subsequent melanoma is 2–4 times higher than that of a first melanoma, people who have had a melanoma can expect to gain greater benefit from reduction in sun exposure than people who have not. Sun exposure in later life appears to add to, not multiply, the effects of sun exposure in earlier life: this is still uncertain, however, and requires more research. Prevention messages should aim to encourage people with light skin to use prudent sun protection at any age.
Acknowledgments
The study was conducted by the GEM Study Group: Coordinating Center, Memorial Sloan—Kettering Cancer Center, New York, NY: Marianne Berwick (PI, currently at the University of New Mexico), Colin B. Begg (Co-PI), Irene Orlow (Co-Investigator), Urvi Mujumdar (Project Coordinator), Amanda J. Hummer (Biostatistician), Nandita Mitra (Biostatistician), Klaus Busam (Dermatopathologist), Pampa Roy (Laboratory Technician), Rebecca Canchola (Laboratory Technician), Brian Clas (Laboratory Technician), Javier Cotignola (Laboratory Technician), and Yvette Monroe (Interviewer).Study centers included the following: The University of Sydney and The Cancer Council New South Wales, Sydney, Australia: Bruce K. Armstrong (PI), Anne Kricker (Co-PI), Melisa Litchfield (Study Coordinator); Menzies Centre for Population Health Research, University of Tasmania, Hobart, Australia: Terence Dwyer (PI), Paul Tucker (Dermatopatholo-gist), Nicola Stephens (Study Coordinator); British Columbia Cancer Agency, Vancouver, Canada: Richard P. Gallagher (PI), Teresa Switzer (Coordinator); Cancer Care Ontario, Toronto, Canada: Loraine D. Marrett (PI), Elizabeth Theis (Co-Investigator), Lynn From (Dermatopathologist), Noori Chowdhury (Coordinator), Louise Vanasse (Coordinator), Mark Purdue (Research Officer), David Northrup (Manager for CATI); Centro per la Prevenzione Oncologia Torino, Piemonte, Italy: Roberto Zanetti (PI), Stefano Rosso (Data Manager), Carlotta Sacerdote (Coordinator); University of California, Irvine: Hoda Anton-Culver (PI), Nancy Leighton (Coordinator), Maureen Gildea (Data Manager); University of Michigan, Ann Arbor: Stephen B. Gruber (PI), Joe Bonner (Data Manager), Joanne Jeter (Coordinator); New Jersey Department of Health and Senior Services, Trenton: Judith Klotz (PI), Homer Wilcox (Co-PI), Helen Weiss (Coordinator); University of North Carolina, Chapel Hill: Robert C. Millikan (PI), Nancy Thomas (Co-Investigator), Dianne Mattingly (Coordinator), Jon Player (Laboratory Technician), Chiu-Kit Tse (Data Analyst); University of Pennsylvania: Timothy R. Rebbeck (PI), Peter Kanetsky (Co- Investigator), Amy Walker (Laboratory Technician), Saa-rene Panossian (Laboratory Technician); Consultants: Harvey Mohrenweiser, University of California, Irvine; Richard Setlow, Brookhaven National Laboratory, Upton, NY. UV data consultants: Dr Julia Lee Taylor and Dr Sasha Madronich, National Centre for Atmospheric Research, Boulder, Colorado.
Financial support: National Cancer Institute, Awards CA83180, CA098438, CA46592 and CA16086. Bruce K. Armstrong is also supported by a University of Sydney Medical Foundation Program Grant. Richard P. Gallagher is supported by a Michael Smith Foundation for Health Research Grant.
Contributor Information
Anne Kricker, School of Public Health, Edward Ford Building A27, University of Sydney, NSW 2006, Australia.
Bruce K. Armstrong, School of Public Health, Edward Ford Building A27, University of Sydney, NSW 2006, Australia
Chris Goumas, School of Public Health, Edward Ford Building A27, University of Sydney, NSW 2006, Australia.
Melisa Litchfield, School of Public Health, Edward Ford Building A27, University of Sydney, NSW 2006, Australia.
Colin B. Begg, Memorial Sloan-Kettering Cancer Center, New York, NY, USA
Amanda J. Hummer, Memorial Sloan-Kettering Cancer Center, New York, NY, USA
Loraine D. Marrett, Cancer Care Ontario, Toronto, ON, Canada
Beth Theis, Cancer Care Ontario, Toronto, ON, Canada.
Robert C. Millikan, University of North Carolina, Chapel Hill, NC, USA
Nancy Thomas, University of North Carolina, Chapel Hill, NC, USA.
Hoda Anton Culver, University of California, Irvine, CA, USA.
Richard P. Gallagher, British Columbia Cancer Agency, Vancouver, BC, Canada
Terence Dwyer, Murdoch Children's Research Institute, Parkville, VIC, Australia.
Timothy R. Rebbeck, University of Pennsylvania, Philadelphia, PA, USA
Peter A. Kanetsky, University of Pennsylvania, Philadelphia, PA, USA
Klaus Busam, Memorial Sloan-Kettering Cancer Center, New York, NY, USA.
Lynn From, Women's College Hospital, Toronto, ON, Canada.
Urvi Mujumdar, Memorial Sloan-Kettering Cancer Center, New York, NY, USA.
Roberto Zanetti, Centro per la Prevenzione Oncologia, Torino, Piemonte, Italy.
Marianne Berwick, University of New Mexico, Albuquerque, NM, USA.
References
- 1.Levi F, Randimbison L, Te VC, La Vecchia C. High constant incidence rates of second cutaneous melanomas. Int J Cancer. 2005;117:877–879. doi: 10.1002/ijc.21262. [DOI] [PubMed] [Google Scholar]
- 2.Blackwood MA, Holmes R, Synnestvedt M, et al. Multiple primary melanoma revisited. Cancer. 2002;94:2248–2255. doi: 10.1002/cncr.10454. [DOI] [PubMed] [Google Scholar]
- 3.Stam-Posthuma JJ, van Duinen C, Scheffer E, Vink J, Bergman W. Multiple primary melanomas. J Am Acad Dermatol. 2001;44:22–27. doi: 10.1067/mjd.2001.110878. [DOI] [PubMed] [Google Scholar]
- 4.Ferrone CR, Ben Porat L, Panageas KS, et al. Clinicopathological features of and risk factors for multiple primary melanomas. JAMA. 2005;294:1647–1654. doi: 10.1001/jama.294.13.1647. [DOI] [PubMed] [Google Scholar]
- 5.International Agency for Research on Cancer . IARC Monographs on the evaluation of carcinogenic risks to humans. IARC; Lyon: 1992. Solar and ultraviolet radiation, vol 55. [PMC free article] [PubMed] [Google Scholar]
- 6.Begg CB, Hummer AJ, Mujumdar U, et al. A design for cancer case-control studies using only incident cases: experience with the GEM study of melanoma. Int J Epidemiol. 2006;35:756–764. doi: 10.1093/ije/dyl044. [DOI] [PubMed] [Google Scholar]
- 7.Kricker A, Vajdic CM, Armstrong BK. Reliability and validity of a telephone questionnaire for estimating lifetime personal sun exposure in epidemiologic studies. Cancer Epidemiol Biomarkers Prev. 2005;14:2427–2432. doi: 10.1158/1055-9965.EPI-05-0265. [DOI] [PubMed] [Google Scholar]
- 8.Hosmer DW, Lemeshow S. Confidence interval estimation of interaction. Epidemiology. 1992;3:452–456. doi: 10.1097/00001648-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Begg CB, Gray R. Methodology for case-control studies with prevalent cases. Biometrika. 1987;74:191–195. [Google Scholar]
- 10.Solomon CC, White E, Kristal AR, Vaughan T. Melanoma and lifetime UV radiation. Cancer Causes Control. 2004;15:893–902. doi: 10.1007/s10552-004-1142-9. [DOI] [PubMed] [Google Scholar]
- 11.Gruber SB, Armstrong BK. Cutaneous and ocular melanoma. In: Schottenfeld D, Fraumeni JF, editors. Cancer epidemiology and prevention. OUP; New York: 2006. pp. 1196–1229. [Google Scholar]
- 12.Holman CD, Armstrong BK. Cutaneous malignant melanoma and indicators of total accumulated exposure to the sun: an analysis separating histogenetic types. J Natl Cancer Inst. 1984;73:75–82. [PubMed] [Google Scholar]
- 13.Parkin DM, Steinitz R, Khlat M, Kaldor J, Katz L, Young J. Cancer in Jewish migrants to Israel. Int J Cancer. 1990;45:614–621. doi: 10.1002/ijc.2910450407. [DOI] [PubMed] [Google Scholar]
- 14.Armstrong BK. How does sun exposure cause skin cancer—epidemiological perspective. In: Hill D, English DR, Elwood JM, editors. Prevention of skin cancer, cancer causes—cancer prevention. Kluwer Academic Publishers; New York: 2004. pp. 89–116. [Google Scholar]
- 15.Gandini S, Sera F, Cattaruzza MS, et al. Meta-analysis of risk factors for cutaneous melanoma. II. Sun exposure. Eur J Cancer. 2005;41:45–60. doi: 10.1016/j.ejca.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Whiteman DC, Whiteman CA, Green AC. Childhood sun exposure as a risk factor for melanoma: a systematic review of epidemiologic studies. Cancer Causes Control. 2001;12:69–82. doi: 10.1023/a:1008980919928. [DOI] [PubMed] [Google Scholar]
- 17.Goggins WB, Tsao H. A population-based analysis of risk factors for a second primary cutaneous melanoma among melanoma survivors. Cancer. 2003;97:639–643. doi: 10.1002/cncr.11116. [DOI] [PubMed] [Google Scholar]
- 18.Begg CB, Berwick M. A note on the estimation of relative risks of rare genetic susceptibility markers. Cancer Epidemiol Biomarkers Prev. 1997;6:99–103. [PubMed] [Google Scholar]
- 19.Autier P, Dore JF. Influence of sun exposures during childhood and during adulthood on melanoma risk. EPIMEL and EORTC Melanoma Cooperative Group. European Organisation for Research and Treatment of Cancer. Int J Cancer. 1998;77:533–537. doi: 10.1002/(sici)1097-0215(19980812)77:4<533::aid-ijc10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]