Abstract
Background
The instant blood-mediated inflammatory response (IBMIR) has been shown as a major factor that causes damage to transplanted islets. Withaferin A (WA), an inhibitor of nuclear factor κB (NFκB), was shown to suppress the inflammatory response in islets and improve syngeneic islet graft survival in mice. We investigated how treating islets with NFκB inhibitors affected IBMIR using an in vitro human autologous blood islet model.
Methods
Human islets were pretreated with or without NFκB inhibitors WA or CAY10512 before mixing autologous blood in a miniaturized in vitro tube model. Plasma samples were collected at multiple time points and used for the measurement of C-peptide, proinsulin, thrombin-antithrombin (TAT) complex, and a panel of proinflammatory cytokines. Infiltration of neutrophils into islets was analyzed using immunohistochemistry.
Results
Rapid release of C-peptide and proinsulin was observed 3 hours after mixing islets and blood in the control group, but not in the NFκB inhibitor–treated groups, whereas TAT levels were elevated in all three groups with a peak at 6 hours. Significant elevation of proinflammatory cytokines was observed in the control group after 3 hours, but not in the treatment groups. Significant inhibition of neutrophil infiltration was also observed in the WA group compared with the control (P <0.001) and CAY10512 (P <0.001) groups.
Conclusions
A miniaturized in vitro tube model can be useful in investigating IBMIR. The presence of NFκB inhibitor could alleviate IBMIR, thus improving the survival of transplanted islets. Protection of islets in the peritransplant phase may improve long-term graft outcomes.
Keywords: islet transplantation, human islets, blood loop model, proinflammatory cytokine
INTRODUCTION
Two types of clinical pancreatic islet transplants are currently being performed: allogeneic islet transplants for those with type 1 diabetes and autologous islet transplants for patients undergoing total or partial pancreatectomy for refractory chronic pancreatitis (1). After isolation, the islets are generally transfused intraportally, as the liver is considered the preferred site (2). A significant number of patients become insulin independent after transplantation, but this result is transient, and most of these patients return to using exogenous insulin (3). This is a clear indication of poor engraftment and/or gradual decay of islet function, which could be due to several factors, including poor islet quality, hypoxia, damage due to instant blood-mediated inflammatory response (IBMIR), and in the case of allogenic islets, autoimmune and alloimmune responses (4, 5).
Previous reports have shown that IBMIR is a significant factor in the damage to allogenic and xenogenic islet transplants during the peritransplant period (6–8). IBMIR is a nonspecific response mediated by the innate immune system characterized by coagulation, complement activation, platelet adhesion, and leukocyte infiltration into the islets. Tissue factor (TF) expressed by the islets is considered a major trigger for IBMIR leading to platelet activation and release of downstream inflammatory mediators such as interleukin (IL)-8, monocyte chemotactic protein (MCP)-1, and macrophage migration inhibitory factor (MIF) (8, 9). We recently demonstrated the occurrence of IBMIR in clinical autologous islet transplantation (10). IBMIR under autologous conditions was characterized by strong coagulation and a rise in proinflammatory cytokines with a minimal impact on complement activation. Analysis of blood samples drawn during intraportal islet infusion and in the hours immediately afterwards showed significant islet damage occurring within 3 hours of the beginning of infusion (10).
Nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) has long been known to be the major transcription factor for the regulation of various proinflammatory cytokines. IL-1β and tumor necrosis factor α (TNF-α) are two major inflammatory cytokines that are released rampantly during IBMIR. These cytokines are regulated by NFκB and can induce transcription of many other downstream products, such as inducible nitric oxide synthases, prostaglandin E2, and interferon gamma-induced protein 10 (IP-10). In a syngeneic islet transplant model, culturing islets with the NFκB inhibitor dehydroxymethylepoxyquinomicin resulted in improved islet engraftment (11). We have also shown that treatment of islets with withaferin A (WA), which is an NFκB inhibitor, resulted in protection of islets from proinflammatory cytokine–induced damage and improved survival of syngeneic mouse islets transplanted intraportally (12). Previous reports have also shown that TF expression is also regulated by NFκB signaling (13).
WA is a steroidal lactone isolated from the plant Withania somnifera that has been reported to have antiinflammatory, antiangiogenic, and anticancer effects (14). WA has been used in Southeast Asia and the Middle East for a long time to cure various inflammatory disorders. A recent mechanistic investigation has shown that WA inhibits inhibitor of NFκB kinase beta and prevents phosphorylation of inhibitor of κB and thereby inhibits NFκB activation (15). In the present study, we also employed CAY10512 (CAY; a potent analog of resveratrol), another known NFκB inhibitor, for further confirmation (16).
We used a miniature tube model (10) comprising a mixture of pure human pancreatic islets mixed with autologous blood to evaluate the effects of NFκB inhibitors. We hypothesized that specific inhibition of NFκB can alleviate the inflammatory component of IBMIR and improve the survival of transplanted islets during the crucial peritransplant phase.
RESULTS
Islet Viability after Culture with Autologous Blood
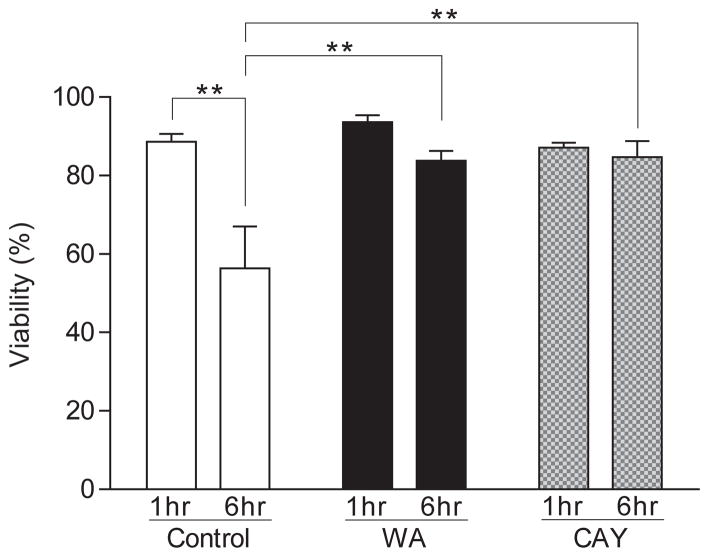
Islets treated with and without WA or CAY were cultured with autologous blood for 6 hours. In the control group, the viability of islets as analyzed by propidium iodide and Hoechst 33342 staining was significantly reduced 6 hours after culture initiation compared to 1 hour after culture initiation (P < 0.01), but no significant differences in viability between 1 and 6 hours were seen in the WA and CAY groups (Fig. 1). In addition, there were significant differences in 6-hour viability between the control, WA, and CAY groups (56.4 ± 10.7, 83.8 ± 2.6, and 84.7 ± 4.1 % [average ± SE], respectively; P < 0.01).
FIGURE 1.
Viability of islets after culture in autologous blood. Islet viability was determined by Hoechst 33342–propidium iodide staining. The viability was significantly decreased at 6 hours compared with 1 hour after the culture in the control group (**P < 0.01) but not in either NFκB inhibitor–treated group. When comparing viability at 6 hours, significant differences were found between the control and the withaferin A (WA) and CAY10512 (CAY) groups (**P < 0.01). Data express average ± SE based on 6 independent experiments. P values were calculated by two-way ANOVA with Tukey’s multiple comparison test.
Human Islet Damage and the Impact of NFκB Inhibitors
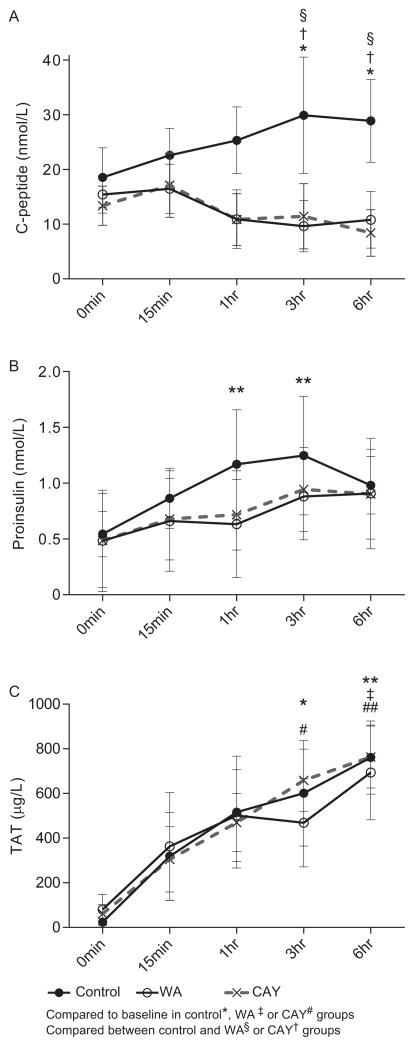
We investigated rapid C-peptide and proinsulin release from islets in the tube model to detect early islet damage. Only the control group showed a significant elevation of C-peptide level in the plasma at 3 and 6 hours compared to baseline (P < 0.05) as well as compared to the WA and CAY groups (P < 0.05, Fig. 2A). Similarly, only the control group showed a significant increase of the proinsulin level at 1 and 3 hours (P < 0.01) compared to the baseline, although no significant differences between the control and NFκB inhibitor groups were found (Fig 2B). TAT levels were significantly elevated, with a peak at 6 hours in all groups (P < 0.01 between control and CAY groups; P < 0.05 between control and WA group; Fig. 2C); there was no significant difference in TAT levels between the three groups. These results were expected because the NFκB signaling pathway is not involved in the formation of the TAT complex.
FIGURE 2.
Autologous islet damage and the impact of NFκB inhibitors. The plasma levels of C-peptide (A), proinsulin (B), and thrombin-antithrombin (TAT) (C) in an autologous islet tube model are shown. Solid line (black circle), solid line (open circle), and dotted lines show the control, withaferin A (WA), and CAY10512 (CAY) groups, respectively. Symbols indicate the P value compared to baseline within each group of control*, WA‡ or CAY# and the significance levels: *P<0.05 and **<0.01. Also, the symbols indicate the P value when compared between the control and WA§ or CAY† groups. Data express average ± SE based on 3 independent experiments. Repeated-measurement two-way ANOVA with Dunnett’s multiple comparison test was performed for statistical evaluation.
Proinflammatory Cytokine Responses
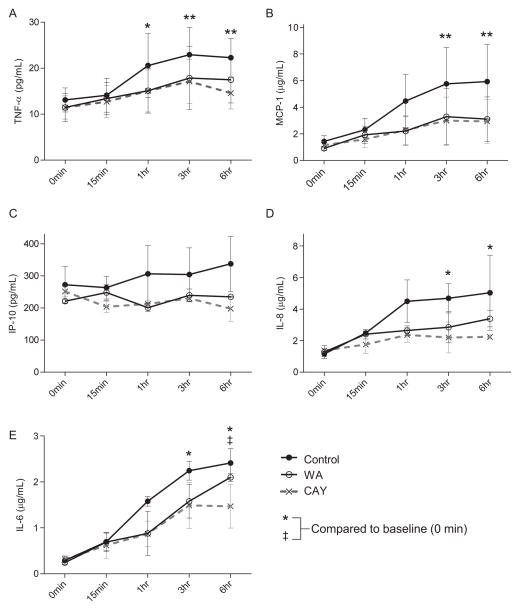
Levels of proinflammatory cytokines in the plasma samples were determined using Millipore multiplex bead assay (Fig. 3). In the control group, there was a significant elevation in the proinflammatory cytokines TNF-α, MCP-1, IL-8, and IL-6 (P <0.01, 0.01, 0.05, and 0.05, respectively), but not in IP-10, when compared to baseline (Fig. 3). Both WA and CAY blocked the significant elevation in cytokine levels up to 6 hours, except for IL-6 in the WA group.
FIGURE 3.
Inhibition of proinflammatory cytokine secretion by NFκB inhibitors. The plasma levels of TNF-α (A), MCP-1 (B), IP-10 (C), IL-8 (D), and IL-6 (E) are shown. Solid line (black circle), solid line (open circle), and dotted lines show control, withaferin A (WA), and CAY10512 (CAY) groups, respectively. Symbols indicate the P value compared to baseline within each group of control* or WA‡ and the significance levels: *P<0.05 and **<0.01. Data express average ± SE based on 3 independent experiments. Repeated-measurement two-way ANOVA with Dunnett’s multiple comparison test was performed for statistical evaluation.
Neutrophil Infiltration in Autologous Islets in Tube Model
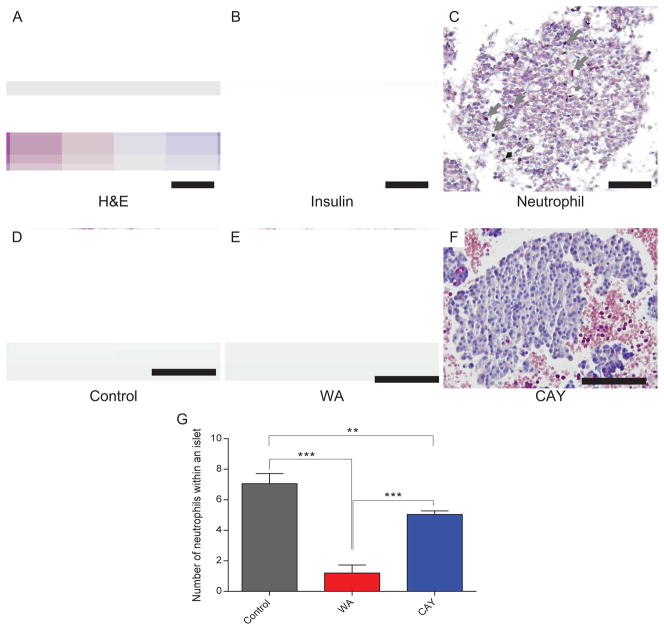
The clots of blood and islets at 3 hours were stained with Naphthol AS-D chloroacetate esterase to evaluate neutrophil infiltration (Fig. 4). The number of infiltrated neutrophils was higher in the islets in the control group (Fig. 4A). In contrast, the islet clots obtained from the WA- and CAY-treated groups showed very few to no neutrophils (Fig. 4B and 4C). The number of neutrophils in an islet was calculated from 20 different fields. The average of the number of infiltrated neutrophils was significantly lower in both the WA (P <0.0001) and CAY (P < 0.001) groups compared to the control (Fig. 4D).
FIGURE 4.
Neutrophil infiltration in islet clots. Neutrophils were stained by Naphthol AS-D chloroacetate esterase reactions (purple), counterstaining with hematoxylin (blue) and insulin (brown). Staining was performed on islet clots obtained after 3 hours of exposure to autologous blood. Representative views of H&E staining in control (A), insulin staining (B), neutrophil staining(C) are shown. Neutrophil staining is also shown in Control (D), Withaferin A treated (E) and CAY10512 (CAY) treated (F) groups. Scale bar indicates 50 μm. The number of neutrophils within an islet was counted (G). Data express average ± SE based on 20 counts from each group. ** P < 0.01 and ***P < 0.0001 evaluated with ANOVA followed by Tukey’s test.
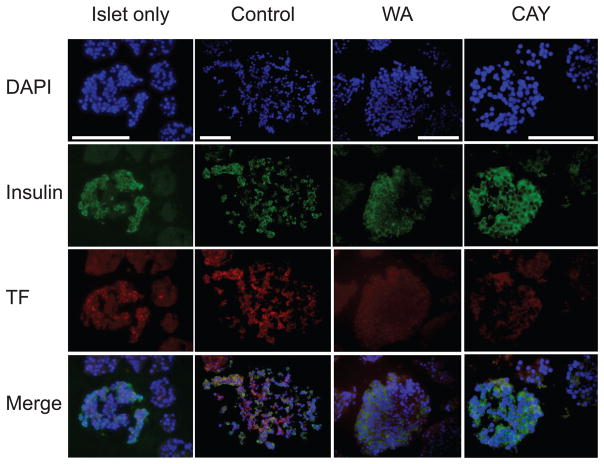
Tissue Factor Expression in Islets
Islets can express TF, which could potentially trigger coagulation and induce inflammation (8). We evaluated TF expression with fluorescence staining in conjunction with insulin using an autologous islet blood sample at 3 hours (Fig. 5). Strong TF expression was observed in the control group, but not in the WA or CAY groups. Staining for TF was more intense on insulin-positive cells.
FIGURE 5.
Tissue factor (TF) expression in islets after exposure to autologous blood. The expression of TF was evaluated with fluorescence stain (red) where nuclei and insulin were stained as blue and green. The autologous islet-blood samples were taken at 3 hours after the culture initiation. White bar indicates 50 μm. Representative sections are shown. WA indicates withaferin A; CAY, CAY10512.
DISCUSSION
IBMIR is a critical problem in both allogeneic and autologous islet transplantation that needs to be addressed to improve the outcome of islet transplantation. Nilsson’s group was the first to describe the characteristics of IBMIR and to extensively investigate different interventions to block IBMIR (17). It has been well established that NFκB is a major transcription factor involved in the modulation of the inflammatory response (18). In this study, we implemented NFκB blockade as a strategy to reduce the activation of IBMIR using WA and CAY, which are NFκB inhibitors (14, 16). A miniaturized in vitro tube model was employed to investigate the effect of these two drugs on IBMIR (10). Our data showed that NFκB inhibitors could be potent agents for pretreatment of islets to ameliorate IBMIR, supported by the significant reduction in proinflammatory cytokine levels in the mixture with autologous blood.
NFκB is one of the key transcription factors activated in response to stress (18). Activation of NFκB, depending on the type of stimuli, could be either protective or harmful to cells. A gene array study showed that pancreatic islet isolation and the following culture of islets led to an increase in the mRNA expression of proinflammatory cytokines in islets (19). This increased expression of proinflammatory cytokines might in turn lead to stress and activation of NFκB in islet cells. Thus, we hypothesized that once islets are mixed with autologous blood, the innate immune system could activate, and this could result in secretion of chemokines by the islets, which recruits monocytes and neutrophils.
After 6-hour culture with autologous blood, viability was significantly preserved in islets treated with WA and CAY when compared with control (Fig. 1), suggesting that WA not only protects islets from IBMIR but also maintains islet viability by other mechanisms, including prevention of apoptosis. A recent study from our laboratory has shown that islets cultured in the presence of WA have much better viability compared to control islets after 48 hours (12). The study also showed that inflammatory cytokine–mediated apoptosis was also reduced in the presence of WA.
Both clinical and in vitro studies have shown that, immediately after islet infusion or mixing in autologous blood, C-peptide and proinsulin levels rise rapidly, and this increase could be due to islet damage (10). Using our in vitro model, we have also shown that mixing of islets in the blood led to a rapid increase in C-peptide and proinsulin levels, suggesting islet damage, but when islets were precultured with WA or CAY, the C-peptide and proinsulin levels were significantly lower, suggesting less islet damage (Fig. 2A, 2B). It is crucial to prevent the initial damage that occurs to the islets, because it could lead to a sequence of immunological events eventually affecting the entire graft. A recent clinical study using positron emission tomography showed that 25% of islets are lost immediately after transplantation (20). Therefore, preventing islet damage in the initial stages may be the key to successful long-term insulin independence.
With IBMIR, the levels of TAT in plasma increase immediately after the islets come in contact with blood, indicating the activation of coagulation (9). Consistent with other reports, our data have also shown an immediate increase in TAT levels when islets were mixed with autologous blood. No influence of WA or CAY on TAT levels was noticed (Fig. 2C); however, this was an expected result because the process of coagulation is not NFκB dependent. On the other hand, it could be argued that coagulation is triggered by the TF expressed and released by the islets. TF expression is known to be partly regulated by NFκB (13). Islets express high levels of TF, and this interacts with factor VIIa and activates the extrinsic coagulation cascade. Therefore, blocking the activation of NFκB represents a sound strategy to inhibit IBMIR. The TF gene is also regulated by several other transcription factors such as Egr, AP-1 and NFAT (21). Various signals lead to activation of different transcription factors that result in the induction of the tissue factor gene and synergistic regulation by several transcription factors would be required for TF expression. The regulation of TF gene by NFkB is induced mainly by cytokine stimulation such as IL-1β, TNFα and by pathogen associated molecular patterns (PAMPs) such as Lipopolysaccharide (LPS)(22). In the case of IBMIR during islet transplantation, the major factor that could induce TF expression in the islets would be the pro-inflammatory cytokines. Previous studies on human monocytes have shown that preventing IkBα degradation resulted in attenuation of the TF gene expression (23) and also use of salicylates such as aspirin have similar effects (21). Therefore, blocking TF expression by NFkB would be protective for the islets during early stages of transplantation. We have shown that both WA and CAY significantly prevent TF expression in islets when compared with the control. Since we cultured islets in WA or CAY only for an hour before mixing with autologous blood, a prolonged treatment of NFκB might further reduce TF expression in islets and prevent the activation of coagulation.
Our recent clinical study showed an increase in the levels of proinflammatory cytokines and chemokines immediately after infusion of islets into the portal vein (10). The presence of high levels of these cytokines amplifies the inflammatory response, making the islets more susceptible to cytokine-mediated apoptosis. In our in vitro study, we have demonstrated that the plasma levels of several proinflammatory cytokines were increased after mixing of islets with blood. TNF-α is a proinflammatory cytokine known to induce apoptosis in beta cells (24). We found that TNF-α levels increased gradually and reached a maximum at 3 and 6 hours. In both the WA and CAY groups, the level of TNF-α did not increase and remained low at all time points (Fig. 3A). IL-6, which can be either proinflammatory or antiinflammatory depending on the stimuli, was also found to increase in control samples, whereas the WA- and CAY-treated samples efficiently suppressed the secretion of this cytokine (Fig. 3E). IP-10 levels in the plasma were high to start with and remained high, but in the WA and CAY groups, the level of IP-10 was lower compared with the control (Fig. 3C). IP-10 is considered one of the major proinflammatory cytokines that can modulate islet graft survival (25). MCP-1 is responsible for attracting monocytes to the site of injury (26). When islets are mixed with blood, MCP-1 levels in the plasma begin to rise and remain high up to 6 hours. When WA- or CAY-treated islets were mixed with blood, the MCP-1 level did not change (Fig. 3b), suggesting prevention of islet damage by macrophages. IL-8 is also a cytokine that recruits polymorphonuclear cells to the site of inflammation. The level of IL-8 also increased rapidly after mixing blood and islets, and this elevation was prevented in the WA and CAY groups (Fig. 3D). Other key proinflammatory cytokines, IL-1β and interferon γ, were undetected in the samples.
Our in vitro tube model was validated for the study of islets mixed with blood based on a comprehensive analysis of pH, pO2 and glucose levels (10). Since our present study using the in vitro tube model showed that both WA and CAY can inhibit the secretion of proinflammatory cytokines/chemokines, it is appropriate to show the resulting outcome. Islet clots were sectioned and stained for neutrophils. The islets that were mixed with blood showed massive infiltration of neutrophils even at 3 hours, whereas the WA- and CAY-treated islets showed a very significant reduction in the number of neutrophils infiltrated per islet (Fig. 4). Thus, the histology results correlate with proinflammatory cytokine or chemokine levels, especially with IL-8, which is required for the maturation and infiltration of neutrophils.
The present study focused on the instant response occurring when purified islets come into contact with blood. Long-term benefits of treatment with NFκB blockers on islet graft outcome remain to be determined. IBMIR against allogenic and xenogenic islets may be stronger than against autologous islets due to the involvement of humoral immune components such as autoantibodies, alloantibodies, and xenoreactive antibodies. Future studies using this miniature tube model could include analyze the effects of inhibitors for various components of IBMIR. In summary, we have demonstrated that a miniaturized in vitro tube model is an effective tool to study IBMIR. We have also shown that blocking NFκB is a promising strategy to alleviate the harmful effects of IBMIR on transplanted islets.
MATERIALS AND METHODS
Human Islet Isolation and Blood Collection
Research-grade human pancreases from brain-dead donors were provided from two local organ procurement organizations (Southwest Transplant Alliance, Dallas, TX, and LifeGift, Houston, TX). Pancreases were procured and preserved with the standard two-layer method. Islets were isolated using the modified Ricordi’s method (27, 28). Blood samples from the donors were collected during pancreas procurement in heparinized tubes (BD Biosciences, Franklin Lakes, NJ) to prevent coagulation. Blood samples were maintained in a refrigerator until the start of the experiment (not more than 36 hours).
Miniaturized In Vitro Tube Model
Islets were cultured in CMRL 1066 islet culture medium (Mediatech, Manassas, VA) overnight at 37°C with 5% CO2 to recover from the isolation stress. Prior to the experiment, islets were washed, counted, and 2000IEQ were treated with CMRL medium containing 1.0 ug/mL WA (Enzo Life Sciences, Farmingdale, NY) or 0.15 μM CAY (Cayman Chemicals, Ann Arbor, MI) or no drug for 1 hour, and blood samples were allowed to equilibrate at 37°C. The blood was aliquoted in heparinized Eppendorf tubes (Cardinal Health, Dublin, OH) (0.5 mL in each tube), the islets (~200 IEQs/200uL conditioned media) were added to the blood, and the tubes with blood and islets were placed in a 37°C shaker incubator. Tubes were removed from the incubator at their respective time points.
Luminex Multiplex Assay, ELISA and Viability
The procedures on Luminex, enzyme-linked immunosorbent assay (ELISA) and viability assay are described in the supplemental digital content (Materials and Methods, SDC).
Neutrophil Infiltration in Purified Human Islets Mixed with Autologous Blood
Formalin-fixed paraffin-embedded islet clots were sectioned in 5-micron sections, and polymorphonuclear neutrophil staining was performed using Naphthol AS-D chloroacetate esterase (Sigma Aldrich, St. Louis, MO). At least 15 separate islets were evaluated for neutrophil infiltration. Neutrophil infiltration was classified by counting polymorphonuclear neutrophils in and around individual islets. Insulin staining was performed on serial sections using rabbit anti-insulin (Cell Signal, Boston, MA) and anti-rabbit HRP secondary antibody (Cell Signal, Boston, MA). Color was developed using DAB chromogen substrate kit (DAKO, Carpinteria, CA).
Fluorescence Staining for Tissue Factor
Islet tissue sections (5 μm) were obtained after fixing in 10% formalin and embedding in paraffin. Tissue sections were deparaffinized, and heat-mediated antigen retrieval was performed. TF was stained with mouse anti-TF antibody (Abcam, Cambridge, England) following anti-mouse Alexa Flour 594 (Invitrogen, Grand Island, NY), along with insulin staining with guinea pig anti-insulin antibody (Sigma, St. Louis, MO) following anti-guinea pig Alexa Flour 488 (Invitrogen, Grand Island, NY) and 4′,6-diamidino-2-phenylindole (Sigma-Aldrich, St. Louis, MO).
Statistical Analysis
Data were expressed as mean ± standard error (SE). Data were analyzed using GraphPad Prism version 6.03 (GraphPad Software, Inc., San Diego, CA). The two-way repeated-measures analysis of variance (ANOVA) followed by Tukey’s or Dunnett post hoc test was performed to evaluate statistical significance between time points or groups. Statistical significance was considered for P values <0.05.
Supplementary Material
Acknowledgments
The authors thank Ana M. Rahman and Yoshiko Tamura from Baylor University Medical Center at Dallas and Nofit Borenstein from Baylor Research Institute for technical support and Cynthia Orticio for professional editing. This work was supported in part by grants from the National Institute of Diabetes and Digestive and Kidney Diseases (1R21DK090513-02 to M.F.L.) and the Juvenile Diabetes Research Foundation (5-2011-372 to B.N. and 3-2011-447 to M.T.).
List of Abbreviations
- CAY
CAY10512 (Potent analog of resveratrol)
- ELISA
enzyme-linked immunosorbent assay
- IBMIR
instant blood-mediated inflammatory response
- IL
interleukin
- IP-10
interferon gamma-induced protein 10
- MCP-1
monocyte chemotactic protein-1
- MIF
macrophage migration inhibitory factor
- NFκB
nuclear factor kappa-light-chain-enhancer of activated B cells
- TAT
thrombin-antithrombin
- TF
tissue factor
- TNF
tumor necrosis factor
- WA
withaferin A
Footnotes
Conflict of Interest Statement: All authors declare no conflicts of interest.
Author contributions: M.A.K., M.T., J.A.S., and T.I. participated in planning the study design, performing experiments, analyzing data, and writing the report. S.M., F.K., R.S., and M.C.L. participated in performing studies and critical review. M.F.L. and B.N. participated in planning the study design, data analysis, critical review, and assistance with funding.
References
- 1.Sutherland DE, Gruessner AC, Carlson AM, et al. Islet autotransplant outcomes after total pancreatectomy: a contrast to islet allograft outcomes. Transplantation. 2008;86 (12):1799. doi: 10.1097/TP.0b013e31819143ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sutherland DE, Radosevich DM, Bellin MD, et al. Total pancreatectomy and islet autotransplantation for chronic pancreatitis. J Am Coll Surg. 2012;214 (4):409. doi: 10.1016/j.jamcollsurg.2011.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryan EA, Paty BW, Senior PA, et al. Five-year follow-up after clinical islet transplantation. Diabetes. 2005;54 (7):2060. doi: 10.2337/diabetes.54.7.2060. [DOI] [PubMed] [Google Scholar]
- 4.McCall M, James Shapiro AM. Update on islet transplantation. Cold Spring Harb Perspect Med. 2012;2 (7):a007823. doi: 10.1101/cshperspect.a007823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Robertson RP. Islet transplantation a decade later and strategies for filling a half-full glass. Diabetes. 2010;59 (6):1285. doi: 10.2337/db09-1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ozmen L, Ekdahl KN, Elgue G, Larsson R, Korsgren O, Nilsson B. Inhibition of thrombin abrogates the instant blood-mediated inflammatory reaction triggered by isolated human islets: possible application of the thrombin inhibitor melagatran in clinical islet transplantation. Diabetes. 2002;51 (6):1779. doi: 10.2337/diabetes.51.6.1779. [DOI] [PubMed] [Google Scholar]
- 7.Goto M, Tjernberg J, Dufrane D, et al. Dissecting the instant blood-mediated inflammatory reaction in islet xenotransplantation. Xenotransplantation. 2008;15 (4):225. doi: 10.1111/j.1399-3089.2008.00482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moberg L, Johansson H, Lukinius A, et al. Production of tissue factor by pancreatic islet cells as a trigger of detrimental thrombotic reactions in clinical islet transplantation. Lancet. 2002;360 (9350):2039. doi: 10.1016/s0140-6736(02)12020-4. [DOI] [PubMed] [Google Scholar]
- 9.Johansson H, Lukinius A, Moberg L, et al. Tissue factor produced by the endocrine cells of the islets of Langerhans is associated with a negative outcome of clinical islet transplantation. Diabetes. 2005;54 (6):1755. doi: 10.2337/diabetes.54.6.1755. [DOI] [PubMed] [Google Scholar]
- 10.Naziruddin B, Iwahashi S, Kanak M, Takita M, Itoh T, Levy M. Evidence for instant blood-mediated inflammatory reaction in clinical autologous islet transplantation. Am J Transplant. doi: 10.1111/ajt.12558. In Press. [DOI] [PubMed] [Google Scholar]
- 11.Takahashi T, Matsumoto S, Matsushita M, et al. Donor pretreatment with DHMEQ improves islet transplantation. J Surg Res. 2010;163 (1):e23. doi: 10.1016/j.jss.2010.04.044. [DOI] [PubMed] [Google Scholar]
- 12.Sorelle JA, Itoh T, Peng H, et al. Withaferin A inhibits pro-inflammatory cytokine-induced damage to islets in culture and following transplantation. Diabetologia. 2013;56 (4):814. doi: 10.1007/s00125-012-2813-9. [DOI] [PubMed] [Google Scholar]
- 13.Kollander R, Solovey A, Milbauer LC, Abdulla F, Kelm RJ, Jr, Hebbel RP. Nuclear factor-kappa B (NFkappaB) component p50 in blood mononuclear cells regulates endothelial tissue factor expression in sickle transgenic mice: implications for the coagulopathy of sickle cell disease. Transl Res. 2010;155 (4):170. doi: 10.1016/j.trsl.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vanden Berghe W, Sabbe L, Kaileh M, Haegeman G, Heyninck K. Molecular insight in the multifunctional activities of Withaferin A. Biochem Pharmacol. 2012;84 (10):1282. doi: 10.1016/j.bcp.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 15.Grover A, Shandilya A, Punetha A, Bisaria VS, Sundar D. Inhibition of the NEMO/IKKbeta association complex formation, a novel mechanism associated with the NF-kappaB activation suppression by Withania somnifera’s key metabolite withaferin A. BMC Genomics. 2010;11 (Suppl 4):S25. doi: 10.1186/1471-2164-11-S4-S25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cucciolla V, Borriello A, Oliva A, Galletti P, Zappia V, Della Ragione F. Resveratrol: from basic science to the clinic. Cell Cycle. 2007;6 (20):2495. doi: 10.4161/cc.6.20.4815. [DOI] [PubMed] [Google Scholar]
- 17.Nilsson B, Ekdahl KN, Korsgren O. Control of instant blood-mediated inflammatory reaction to improve islets of Langerhans engraftment. Curr Opin Organ Transplant. 2011;16 (6):620. doi: 10.1097/MOT.0b013e32834c2393. [DOI] [PubMed] [Google Scholar]
- 18.Pasparakis M. Regulation of tissue homeostasis by NF-kappaB signalling: implications for inflammatory diseases. Nat Rev Immunol. 2009;9 (11):778. doi: 10.1038/nri2655. [DOI] [PubMed] [Google Scholar]
- 19.Jackson A, McWilliams C, Kaizer E, et al. Gene expression profiling of human pancreatic islets undergoing a simulated process of instant blood-mediated inflammatory reaction. Transplant Proc. 2008;40 (2):430. doi: 10.1016/j.transproceed.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 20.Eriksson O, Eich T, Sundin A, et al. Positron emission tomography in clinical islet transplantation. Am J Transplant. 2009;9 (12):2816. doi: 10.1111/j.1600-6143.2009.02844.x. [DOI] [PubMed] [Google Scholar]
- 21.Mackman N. Regulation of the tissue factor gene. Thromb Haemost. 1997;78 (1):747. [PubMed] [Google Scholar]
- 22.Ruf W, Riewald M. Regulation of Tissue Factor Expression. In: ten Cate H, Levi M, editors. Molecular mechanisms of disseminated intravascular coagulation. Georgetown, TX: Landes Bioscience; 2003. p. 61. [Google Scholar]
- 23.Parry GC, Mackman N. Transcriptional regulation of tissue factor expression in human endothelial cells. Arterioscler Thromb Vasc Biol. 1995;15 (5):612. doi: 10.1161/01.atv.15.5.612. [DOI] [PubMed] [Google Scholar]
- 24.Ishizuka N, Yagui K, Tokuyama Y, et al. Tumor necrosis factor alpha signaling pathway and apoptosis in pancreatic beta cells. Metabolism. 1999;48 (12):1485. doi: 10.1016/s0026-0495(99)90234-2. [DOI] [PubMed] [Google Scholar]
- 25.Baker MS, Chen X, Rotramel AR, et al. Genetic deletion of chemokine receptor CXCR3 or antibody blockade of its ligand IP-10 modulates posttransplantation graft-site lymphocytic infiltrates and prolongs functional graft survival in pancreatic islet allograft recipients. Surgery. 2003;134 (2):126. doi: 10.1067/msy.2003.213. [DOI] [PubMed] [Google Scholar]
- 26.Ziraldo C, Vodovotz Y, Namas RA, et al. Central Role for MCP-1/CCL2 in Injury-Induced Inflammation Revealed by In Vitro, In Silico, and Clinical Studies. PLoS One. 2013;8 (12):e79804. doi: 10.1371/journal.pone.0079804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsumoto S, Okitsu T, Iwanaga Y, et al. Successful islet transplantation from nonheartbeating donor pancreata using modified Ricordi islet isolation method. Transplantation. 2006;82 (4):460. doi: 10.1097/01.tp.0000231710.37981.64. [DOI] [PubMed] [Google Scholar]
- 28.Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37 (4):413. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.