SUMMARY
Human imaging studies have revealed that intranasal administration of the “prosocial” hormone oxytocin (OT) activates the frontal cortex, and that this action of OT correlates with enhanced brain function in autism. Here we report the discovery of a population of somatostatin (Sst) positive, regular spiking interneurons that express the oxytocin receptor (OxtrINs). Silencing of OxtrINs in the medial prefrontal cortex (mPFC) of female mice resulted in loss of social interest in male mice specifically during the sexually receptive phase of the estrous cycle. This sociosexual deficit was also present in mice in which the Oxtr gene was conditionally deleted from the mPFC, and in control mice infused with an Oxtr antagonist. Our data demonstrate a gender, cell type and state specific role for OT/Oxtr signaling in the mPFC, and identify a latent cortical circuit element that may modulate other complex social behaviors in response to OT.
INTRODUCTION
Complex behaviors in mammals are generated by the cerebral cortex in response to dynamic sensory cues and internal state. Although the neocortex can be subdivided into architectonic areas that conform generally to the sensory modality providing their input, and into associative areas that process information from multiple cortical and subcortical structures to generate appropriate behavioral outputs (Kandel, 2013), in each area the balance of excitation and inhibition governs its contributions to behavior and sensory perception (Haider et al., 2006; Isaacson and Scanziani, 2011). Disturbances in this balance are thought to play a role in the social impairments at the core of psychiatric disorders such as autism and schizophrenia (Kehrer et al., 2008; Markram and Markram, 2010; Rubenstein and Merzenich, 2003). Recent evidence has indicated altered inhibition may be especially important in the pathophysiology of these disorders (Chao et al., 2010; Oblak et al., 2011; Takahashi et al., 2013).
Inhibition in the cortex is generated by a large variety of cortical interneurons that release the neurotransmitter γ-aminobutyric acid (GABA). To gain insight into the circuit functions of inhibitory neurons in the cerebral cortex, and to understand how their contributions to behavior can be modulated by external and internal cues, it is necessary to identify discrete cortical interneuron types and investigate their physiological and molecular properties (Fishell and Heintz, 2013; Kepecs and Fishell, 2014; Pfeffer et al., 2013). For example, detailed knowledge of neurotransmitter and neuropeptide profiles of defined neurons in the crab stomatogastric ganglion (Marder, 2012), and identification neuropeptide receptors expressed in C. elegans neurons (Flavell et al., 2013), have guided electrophysiological and interventional studies that revealed state dependent contributions of these cells to behavior. Given these precedents, and the evolving concept of latent circuits that contribute to behavior in response to internal modulatory influences (Bargmann, 2012; Bargmann and Marder, 2013), it is important to identify cortical interneuron populations responding to specific neuromodulators, and understand the contributions of these neurons to complex behaviors.
GABAergic cortical interneurons emerge from one of two embryonic subcortical progenitor zones, the medial ganglionic eminence (MGE) and caudal ganglionic eminence (CGE) (Fishell and Rudy, 2011) and diversify within the developing cerebral cortex to generate an as yet undetermined number of functionally distinct cell types (Ascoli et al., 2008; Fishell and Rudy, 2011; Nelson et al., 2006). While it is clear that they can be broadly categorized based on expression of calcium binding proteins and neuropeptides (Kubota et al., 1994; Markram et al., 2004), abundant evidence that these “cardinal” interneuron types can be further subdivided into functionally relevant subtypes has been obtained (Kvitsiani et al., 2013; Xu et al., 2013). However, molecular definition of these classes of cortical interneurons and determination of their contributions to cortical function and behavior has been difficult.
To identify and characterize an interneuron population that is involved in modulation of a complex behavior, we employed TRAP translational profiling for comparative analysis of the “cardinal” interneuron populations in the mouse cerebral cortex (Doyle et al., 2008; Heiman et al., 2008). This resulted in the discovery of a novel population of somatostatin expressing, regular spiking interneurons that express the oxytocin receptor (Oxtr). To understand the role of these neurons in the complex social behaviors modulated by oxytocin, transgenic mice expressing Cre recombinase in Oxtr expressing interneurons were generated (OxtrCre) and shown to target this small population of oxytocin responsive cortical interneurons. Silencing of neurotransmission in OxtrCre interneurons in the medial prefrontal cortex (mPFC) resulted in a female specific social deficit during estrus (but not during diestrus) that did not occur upon silencing of other interneuron subtypes or pyramidal neurons in the mPFC. The loss of normal sociosexual behavior in mice carrying OxtrCre neurons that have been silenced in the mPFC was also evident in wt female mice infused with an Oxtr antagonist, and in female mice in which the oxytocin receptor gene (Oxtr) was conditionally deleted from the mPFC. Taken together, our data identify a novel subpopulation of Oxtr expressing cortical interneurons that play a gender and state specific role in oxytocin signaling in the medial prefrontal cortex. They reveal a critical function for these neurons in a latent cortical circuit that governs female sociosexual behavior, and suggest that this circuit may modulate other complex social behaviors.
RESULTS
Oxtr is expressed in a novel subpopulation of Sst positive cortical interneurons
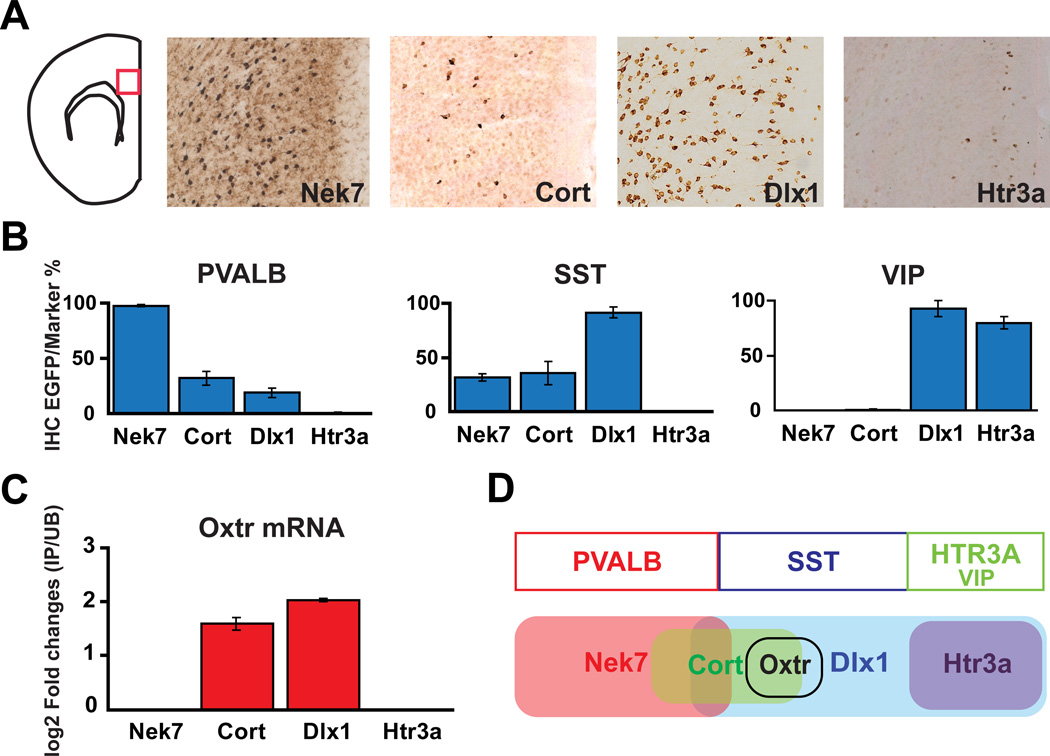
To begin to probe further the diversity of interneurons in the cerebral cortex, we employed the translating ribosome affinity purification (TRAP) approach (Doyle et al., 2008; Heiman et al., 2008) in BAC transgenic mice expressing the EGFP/L10a ribosomal fusion protein (bacTRAP) targeting broad categories of interneurons. bacTRAP mice were generated using bacterial artificial chromosomes (BAC) containing the well characterized interneuron markers distal-less homeobox 1 (Dlx1) (Cobos et al., 2005) and the serotonin receptor 3A (Htr3a) (Rudy et al., 2011), and the NIMA (never in mitosis gene a)-related expressed kinase 7 (Nek7) genes, as described previously (Doyle et al., 2008; Gong et al., 2003). The Nek7 gene was chosen as an alternative to Parv (parvalbumin) because its expression significantly overlaps in fast-spiking cortical interneurons, and because this locus performs very well as a driver in transgenic mice.
It has been reported that three non-overlapping marker populations parvalbumin (Pvalb), somatostatin (Sst), and serotonin receptor 3A (Htr3a) can account for nearly 100% of neocortical interneurons, and that all vasoactive intestinal peptide (Vip) positive neurons are included within the Htr3a group (Rudy et al., 2011). Therefore, we examined the colocalization of Pvalb, Sst and Vip proteins in these bacTRAP lines by immunofluorescence (Fig. 1B). Consistent with previous studies (Cobos et al., 2005; Ferezou et al., 2002), these bacTRAP mice targeted different, partially overlapping, GABAergic interneuron subtypes in the cortex. The Dlx1 GM520 line labeled nearly all Vip (92.9%) or Sst (91.6%) positive neurons in the cortex, but only 18.8% of the Pvalb expressing cortical interneurons. The Nek7 MN733 bacTRAP line targeted nearly all Pvalb positive (98.8%) neurons. The Nek7 line also labeled a subpopulation of Sst expressing neurons (30%), although Nek7 expressing neurons did not express Vip. In contrast, the Htr3a GM433 line is expressed in the majority of caudal ganglionic eminence (CGE) derived neurons (including Vip positive neurons), but it does not label the medial ganglionic eminence (MGE) derived Pvalb or Sst positive interneurons. Finally, to provide further discrimination in our comparative analysis, we generated a bacTRAP line for cortistatin (Cort GM130), which is expressed in subpopulations of both Pvalb and Sst positive neurons (de Lecea et al., 1997), but is not expressed in Vip expressing cortical interneurons.
Figure 1. Expression of the endogenous Oxtr gene in new bacTRAP transgenic lines (Nek7, Cort, Dlx1, Htr3a) targeting cortical interneuron populations.
(A) DAB immunohistochemistry (IHC) with anti-EGFP antibody on each mouse line in the medial prefrontal cortex (mPFC) reveals a unique and different pattern of expression for the EGFP-L10a transgene. (B) Summary of immunofluorescence studies using anti-EGFP and anti-PVALB, anti-SST, or anti-VIP antibody for each line. The Y-axis is the percentage of specific neuronal markers (Pvalb, Sst, Vip) that is also positive for EGFP. Error bars represent standard error of the mean (SEM). (C) Enrichment of Oxtr mRNA is observed in TRAP profiling data collected from Cort and Dlx1 interneurons, but not Nek7 RNA or Htr3a RNA. Error bars represent standard deviation (SD). (D) Diagram indicating that the Oxtr mRNA is present in a novel population of Sst expressing cortical interneurons.
To identify more specific interneuron expressed genes that might identify a functionally coherent cortical interneuron cell type, TRAP translational profiles were collected from each of these lines and compared (Fig. S1). As expected, previously studied conventional interneuron markers were enriched in the TRAP expression data for each line, consistent with both our characterization of these lines (Fig. 1) and studies of the complex patterns of expression of these genes in the cerebral cortex (Cobos et al., 2005; de Lecea et al., 1997; Ferezou et al., 2002). Although many novel interneuron expressed genes are evident in these data, the biological role of oxytocin in mammalian social behaviors (Gimpl and Fahrenholz, 2001; Insel, 2010) and the unique expression pattern of the oxytocin receptor gene (Oxtr) in our datasets stimulated our interest in investigation of Oxtr expressing cortical interneurons. Thus, the enriched expression of the Oxtr gene in the Dlx1 and Cort neurons but not Nek7 or Htr3a TRAP profiles, and its absence in expression profiles collected from cortical pyramidal neurons (Schmidt et al., 2012), strongly suggested that Oxtr is expressed in a specific subpopulation of Sst positive cortical interneurons (Fig. 1C, D).
An Oxtr Cre driver line targeting a specific population of cortical interneurons
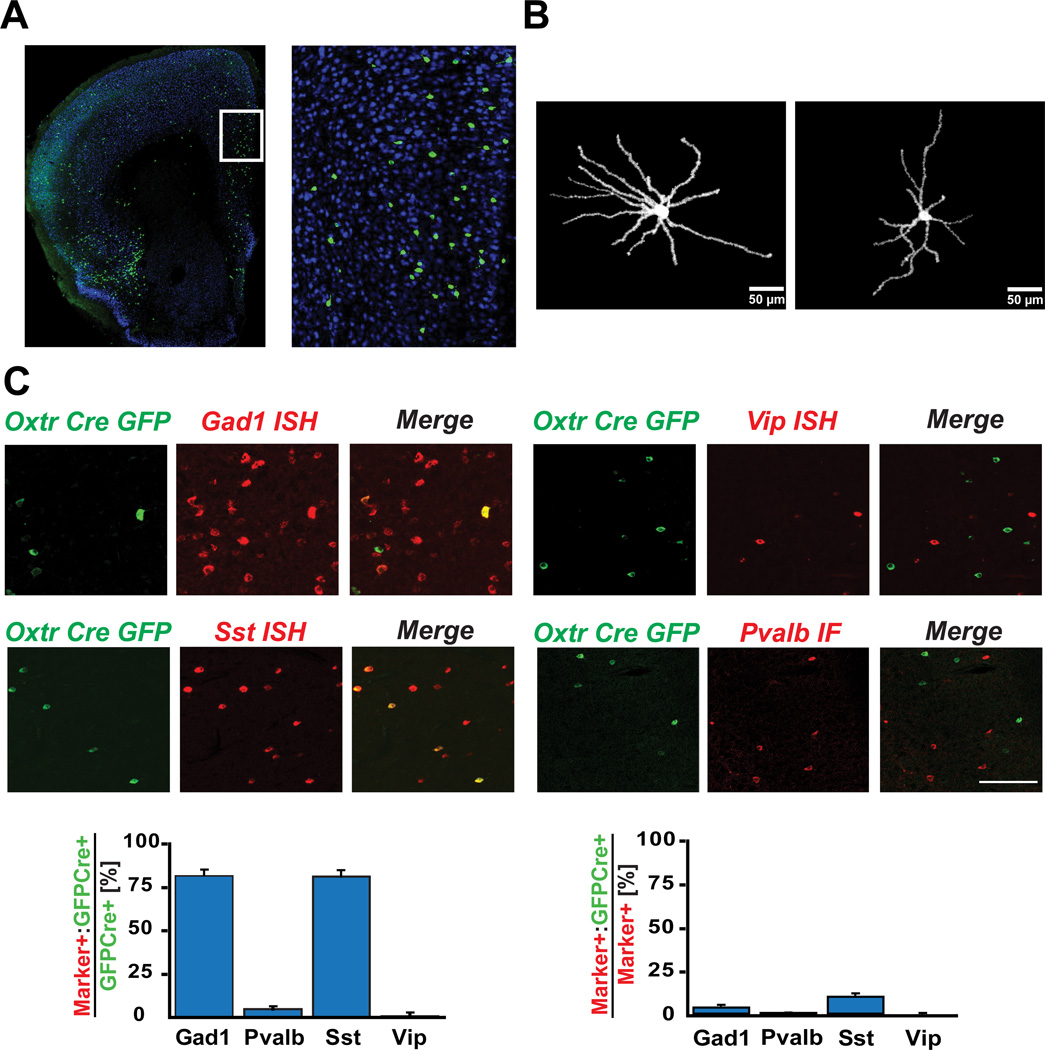
To investigate further the properties and function of Oxtr expressing interneurons, a BAC transgenic Cre driver line was prepared as previously described (Fig. 2) (Gerfen et al., 2013). The Oxtr Cre line was then crossed to an EGFP/L10a reporter line (Stanley et al., 2013) to characterize Cre expressing cells in the cerebral cortex, and determine their expression profile. OxtrCre recombination was evident in a scattered cell population in the mPFC (Fig. 2A). There were no gender differences in the number of Oxtr Cre positive neurons in the mPFC (Fig. S2). Upon injection of a Cre dependent AAV virus encoding ChR2-mCherry into the mPFC, OxtrCre neurons displayed a stellate morphology with multiple dendrites extending from the cell soma (Fig. 2B). Consistent with the comparative TRAP data used to select the Oxtr gene to target this class of neurons, EGFP/L10a recombination and expression in the Oxtr Cre line was evident in a subpopulation of Gad1 (81.8%) or Sst positive (81.3%) interneurons (Fig. 2C, left panels). These cells represented only a small minority of cortical interneurons, as we observed that only 3.4% of Gad1 expressing neurons and 9.5% of Sst positive cortical neurons co-expressed EGFP/L10a (Fig. 2C, right panels). Although expression of Oxtr was evident by in situ hybridization (ISH) in ~60% of the OxtrCre/EGFPL10a neurons (Fig. S3), the very low signal to noise ratio in the Oxtr ISH studies prevented accurate quantitation of these results.
Figure 2. Recombination in OxtrCre BAC transgenic mice is accurately targeted to a subpopulation of Sst expressing cortical interneurons.
(A) Immunofluorescence image showing EGFP-L10a expressing OxtrCre positive neurons in the medial prefrontal cortex (mPFC). The right panel is a higher magnification image of the area indicated by the box in the left panel. (B) Stellate morphology of ChR2-mCherry expressing OxtrCre neurons in mPFC. (C) Immunofluorescence for EGFP (Green) and in situ hybridization (ISH) or immunofluorescence images (Red) for known interneuron markers (scale bar = 100 µm). The EGFP signal overlapped with the Gad1/Sst ISH signal, but rarely with Vip ISH or Pvalb IF signal. Bar graphs showing quantitation of these results (n=4): the majority of EGFP positive cells (left panel) are positive for Gad1 (81.8%) and Sst (81.3%), but do not express Pvalb (4.9%) or Vip (0.7%); EGFP positive cells constitute only a small fraction of the total interneuron population Gad1 (3.4%) or of the Sst (9.5%) positive subpopulation of interneurons (right panel).
To provide further insight into the molecular nature of OxtrCre/EGFPL10a neuron population, TRAP expression profiles were collected from OxtrCre/EGFPL10a neurons (Fig. S4A). These results support the anatomical studies: expression of Gad1, Gad2, Oxtr and Sst are all enriched in the TRAP data from the OxtrCre neurons, whereas Htr3a and Vip are not enriched. Markers for cortical pyramidal cells (e.g. Emx1) are depleted in these data. Furthermore, comparative analysis of TRAP data collected from independently generated additional cortical interneuron and pyramidal cell bacTRAP lines (Schmidt et al., 2012), confirmed that expression of the endogenous Oxtr gene is evident in the OxtrCre/EGFPL10a, Cort and Dlx1 TRAP data, and not detected in the Nek7 and Htr3a interneuron or the S100a10 pyramidal cell data (Fig. S4B). That the OxtrCre/EGFPL10a line reflected accurately the known markers for specific classes of cortical neurons is evident from the enrichment or depletion of known markers. Thus, Pvalb is expressed in Nek7, Cort and Dlx1 interneurons, and not detected or depleted in Oxtr and Htr3a TRAP data; Sst is enriched in Oxtr, Cort and Dlx1 interneurons, and depleted in Nek7, Htr3a, and S100a10 data; Htr3a is expressed in the Htr3a and Dlx1 TRAP data, but not the Oxtr, Nek7, Cort or S100a10 expression data; and Emx1 is uniquely expressed in the TRAP dataset collected from a pyramidal cell bacTRAP line (S100a10) (Fig. S4C). Taken together, the immunofluorescence analysis of the Cre expressing cells, the enrichment and depletion of conventional interneuron and pyramidal cell markers in the TRAP expression data prepared from the Oxtr/Cre targeted cell population, and the comparative studies of Oxtr expression in bacTRAP lines targeting known populations of interneurons and pyramidal cells identify the targeted neurons in this OxtrCre line as a novel subpopulation of Sst positive cortical interneurons that express the endogenous Oxtr gene.
OxtrINs display a distinct physiological profile and spontaneously fire upon Oxytocin application
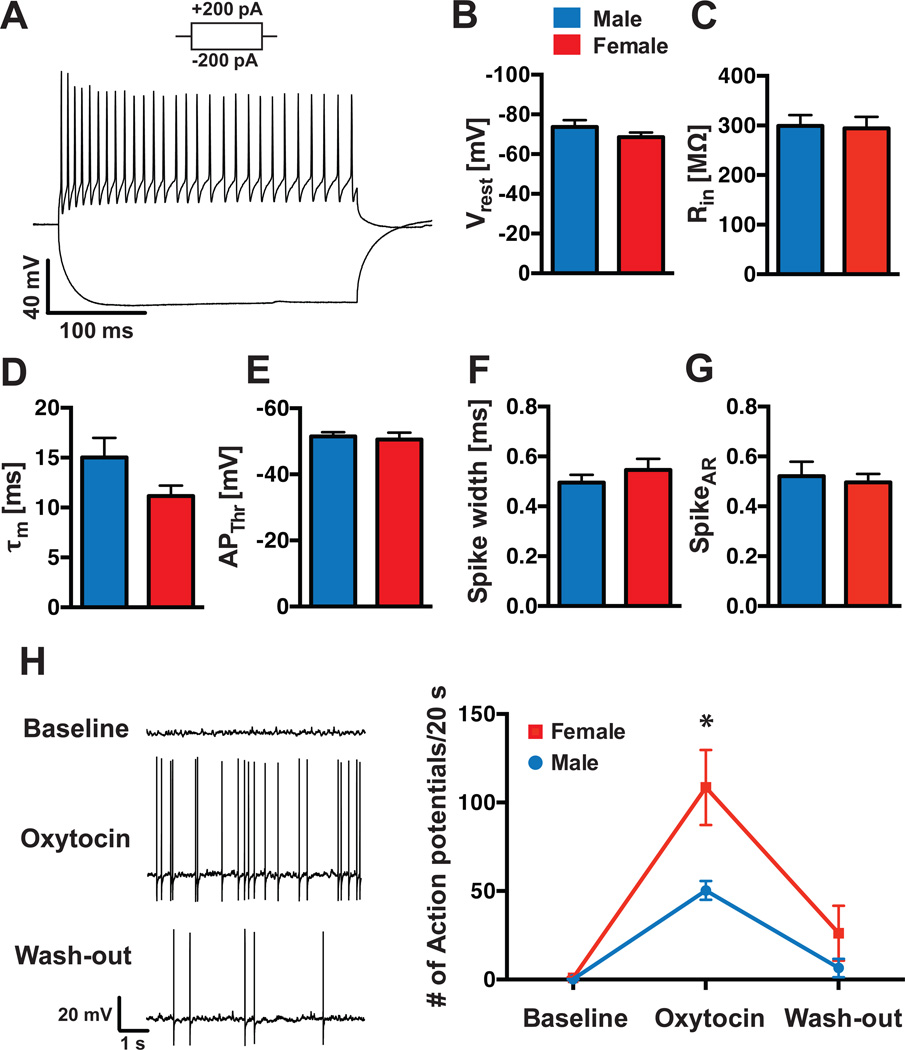
The novel molecular profile of OxtrINs suggests that their electrophysiological properties might also be distinctive. To determine whether this is the case, we performed whole-cell current-clamp recordings from OxtrINs from male and female mice to analyze their basic physiological properties, their action-potential (AP) firing pattern (Fig. 3A) and potential gender differences in their physiology. In contrast to the previously studied Sst positive Martinotti, X94, X98 or GIN interneuron types (Ma et al., 2006; Xu et al., 2013), no Ih-currents were observed in OxtrINs in either gender (Fig. 3A). These currents are typically driven by hyperpolarization-activated cyclic nucleotide-gated (HCN) channels, which were not enriched in the OxtrIN TRAP data. Similar to Martinotti cells, OxtrINs displayed continuously adapting, regular AP-firing upon 200 pA current injections (Fig. 3A, G; spike-adaptation rate (SpikeAR) = 0.52 ± 0.06 for male, 0.50 ± 0.03 for female), although their resting membrane potential was significantly more negative than Martinotti cells (Vrest = −73.7 ± 3.39 mV for male, −68.6 ± 2.38 mV for female; Fig. 3B; Xu et al., 2013). Thus, although the general electrophysiological parameters of OxtrINs (input resistance (Rin); membrane time constant (τm); AP-threshold (APThr); Spike width; Fig. 3C–F; Table S1) are consistent with their identity as a subpopulation of Sst positive cortical interneurons, their properties are not shared by previously characterized cortical interneuron types.
Figure 3. OxtrCre interneurons initiate action potential firing in response to oxytocin.
(A): A representative trace of OxtrCre interneuron activity upon a +200 pA and −200 pA current pulse at resting membrane potential. Plots showing the resting potential (Vrest) (B); the input resistance (Rin) (C); the membrane time constant (τm) (D); the action potential threshold (APThr) (E); the spike width (F); and the spike adaptation ratio (SpikeAR) (G) in OxtrINs from male (blue bars) and female (red bars) animals. Oxytocin receptor positive neurons at Vrest were not spontaneously active, but initiated action potential firing upon bath application of 100 nM Oxytocin (H, left panel). This effect was more pronounced in females (108.5 ± 21.25 APs/20 s, n=10), compared to males (50.3 ± 5.32 APs/20 s, n=6; p=0.024); wash-out of oxytocin partially reduced action potential firing to baseline level in both groups (H, right panel).
As predicted from their identification as interneurons that express the oxytocin receptor, OxtrINs respond robustly to OT. Thus, patch-clamped recordings of OxtrINs under basal conditions reveal that they are inactive. However, upon bath-application of oxytocin (100 nM) to the slice, the cells begin to fire APs (Fig. 3H). Interestingly, although general physiological properties were not sexually dimorphic between males and females (Table S1), the oxytocin effect on AP-firing was more pronounced in OxtrINs from female, compared to male mice (female: 108.5 ± 21.25 APs/20 s, n=10; male: 50.3 ± 5.32 APs/20 s, n=6; p=0.024; Fig. 3H). The resting membrane potential did not significantly change over the course of the experiment, and a 10-minute wash-out of oxytocin partially restored initial baseline conditions in both genders (Fig. 3H). Taken together, these data identify OxtrINs as a novel population of continuously adapting cortical interneurons, with a gender specific response to oxytocin.
OxtrCre neurons in the medial prefrontal cortex (mPFC) are required for normal female sociosexual behavior during estrus
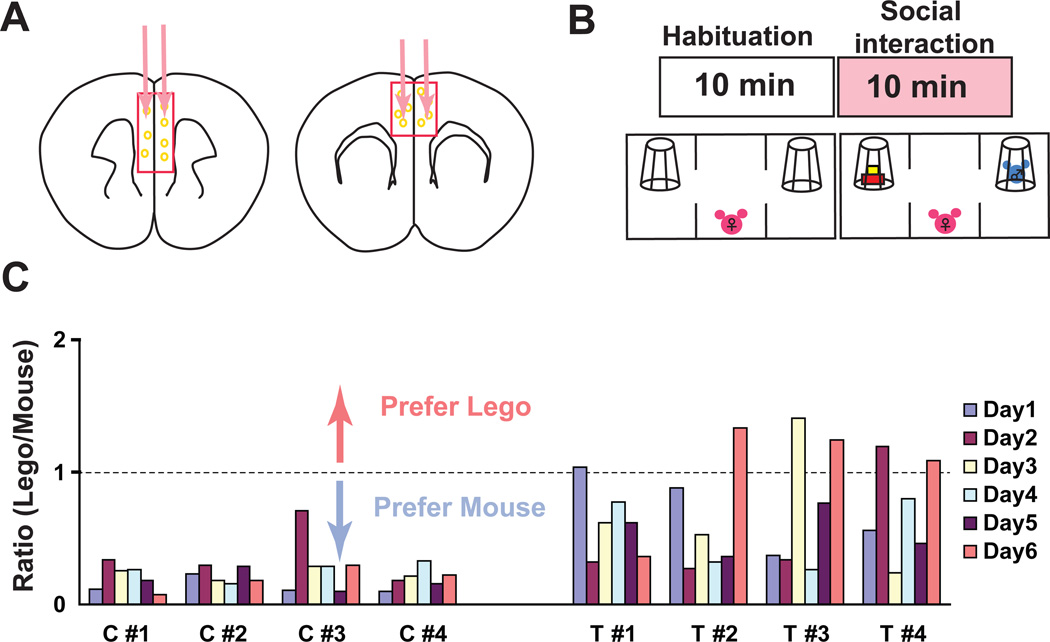
Oxytocin is a nonapeptide hormone that is known to play important roles in a variety of mammalian social behaviors, including maternal care, pair bonding, social recognition, stress and anxiety (Gimpl and Fahrenholz, 2001; Insel, 2010). Given our demonstration that the action of oxytocin on OxtrCre neurons is to stimulate their activity, and recent studies showing that manipulation of the general balance of excitation and inhibition in the mPFC alters social behavior (Yizhar et al., 2011), we next tested directly the impact of OxtrCre neurons in the mPFC in mouse social interactions. To do so, we chose the viral mediated tethered toxin (t-toxin) strategy for chronic inhibition of neurotransmission (Auer et al., 2010; Warner-Schmidt et al., 2012) in mPFC OxtrCre interneurons, and the three chamber mouse social interaction test (Moy et al., 2004). Adult OxtrCre mice were injected stereotactically at four sites in the mPFC (Fig. 4A) with AAV2/8 viruses that express tethered peptide toxins that inhibit the Cav2.1 and Cav2.2 calcium channels only after recombination in Cre expressing neurons (thus blocking neurotransmission from mPFC OxtrCre neurons) or control viruses expressing EGFP alone. Virus infection was found in cingulate, prelimbic, infralimbic and medial orbital cortex (from bregma 2.5 to bregma −0.5mm). Given the known roles of oxytocin in female sociosexual behavior (Ross et al., 2009), and the increased physiological response in female OxtrINs, we were particularly interested in female social interactions with male mice. Thus, following habituation to the three chamber social interaction box, virus injected OxtrCre mice were given a choice to explore a chamber containing an unknown mouse or one containing a plastic Lego block (Fig. 4B). Initial results from the tests were quite variable (Fig. 4C). Although no effect of silencing was observed in the social interaction tests for any of the male mice tested (Fig S5), a strong but quite variable effect was observed in female mice injected with the silencing viruses (Fig. 4C).
Figure 4. Silencing of OxtrCre in the mPFC of female mice results in variations in social preference toward male mice over a six day period.
(A) Schematic diagram showing 4 injection sites of Cre dependent AAV viruses encoding membrane-tethered toxins that block neurotransmission (APC/MPE) or control AAV viruses expressing EGFP. (B) Diagram indicating the social interaction test. After 10min habituation to the apparatus, a subject female mouse is exposed to a Lego (novel object, left panel) and a strange wt male mouse (stranger mouse, right panel) in wire cups for 10 min. (C) Results of the social interaction at 6 consecutive days of testing for four control virus injected OxtrCre female mice (C1–4) and 4 t-toxin virus injected OxtrCre female mice (T1–4). The ratio of exploration of the novel object relative to the stranger male mouse indicated on the Y-axis (Lego cup/Mouse cup). A ratio of 1 indicates equal preference for the Lego and the stranger mouse, whereas values below 1 indicate a preference for the mouse versus the Lego. Control mice (C1–4) prefer interaction with the male stranger mouse on all days of testing. OxtrCre interneuron silenced mice (T1–4) lost their preference for social interaction with the male stranger mouse on certain test days.
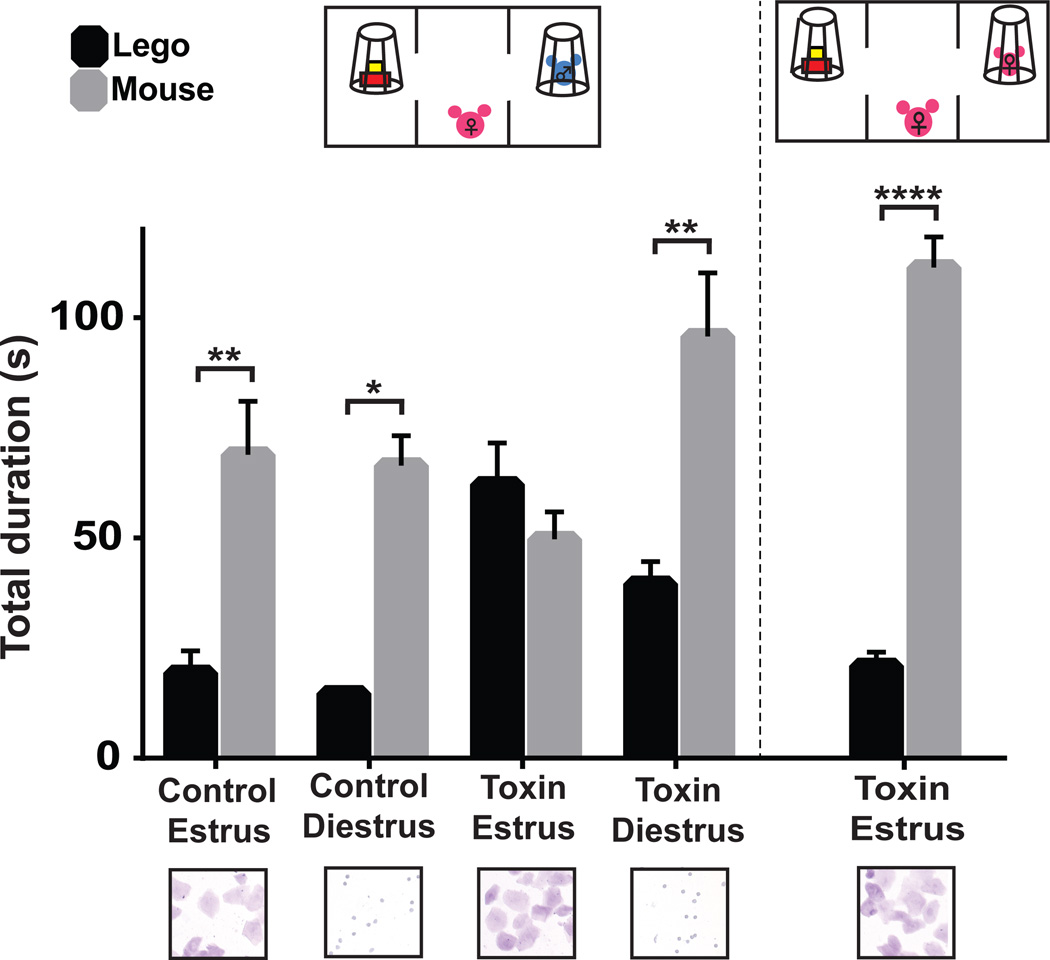
To ascertain whether this variability in female social approach behavior might result from interference with the actions of oxytocin in the mPFC during a specific time in their estrous cycle, new cohorts of OxtrCre females were injected with the t-toxin and control viruses in the mPFC, their social interactions measured, and vaginal smears collected and examined to determine whether the mice were in the estrus or diestrus phase of their cycle (McLean et al., 2012). As shown in Fig. 5, silencing of OxtrCre neurons in the mPFC had a major effect on social interactions with male mice during the sexually responsive, estrus phase of their cycle. Thus, female mice in which these interneurons were silenced lost interest in male mice during estrus. However, these same animals retained their social interest in female mice during estrus, and in male mice during diestrus (Fig. 5). There was no impact of OxtrCre neuron silencing on the distance traveled (Fig. S6) or in the novel object recognition test (Fig. S7), demonstrating that the loss of social preference we observed is not due to hyperactivity. We conclude that Oxtr expressing interneurons in the mPFC play an important role in the regulation of male-directed, female social behavior specifically during the sexually responsive phase of the estrous cycle.
Figure 5. Silencing of OxtrCre interneurons in the mPFC of female mice causes an estrous cycle dependent loss of social interest in male mice.
Control virus injected in OxtrCre female mice (littermates of the experimental group) showed normal preference to male mice regardless of the stage of the estrous cycle (Estrus, n=11 p**≤0.01; Diestrus, n=6; p*≤0.05). T-toxin virus injected OxtrCre female mice showed no preference to WT male mouse during estrus, the sexually responsive stage of the estrous cycle (n=14). However, during diestrus (when they are not sexually receptive), these same animals display significantly stronger interest in male mice (n=9, p**≤0.01). T-toxin injected mice display normal interest in female mice during estrus (right diagram and columns), indicating that general social recognition is not impaired during this phase of their cycle (n=10, p****≤0.0001). Below each column is a photograph of cell shapes evident in vaginal smears taken from a representative female mouse in the test group. Summary data are presented as mean ± s.e.m..
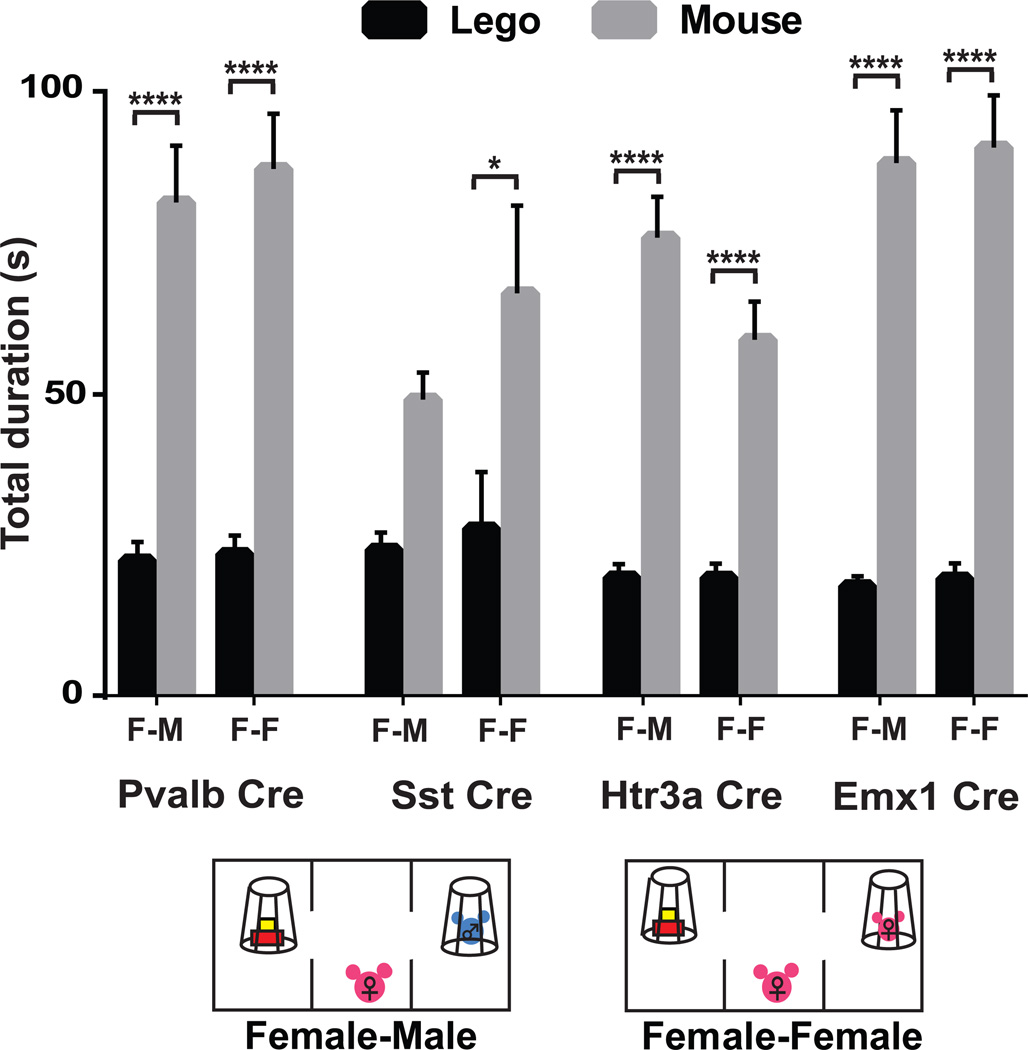
To understand whether this phenotype occurred specifically as a result of altering the activity of this specific class of cortical interneurons, or whether it might result from an general imbalance in excitation/inhibition in the mPFC that causes a less specific alteration in social behavior, we measured the effects of silencing of several additional cell types on female specific, estrus specific social interest in males. Injections of the AAV t-toxin viruses into Htr3a (Chittajallu et al., 2013), Pvalb (Hippenmeyer et al., 2005) and Emx1 (Gorski et al., 2002) Cre driver lines had no impact on female specific social behavior during estrus (Fig. 6). Interestingly, Sst Cre (Taniguchi et al., 2011) injected mice displayed a mild phenotype in the social interaction test. Although their social response to male mice during estrus was blunted, they retained interest in female mice as expected. This supports the finding that Sst positive interneurons can be subdivided into subtypes with distinct functions (Xu et al., 2013). These data suggest that OxtrCre neurons regulate specifically female sociosexual behavior, and that a general imbalance in excitation/inhibition in the mPFC is not sufficient to alter this aspect of female sociality.
Figure 6. The sociosexual behavioral deficit resulting from T-toxin silencing of OxtrCre interneurons in the mPFC is cell type specific.
Female-male (F–M) and female-female (F-F) social interactions (see diagrams below figures) during estrus in additional Cre driver lines injected with the T-toxin virus in the mPFC: Parv interneurons (Pvalb Cre), Sst interneurons (Sst Cre), Htr3a interneurons (Htr3a Cre) and pyramidal cells (Emx1 Cre). No statistically significant social deficit is observed in animals in which populations of cortical neurons that do not overlap with OxtrINs have been silenced: Pvalb Cre (n=9, p****≤0.0001, n=15, p****≤0.0001), Htr3a Cre (n=9, p****≤0.0001, n=8, p****≤0.0001), Emx1Cre (n=11, p****≤0.0001, n=12, p****≤0.0001). However, silencing of the parental interneuron population for OxtrINs in female mice (Sst Cre) resulted in a less robust social interaction with male mice during estrus (n=9) while maintaining normal interactions with female mice (n=11, p*≤0.05). Summary data are presented as mean ± s.e.m.
Oxytocin action in the mPFC is required to regulate female sociosexual behavior
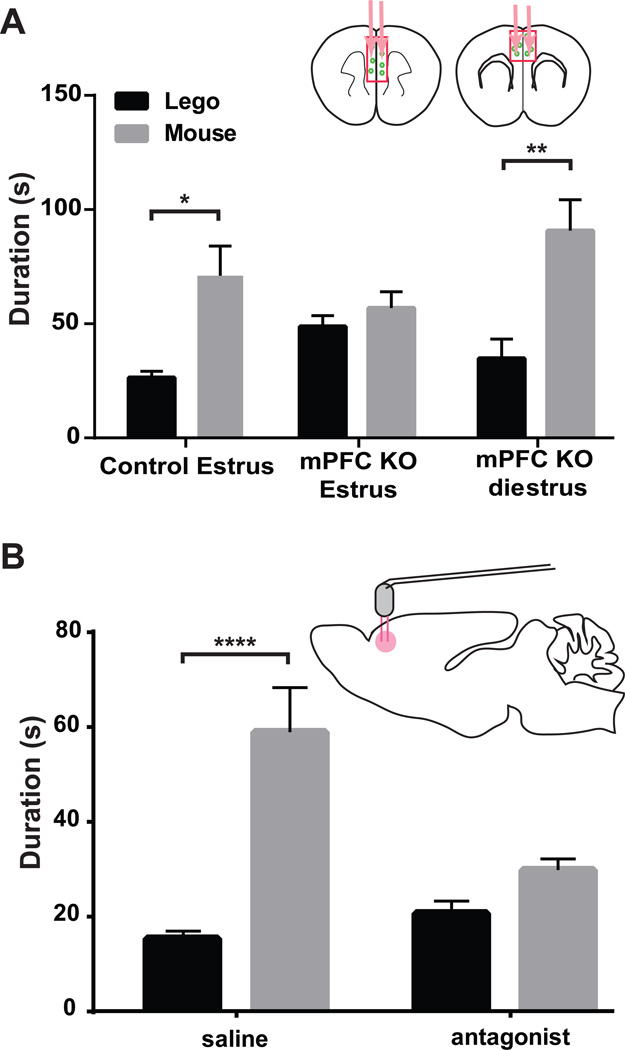
The known roles of oxytocin in regulating female social behaviors (Campbell, 2008; McCall and Singer, 2012), and the present demonstration that OxtrCre neuron activity in mPFC is required for female social approach to male mice during estrus, strongly suggest that oxytocin acts within the mPFC to regulate female social behavior. To test directly this hypothesis, two approaches were employed. First, Cre recombinase expressing AAV viruses were injected into the mPFC of mice carrying a conditional allele of Oxtr gene (Lee et al., 2008) to delete Oxtr (and hence signaling by oxytocin) specifically in the mPFC. As shown in Figure 7A, genetic ablation of Oxtr in the mPFC of female mice again resulted in loss of social interest in male mice specifically during estrus, having no effect on social approach during diestrus (Fig. 7A). To confirm this result, a second set of experiments were conducted using a specific antagonist of the Oxtr (Dolen et al., 2013). Thus, Oxtr-A (L-368,899 hydrochloride, 10 nM, and 0.5 µl) was infused bilaterally into the mPFC of female mice through a cannula to block pharmacologically the Oxtr during the social interaction test. Control mice infused with saline displayed normal sociality in these assays, whereas those females infused with Oxtr-A lost interest in male mice in the social approach test during estrus (Fig. 7B). Taken together, the aberrant social approach behavior evident in the three chamber social interaction test as a result of either genetic or pharmacological disruption of oxytocin signaling in the mPFC proves that oxytocin action in the mPFC is required for normal female sociosexual behavior.
Figure 7. Female social interactions with male mice during estrus are oxytocin dependent.
Two approaches were used to disrupt oxytocin signaling in the mPFC and measure its effects on female social behavior. (A) Injection of an AAV Cre virus into the mPFC of female Oxtr conditional knockout (mPFC Oxtr KO) mice results in loss of social interest in male mice during estrus. Female mPFC Oxtr KO mice injected with the control virus (n=7) displayed normal social behavior during estrus (p*≤0.05). mPFC Oxtr KO female mice at the estrus stage (n=8) failed to show preference for male mice, however, they retained their social interest in male mice (n=7) during diestrus (p**≤0.01). (B) Wild type female mice injected with the Oxtr antagonist into the mPFC (L-368,899 hydrochloride) (n=11), also lost the social preference for male mice during estrus. Saline injected control mPFC injected female mice retained normal behavior during estrus (n=9, p****≤0.0001). Social interactions were tested using the three chamber social interaction test. Summary data are presented as mean ± s.e.m.
DISCUSSION
A mechanistic understanding of mammalian brain function must include identification of cell types and circuits that control specific aspects of behavior, and analysis of the internal and external cues that regulate their functions. In this study, we have employed TRAP translational profiling (Doyle et al., 2008; Heiman et al., 2008) of cardinal interneuron populations in the cerebral cortex (Rudy et al., 2011) to identify a subpopulation of somatostatin positive, oxytocin responsive, regular spiking interneurons. Chronic silencing of this interneuron population (OxtrINs) in the mPFC revealed that it modulates female social interactions with male mice during estrus, the sexually receptive phase of the estrous cycle. Deletion of the Oxtr gene in the mPFC, or pharmacological inhibition of Oxtr activity at this site, demonstrated that modulation of this aspect of female social behavior requires oxytocin. Taken together, our data identify a novel interneuron population in the mouse prefrontal cortex that regulates an important aspect of female sociosexual behavior, and suggest that OxtrINs in the mPFC may be important for the modulation of social interactions in response to the changing levels of oxytocin that occur in a variety of contexts in non-human primates (Moscovice and Ziegler, 2012) and humans (Heinrichs and Domes, 2008).
Are OxtrINs a new cell type?
It is generally agreed that identification of new CNS cell types must include data demonstrating their anatomical and molecular properties, their physiological characteristics, and their contributions to CNS function (Ascoli et al., 2008; Markram et al., 2004). In this study, we have determined that OxtrINs in the mPFC express a distinctive set of molecular markers, that they are continuously adapting, regular spiking GABAergic neurons, that they respond to oxytocin by increasing their basal firing rate, and that oxytocin action on these neurons modulates a very specific aspect of female social behavior. These properties, particularly the specific function of OxtrINs relative to other Sst positive subpopulations, and Htr3a or Pvalb expressing interneurons, allow us to conclude provisionally that OxtrINs are a novel, functionally coherent cell type. However, there are two issues that preclude a definitive conclusion regarding OxtrIN identity. First, we have not provided detailed anatomical data placing the OxtrINs within cortical circuitry, or examined possible variations in OxtrINs in other regions of the cerebral cortex. Second, while our comparative data demonstrate that OxtrINs share a gene expression signature in their ground state that is distinct from other known cortical interneuron populations (Fig. S4), these analyses have been done using data from broad classes of interneurons that are not sufficiently well defined to allow us to assign a unique molecular identity to OxtrINs. Nevertheless, the data that we have collected thus far, and the fact that there is only one known receptor for oxytocin (Gimpl and Fahrenholz, 2001), suggest that further molecular and functional studies of OxtrINs may confirm our provisional definition of these cells as a coherent, modulatory cell type that participates in a latent cortical circuit that controls specific oxytocin dependent behaviors (Bargmann, 2012).
Oxytocin function during the estrous cycle
A central finding of our study is that experimental disruptions of oxytocin responsive interneurons in the mPFC, or of oxytocin signaling to these neurons, results in loss of female social approach to male mice in sexually receptive estrus females but not in sexually unresponsive diestrus females. In a general sense, this finding fits in well with previous studies of prosocial behavioral effects of the hormone (Donaldson and Young, 2008; Insel and Young, 2001) and with the variations in peripheral oxytocin levels that are known to occur during estrus in a variety of species. Studies of rodents (Crowley et al., 1978; Van Tol et al., 1988), non-human primates (Perlow et al., 1982) and women (McCarthy and Altemus, 1997; Richard and Zingg, 1990) have provided evidence that there is an estrogen dependent surge in oxytocin in the periovulatory period that facilitates female sexual receptivity. Recent evidence in freely cycling female baboons in the wild (Moscovice and Ziegler, 2012), or in human couples (Scheele et al., 2012), have provided the additional insight that oxytocin acts primarily in these contexts to improve interactive reciprocity between consorting partners. However, the levels of OT binding varies between brain area and during estrus (Dumais et al., 2013), and Greer et al., 1986 have reported that OT concentrations in the paraventricular hypothalamus was significantly greater during diestrus. In spite of the apparent complexities in OT regulation during the estrous cycle, we believe the simplest interpretation of our results is that OxtrINs respond to the fluctuations in oxytocin levels that occur during the estrous cycle, and that they modulate latent cortical circuits that play a fundamental role in the female receptivity to males. Our observation that OxtrINs in the mPFC of female mice respond to OT more robustly than those in male mice (Fig. 3) suggests that female mice may be more sensitive generally than male mice to changing levels of OT. Although OxtrINs have not been identified or studied in the cortex of other mammals, it seems probable that this new class of interneurons and their associated circuitry may modulate preferentially female social interactions.
Oxytocin and the Cerebral Cortex
A large number of studies in experimental animals and humans have established that oxytocin regulates many aspects of mammalian social behavior, from the regulation of affiliate behaviors between individuals to social behaviors in groups (Anacker and Beery, 2013). Oxtr knockout mice display deficits in social memory (Lee et al., 2008) and social approach behavior (Nishimori et al., 2008). It is apparent that oxytocin production in the hypothalamus is regulated in response to social interactions in many species (Leng et al., 2008), that oxytocin expressing neurons project widely (Knobloch et al., 2012; Knobloch and Grinevich, 2014), and that Oxtr is expressed in many brain structures (Gimpl and Fahrenholz, 2001; Yoshida et al., 2009). The fact that OxtrINs are dispersed throughout the cerebral cortex, and that within the mPFC these cells are important for female interactions with male mice specifically during the peak of peripheral oxytocin that occurs around estrus, suggests that these neurons participate in a latent cortical circuit (Bargmann, 2012) that can modulate behavior in a state dependent manner. It seems probable that this circuit will be activated in response to elevated oxytocin levels in a variety of contexts, and that it will mediate the contributions of the cerebral cortex to oxytocin dependent behaviors. Recent studies of the effects of intranasal oxytocin administration in children and adults with autism spectrum (ASD) disorder are of particular interest. Although it remains unclear whether oxytocin therapy will be safe and effective for long term use in the treatment of social deficits associated with ASD (MacDonald et al., 2011), encouraging results in clinical trials have emerged recently (Gordon et al., 2013; Guastella and MacLeod, 2012; Watanabe et al., 2014). Our studies strongly implicate the mPFC and other cortical areas in the behavioral effects of oxytocin administration and treatment, and suggest that further investigation of the contributions of OxtrINs to behavior in a variety of social contexts, and definition of the exact mechanisms responsible for activation of this interesting circuit, may provide insights into ASD and other social behavioral disorders.
Activation of OxtrINs
Oxtr is expressed in many brain structures, and in distinct cell types (Yoshida et al., 2009). The cortical OxtrINs identified here are nearly silent under basal conditions in acute mPFC brain slices, and they respond to oxytocin by initiating action potential firing in response to bath application of the hormone (Fig. 3). In the nucleus accumbens, presynaptic Oxtr on serotonergic neurons from the dorsal raphe modulate long term depression, thus contributing to social reward behaviors (Dolen et al., 2013). In the median raphe nucleus, oxytocin acts through its receptors on serotonergic neurons to induce serotonin release and reduce anxiety related behaviors (Yoshida et al., 2009). In the hippocampus, oxytocin increases the firing of fast spiking interneurons and improves the fidelity of information transfer from these neurons to pyramidal cells (Owen et al., 2013). Although there is only one Oxtr, it can couple to different G proteins and, therefore, lead to different functional effects within the cell (Gimpl and Fahrenholz, 2001; Stoop, 2012). Given the variety of cell types expressing Oxtr, the different cellular mechanisms that are engaged by Oxtr depending on its G protein coupling, and the distinct physiological effects of oxytocin on receptor bearing neurons, two areas of investigation would significantly improve our understanding of the functions of OxtrINs. First, additional studies of Oxtr signal transduction in OxtrINs can provide additional insights into its mechanism of action, and its possible interactions with other neuromodulators. Second, detailed neuroanatomical studies of the connectivity of OxtrINs in the cerebral cortex, and the influence of these neurons on their targets, would shed important light on the circuit mechanisms that are engaged by oxytocin in the cerebral cortex to regulate behavior.
CONCLUDING REMARKS
In this study, we have characterized a novel interneuron population in the cerebral cortex (OxtrINs) that regulates a gender specific, estrus specific social behavior. The modulation of these neurons by oxytocin, and their state dependent contributions to behavior, identify them as components of a circuit that is activated in response to an internal physiological cue to regulate an important aspect of female sociosexual behavior. Thus, our data extend the concept of latent circuits that has arisen from studies on neuromodulation in crustaceans, flies, worms and mammalian sensory neurons (Bargmann, 2012) to the cerebral cortex, and suggest that external social stimuli that stimulate oxytocin production will recruit this newly identified circuit to help coordinate the complex behavioral responses elicited by changing social situations in all mammals, including humans (Gimpl and Fahrenholz, 2001). Finally, we identify OxtrINs as an important focus for investigation of cellular and molecular mechanisms that can play a role in normalization of the aberrant cortical circuit activities evident in ASD and other human social disorders (Kehrer et al., 2008; Markram and Markram, 2010; Rubenstein and Merzenich, 2003).
Experimental Procedures
Animals. All procedures involving animals were approved by the Rockefeller University Institutional Animal Care and Use Committee and were in accordance with the National Institutes of Health guidelines. The Dlx1 GM520, Nek7 MN733, Cort GM130 and Htr3a GM443 bacTRAP mice, and OxtrCre ON82 and Htr3aCre NO152 mice were generated and maintained at the Rockefeller University (Doyle et al., 2008; Schmidt et al., 2012). Conditional Oxtr KO mice, Pvalb-Cre, SST Cre and Emx1 Cre mice were purchased from The Jackson Laboratory (#008471, #008069, #013044, #005628)
Immunofluorescence staining and In Situ Hybridization. Immunofluorescence staining was carried out on 35 µm thick sections using chicken anti-GFP (1:1000, Abcam), rabbit anti-PV (1:1000, Swant), rat anti-SST (1:100, Chemicon) and rabbit anti-VIP (1:500, Immunostar) followed by Alexa-fluor conjugated secondary antibodies (Invitrogen). Fluorescence in situ hybridization (FISH) was performed as described previously (Ishii et al., 2004; Serizawa et al., 2003). Detailed protocols are in the extended experimental procedures.
Immunohistochemistry. For Diaminobenzidene (DAB) staining, brains were processed identically with MultiBrain Technology (NSA, Neuro Science Associates, Knoxville, TN) with a 1:75,000 dilution of Goat anti-EGFP serum (Heiman et al., 2008) according to the Vectastain elite protocol (Vector Labs, Burlingame, CA).
TRAP Translational profiling. TRAP was conducted as described previously (Doyle et al., 2008; Heiman et al., 2008). Whole cortices were dissected and homogenized rapidly. Collected polysomes were immunoprecipitated with a mix of two monoclonal antibodies (19C8, 19F7) against EGFP. For microarray analysis, 20 ng of total RNA was amplified and hybridized to GeneChip Mouse Genome 430 2.0 microarrays (Affymetrix). Detailed protocols are in the extended experimental procedures. Raw data are available from Gene Expression Omnibus with accession number GSE56996.
Slice preparation and electrophysiological recordings. Patch pipettes had resistances of 4–8 MΩ when filled with a solution containing (in mM): 105 K-gluconate, 30 KCl, 10 Hepes, 10 19 phosphocreatine, 4 ATP-Mg2+, 0.3 GTP (pH adjusted to 7.2 with KOH). Electrophysiological responses were recorded at 33–36 °C. Recordings were basically analyzed as described in Xu et al., 2013. Oxytocin was bath applied at 100 nM.
Behavioral testing (detailed descriptions of behavioral methods can be found in Extended Experimental Procedures). Virgin female mice were used for behavioral experiments. The threechamber social interaction test was conducted as described previously (Moy et al., 2004). Results from each time investigating each cup and the distance they traveled was recorded manually and automatically using Ethovision v7.0 (Noldus, Leesburg VA). Statistical comparisons were made by ANOVA using Prism 5 software (GraphPad).
Supplementary Material
Highlights.
Oxtr Cre BAC transgenic mice target a subset of Sst positive cortical interneurons
OxtrINs respond to Oxytocin by increasing their firing rate
OxtrINs in mPFC are required for female social interest in male mice during estrus
Oxytocin action in mPFC is required for female sociosexual behavior
Acknowledgments
This work was supported by the Howard Hughes Medical Institute (NH). We wish to thank Guojun Ma and Clint Earnheart for their contributions to the initial characterization of bacTRAP lines targeting cortical interneuron populations and the GENSAT Project for the Htr3a Cre mice used in this study. We would also like to thank Jie Xing, Paola Emhardt, Beatriz Lopez for their skilled assistance, Soohyun Lee, Robin Tremblay, Eric Schmidt, Joseph Doughterty, Jodi Gresack, Hirofumi Nakayama, Awni Mousa and all other Heintz lab members for their excellent advice, and Wenxiang Zhang from the Rockefeller University Genomics Resource Center for help with the TRAP data production.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anacker AM, Beery AK. Life in groups: the roles of oxytocin in mammalian sociality. Front Behav Neurosci. 2013;7:185. doi: 10.3389/fnbeh.2013.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ascoli GA, Alonso-Nanclares L, Anderson SA, Barrionuevo G, Benavides-Piccione R, Burkhalter A, Buzsaki G, Cauli B, Defelipe J, Fairen A, et al. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat Rev Neurosci. 2008;9:557–568. doi: 10.1038/nrn2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer S, Sturzebecher AS, Juttner R, Santos-Torres J, Hanack C, Frahm S, Liehl B, Ibanez-Tallon I. Silencing neurotransmission with membrane-tethered toxins. Nat Methods. 2010;7:229–236. doi: 10.1038/nmeth.1425. [DOI] [PubMed] [Google Scholar]
- Bargmann CI. Beyond the connectome: how neuromodulators shape neural circuits. Bioessays. 2012;34:458–465. doi: 10.1002/bies.201100185. [DOI] [PubMed] [Google Scholar]
- Bargmann CI, Marder E. From the connectome to brain function. Nat Methods. 2013;10:483–490. doi: 10.1038/nmeth.2451. [DOI] [PubMed] [Google Scholar]
- Campbell A. Attachment, aggression and affiliation: the role of oxytocin in female social behavior. Biol Psychol. 2008;77:1–10. doi: 10.1016/j.biopsycho.2007.09.001. [DOI] [PubMed] [Google Scholar]
- Chao HT, Chen H, Samaco RC, Xue M, Chahrour M, Yoo J, Neul JL, Gong S, Lu HC, Heintz N, et al. Dysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypes. Nature. 2010;468:263–269. doi: 10.1038/nature09582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chittajallu R, Craig MT, McFarland A, Yuan X, Gerfen S, Tricoire L, Erkkila B, Barron SC, Lopez CM, Liang BJ, et al. Dual origins of functionally distinct O-LM interneurons revealed by differential 5-HT(3A)R expression. Nat Neurosci. 2013;16:1598–1607. doi: 10.1038/nn.3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobos I, Calcagnotto ME, Vilaythong AJ, Thwin MT, Noebels JL, Baraban SC, Rubenstein JL. Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nat Neurosci. 2005;8:1059–1068. doi: 10.1038/nn1499. [DOI] [PubMed] [Google Scholar]
- Crowley WR, O'Donohue TL, George JM, Jacobowitz DM. Changes in pituitary oxytocin and vasopressin during the estrous cycle and after ovarian hormones: evidence for mediation by norepinephrine. Life Sci. 1978;23:2579–2585. doi: 10.1016/0024-3205(78)90373-9. [DOI] [PubMed] [Google Scholar]
- de Lecea L, del Rio JA, Criado JR, Alcantara S, Morales M, Danielson PE, Henriksen SJ, Soriano E, Sutcliffe JG. Cortistatin is expressed in a distinct subset of cortical interneurons. J Neurosci. 1997;17:5868–5880. doi: 10.1523/JNEUROSCI.17-15-05868.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501:179–184. doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- Doyle JP, Dougherty JD, Heiman M, Schmidt EF, Stevens TR, Ma G, Bupp S, Shrestha P, Shah RD, Doughty ML, et al. Application of a translational profiling approach for the comparative analysis of CNS cell types. Cell. 2008;135:749–762. doi: 10.1016/j.cell.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumais KM, Bredewold R, Mayer TE, Veenema AH. Sex differences in oxytocin receptor binding in forebrain regions: correlations with social interest in brain region- and sex- specific ways. Horm Behav. 2013;64:693–701. doi: 10.1016/j.yhbeh.2013.08.012. [DOI] [PubMed] [Google Scholar]
- Ferezou I, Cauli B, Hill EL, Rossier J, Hamel E, Lambolez B. 5-HT3 receptors mediate serotonergic fast synaptic excitation of neocortical vasoactive intestinal peptide/cholecystokinin interneurons. J Neurosci. 2002;22:7389–7397. doi: 10.1523/JNEUROSCI.22-17-07389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishell G, Heintz N. The neuron identity problem: form meets function. Neuron. 2013;80:602–612. doi: 10.1016/j.neuron.2013.10.035. [DOI] [PubMed] [Google Scholar]
- Fishell G, Rudy B. Mechanisms of inhibition within the telencephalon: "where the wild things are". Annu Rev Neurosci. 2011;34:535–567. doi: 10.1146/annurev-neuro-061010-113717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell SW, Pokala N, Macosko EZ, Albrecht DR, Larsch J, Bargmann CI. Serotonin and the neuropeptide PDF initiate and extend opposing behavioral states in C. elegans. Cell. 2013;154:1023–1035. doi: 10.1016/j.cell.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR, Paletzki R, Heintz N. GENSAT BAC cre-recombinase driver lines to study the functional organization of cerebral cortical and basal ganglia circuits. Neuron. 2013;80:1368–1383. doi: 10.1016/j.neuron.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Gong S, Zheng C, Doughty ML, Losos K, Didkovsky N, Schambra UB, Nowak NJ, Joyner A, Leblanc G, Hatten ME, et al. A gene expression atlas of the central nervous system based on bacterial artificial chromosomes. Nature. 2003;425:917–925. doi: 10.1038/nature02033. [DOI] [PubMed] [Google Scholar]
- Gordon I, Vander Wyk BC, Bennett RH, Cordeaux C, Lucas MV, Eilbott JA, Zagoory- Sharon O, Leckman JF, Feldman R, Pelphrey KA. Oxytocin enhances brain function in children with autism. Proc Natl Acad Sci U S A. 2013;110:20953–20958. doi: 10.1073/pnas.1312857110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski JA, Talley T, Qiu M, Puelles L, Rubenstein JL, Jones KR. Cortical excitatory neurons and glia, but not GABAergic neurons, are produced in the Emx1-expressing lineage. J Neurosci. 2002;22:6309–6314. doi: 10.1523/JNEUROSCI.22-15-06309.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer ER, Caldwell JD, Johnson MF, Prange AJ, Jr, Pedersen CA. Variations in concentration of oxytocin and vasopressin in the paraventricular nucleus of the hypothalamus during the estrous cycle in rats. Life Sci. 1986;38:2311–2318. doi: 10.1016/0024-3205(86)90638-7. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, MacLeod C. A critical review of the influence of oxytocin nasal spray on social cognition in humans: evidence and future directions. Horm Behav. 2012;61:410–418. doi: 10.1016/j.yhbeh.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Haider B, Duque A, Hasenstaub AR, McCormick DA. Neocortical network activity in vivo is generated through a dynamic balance of excitation and inhibition. J Neurosci. 2006;26:4535–4545. doi: 10.1523/JNEUROSCI.5297-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiman M, Schaefer A, Gong S, Peterson JD, Day M, Ramsey KE, Suarez-Farinas M, Schwarz C, Stephan DA, Surmeier DJ, et al. A translational profiling approach for the molecular characterization of CNS cell types. Cell. 2008;135:738–748. doi: 10.1016/j.cell.2008.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M, Domes G. Neuropeptides and social behaviour: effects of oxytocin and vasopressin in humans. Prog Brain Res. 2008;170:337–350. doi: 10.1016/S0079-6123(08)00428-7. [DOI] [PubMed] [Google Scholar]
- Hippenmeyer S, Vrieseling E, Sigrist M, Portmann T, Laengle C, Ladle DR, Arber S. A developmental switch in the response of DRG neurons to ETS transcription factor signaling. PLoS Biol. 2005;3:e159. doi: 10.1371/journal.pbio.0030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR. The challenge of translation in social neuroscience: a review of oxytocin, vasopressin, and affiliative behavior. Neuron. 2010;65:768–779. doi: 10.1016/j.neuron.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Young LJ. The neurobiology of attachment. Nat Rev Neurosci. 2001;2:129–136. doi: 10.1038/35053579. [DOI] [PubMed] [Google Scholar]
- Isaacson JS, Scanziani M. How inhibition shapes cortical activity. Neuron. 2011;72:231–243. doi: 10.1016/j.neuron.2011.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii T, Omura M, Mombaerts P. Protocols for two- and three-color fluorescent RNA in situ hybridization of the main and accessory olfactory epithelia in mouse. J Neurocytol. 2004;33:657–669. doi: 10.1007/s11068-005-3334-y. [DOI] [PubMed] [Google Scholar]
- Kandel ER. Principles of neural science. 5th edn. New York: McGraw-Hill; 2013. [Google Scholar]
- Kehrer C, Maziashvili N, Dugladze T, Gloveli T. Altered Excitatory-Inhibitory Balance in the NMDA-Hypofunction Model of Schizophrenia. Front Mol Neurosci. 2008;1:6. doi: 10.3389/neuro.02.006.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kepecs A, Fishell G. Interneuron cell types are fit to function. Nature. 2014;505:318–326. doi: 10.1038/nature12983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, et al. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- Knobloch HS, Grinevich V. Evolution of oxytocin pathways in the brain of vertebrates. Front Behav Neurosci. 2014;8:31. doi: 10.3389/fnbeh.2014.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota Y, Hattori R, Yui Y. Three distinct subpopulations of GABAergic neurons in rat frontal agranular cortex. Brain Res. 1994;649:159–173. doi: 10.1016/0006-8993(94)91060-x. [DOI] [PubMed] [Google Scholar]
- Kvitsiani D, Ranade S, Hangya B, Taniguchi H, Huang JZ, Kepecs A. Distinct behavioural and network correlates of two interneuron types in prefrontal cortex. Nature. 2013;498:363–366. doi: 10.1038/nature12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Caldwell HK, Macbeth AH, Tolu SG, Young WS., 3rd A conditional knockout mouse line of the oxytocin receptor. Endocrinology. 2008;149:3256–3263. doi: 10.1210/en.2007-1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng G, Meddle SL, Douglas AJ. Oxytocin and the maternal brain. Curr Opin Pharmacol. 2008;8:731–734. doi: 10.1016/j.coph.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Ma Y, Hu H, Berrebi AS, Mathers PH, Agmon A. Distinct subtypes of somatostatin-containing neocortical interneurons revealed in transgenic mice. J Neurosci. 2006;26:5069–5082. doi: 10.1523/JNEUROSCI.0661-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald E, Dadds MR, Brennan JL, Williams K, Levy F, Cauchi AJ. A review of safety, side-effects and subjective reactions to intranasal oxytocin in human research. Psychoneuroendocrinology. 2011;36:1114–1126. doi: 10.1016/j.psyneuen.2011.02.015. [DOI] [PubMed] [Google Scholar]
- Marder E. Neuromodulation of neuronal circuits: back to the future. Neuron. 2012;76:1–11. doi: 10.1016/j.neuron.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Markram K, Markram H. The intense world theory - a unifying theory of the neurobiology of autism. Front Hum Neurosci. 2010;4:224. doi: 10.3389/fnhum.2010.00224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall C, Singer T. The animal and human neuroendocrinology of social cognition, motivation and behavior. Nat Neurosci. 2012;15:681–688. doi: 10.1038/nn.3084. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Altemus M. Central nervous system actions of oxytocin and modulation of behavior in humans. Mol Med Today. 1997;3:269–275. doi: 10.1016/S1357-4310(97)01058-7. [DOI] [PubMed] [Google Scholar]
- McLean AC, Valenzuela N, Fai S, Bennett SA. Performing vaginal lavage, crystal violet staining, and vaginal cytological evaluation for mouse estrous cycle staging identification. J Vis Exp. 2012:e4389. doi: 10.3791/4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscovice LR, Ziegler TE. Peripheral oxytocin in female baboons relates to estrous state and maintenance of sexual consortships. Horm Behav. 2012;62:592–597. doi: 10.1016/j.yhbeh.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moy SS, Nadler JJ, Perez A, Barbaro RP, Johns JM, Magnuson TR, Piven J, Crawley JN. Sociability and preference for social novelty in five inbred strains: an approach to assess autistic-like behavior in mice. Genes Brain Behav. 2004;3:287–302. doi: 10.1111/j.1601-1848.2004.00076.x. [DOI] [PubMed] [Google Scholar]
- Nelson SB, Sugino K, Hempel CM. The problem of neuronal cell types: a physiological genomics approach. Trends Neurosci. 2006;29:339–345. doi: 10.1016/j.tins.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Nishimori K, Takayanagi Y, Yoshida M, Kasahara Y, Young LJ, Kawamata M. New aspects of oxytocin receptor function revealed by knockout mice: sociosexual behaviour and control of energy balance. Prog Brain Res. 2008;170:79–90. doi: 10.1016/S0079-6123(08)00408-1. [DOI] [PubMed] [Google Scholar]
- Oblak AL, Gibbs TT, Blatt GJ. Reduced GABAA receptors and benzodiazepine binding sites in the posterior cingulate cortex and fusiform gyrus in autism. Brain Res. 2011;1380:218–228. doi: 10.1016/j.brainres.2010.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen SF, Tuncdemir SN, Bader PL, Tirko NN, Fishell G, Tsien RW. Oxytocin enhances hippocampal spike transmission by modulating fast-spiking interneurons. Nature. 2013;500:458–462. doi: 10.1038/nature12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlow MJ, Reppert SM, Artman HA, Fisher DA, Self SM, Robinson AG. Oxytocin, vasopressin, and estrogen-stimulated neurophysin: daily patterns of concentration in cerebrospinal fluid. Science. 1982;216:1416–1418. doi: 10.1126/science.7201163. [DOI] [PubMed] [Google Scholar]
- Pfeffer CK, Xue M, He M, Huang ZJ, Scanziani M. Inhibition of inhibition in visual cortex: the logic of connections between molecularly distinct interneurons. Nat Neurosci. 2013;16:1068–1076. doi: 10.1038/nn.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richard S, Zingg HH. The human oxytocin gene promoter is regulated by estrogens. J Biol Chem. 1990;265:6098–6103. [PubMed] [Google Scholar]
- Ross HE, Cole CD, Smith Y, Neumann ID, Landgraf R, Murphy AZ, Young LJ. Characterization of the oxytocin system regulating affiliative behavior in female prairie voles. Neuroscience. 2009;162:892–903. doi: 10.1016/j.neuroscience.2009.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003;2:255–267. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B, Fishell G, Lee S, Hjerling-Leffler J. Three groups of interneurons account for nearly 100% of neocortical GABAergic neurons. Dev Neurobiol. 2011;71:45–61. doi: 10.1002/dneu.20853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele D, Striepens N, Gunturkun O, Deutschlander S, Maier W, Kendrick KM, Hurlemann R. Oxytocin modulates social distance between males and females. J Neurosci. 2012;32:16074–16079. doi: 10.1523/JNEUROSCI.2755-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt EF, Warner-Schmidt JL, Otopalik BG, Pickett SB, Greengard P, Heintz N. Identification of the cortical neurons that mediate antidepressant responses. Cell. 2012;149:1152–1163. doi: 10.1016/j.cell.2012.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serizawa S, Miyamichi K, Nakatani H, Suzuki M, Saito M, Yoshihara Y, Sakano H. Negative feedback regulation ensures the one receptor-one olfactory neuron rule in mouse. Science. 2003;302:2088–2094. doi: 10.1126/science.1089122. [DOI] [PubMed] [Google Scholar]
- Stanley S, Domingos AI, Kelly L, Garfield A, Damanpour S, Heisler L, Friedman J. Profiling of Glucose-Sensing Neurons Reveals that GHRH Neurons Are Activated by Hypoglycemia. Cell Metab. 2013;18:596–607. doi: 10.1016/j.cmet.2013.09.002. [DOI] [PubMed] [Google Scholar]
- Stoop R. Neuromodulation by oxytocin and vasopressin. Neuron. 2012;76:142–159. doi: 10.1016/j.neuron.2012.09.025. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Ukai S, Kose A, Hashimoto T, Iwatani J, Okumura M, Tsuji T, Shinosaki K. Reduction of cortical GABAergic inhibition correlates with working memory impairment in recent onset schizophrenia. Schizophr Res. 2013;146:238–243. doi: 10.1016/j.schres.2013.02.033. [DOI] [PubMed] [Google Scholar]
- Taniguchi H, He M, Wu P, Kim S, Paik R, Sugino K, Kvitsiani D, Fu Y, Lu J, Lin Y, et al. A resource of Cre driver lines for genetic targeting of GABAergic neurons in cerebral cortex. Neuron. 2011;71:995–1013. doi: 10.1016/j.neuron.2011.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Tol HH, Bolwerk EL, Liu B, Burbach JP. Oxytocin and vasopressin gene expression in the hypothalamo-neurohypophyseal system of the rat during the estrous cycle, pregnancy, and lactation. Endocrinology. 1988;122:945–951. doi: 10.1210/endo-122-3-945. [DOI] [PubMed] [Google Scholar]
- Warner-Schmidt JL, Schmidt EF, Marshall JJ, Rubin AJ, Arango-Lievano M, Kaplitt MG, Ibanez-Tallon I, Heintz N, Greengard P. Cholinergic interneurons in the nucleus accumbens regulate depression-like behavior. Proc Natl Acad Sci U S A. 2012;109:11360–11365. doi: 10.1073/pnas.1209293109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, Abe O, Kuwabara H, Yahata N, Takano Y, Iwashiro N, Natsubori T, Aoki Y, Takao H, Kawakubo Y, et al. Mitigation of sociocommunicational deficits of autism through oxytocin-induced recovery of medial prefrontal activity: a randomized trial. JAMA Psychiatry. 2014;71:166–175. doi: 10.1001/jamapsychiatry.2013.3181. [DOI] [PubMed] [Google Scholar]
- Xu H, Jeong HY, Tremblay R, Rudy B. Neocortical somatostatin-expressing GABAergic interneurons disinhibit the thalamorecipient layer 4. Neuron. 2013;77:155–167. doi: 10.1016/j.neuron.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O'Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Takayanagi Y, Inoue K, Kimura T, Young LJ, Onaka T, Nishimori K. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. J Neurosci. 2009;29:2259–2271. doi: 10.1523/JNEUROSCI.5593-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.