Abstract
Detecting the signal backscattered by nanoparticles immersed in highly scattering media such as biological tissue remains a challenge. In this article we report on the use of Full Field OCT (FF-OCT) to slice in depth in phantoms and in tissues in order a) to selectively observe the particles through the backscattered light at suitable wavelengths, and b) to detect the effects of the time-dependent response to full field optical heating through the strong absorption cross-section of these plasmonic nanoparticles. The analysis of the thermal wave behavior leads to the localization of the heat sources even when FF-OCT signals cannot reach the heated area.
OCIS codes: (110.4500) Optical coherence tomography, (350.4990) Particles
1. Introduction
Plasmonic probes such as gold nanoparticles have advantage of being biocompatible, non-toxic and moreover having/showing a good chemical stability [1]. Gold nanoparticles were extensively used in preclinical therapeutic applications via conjugations with antibodies, chemo-therapeutic drugs, microRNA (miRNA) or genes [2,3]. For the moment, gold nanoshells (also known as AuroShells) are currently in clinical trials to treat head and neck carcinoma [4] or lung cancer [5] by hyperthermia. Gold nanoshells, composed of a silica core and a gold shell, display a plasmonic resonance that can be shifted in the near infrared windows where light exhibits an optimum tissue penetration [6]. The spectral region between 650 and 900 nm, called therapeutic window, shows low absorption of hemoglobin (<650 nm) and water (>900 nm). Based on their unique optical and plasmonic properties, gold nanoshells were used as contrast agent for optical imaging such as photoacoustic imaging (PAI), photoacoustic tomography (PAT) [7,8], optical coherence tomography (OCT) [9–13] and photothermal OCT [14–19].
In this study we intend to check the ability of Full Field Optical Coherence Tomography (FF-OCT) to image single gold nanoshell. FF-OCT uses a much larger numerical aperture lenses than OCT; therefore, it is able to detect the backscattered signal from a single nanoshell.
We show that in a scattering phantom mimicking tissue, such signals have a higher amplitude than the amplitude of the background signal of the other scatterers. However, in highly scattering tissues, such as skin, the speckle nature of the OCT signals generates large fluctuations of the signal amplitude which hides the scattering signal of the particle. To overcome this difficulty we have modified our FF-OCT setup and have introduced a heating beam at the plasmon wavelength and a probe beam at a different wavelength. For the first time, to the best of our knowledge, a transient photothermal approach using OCT proved to be successful. The time response of the results allows in-depth separation of the heated areas. The time dependent signals fit the thermal model that we have used.
2. Experimental setup
The objective of this study is to show the feasibility of the detection of gold nanoparticles in scattering sample using a FF-OCT system.
The nanoparticles used are PEGylated (molecular weight MW = 5000) gold nanoshells composed of a 20 nm ( ± 10 nm) gold shell surrounding a 130 nm ( ± 20 nm) silica core (Nanospectra Biosciences ). For this geometry, the plasmon resonance is broad and approximately centered at 800 nm [20].
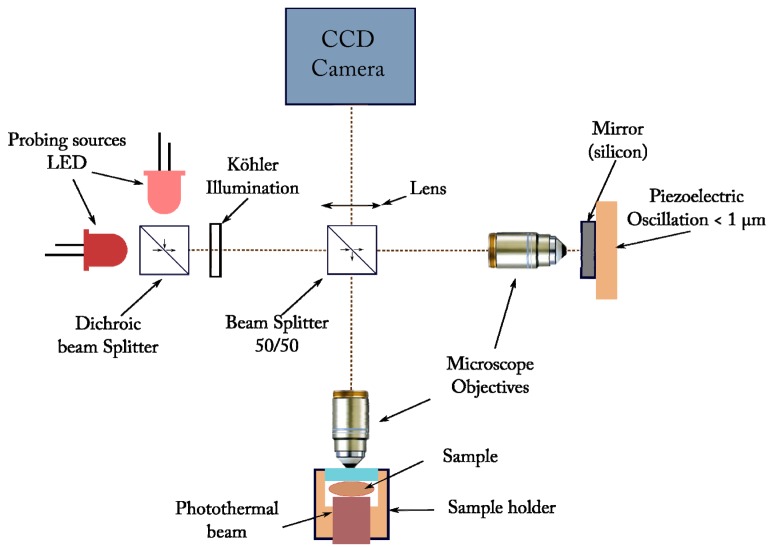
FF-OCT is a low coherence interference imaging technique based on a Linnik interferometer (see Fig. 1) [21]. An identical water-immersion microscope objective (Olympus, magnification10x, NA = 0.3) was placed on each arm of our interferometer. A silicon mirror (reflectivity of about 20%) was used on the reference arm. Images were recorded with a CCD camera (Dalsa 1M60, 1024x1024 pixels, 50Hz, 12bits, full well capacity of 150000e). The interference signal was isolated from the background by a piezoelectric modulation of the path difference.
Fig. 1.
Schematic representation of the FF-OCT setup. Compared to the standard FF-OCT setup of reference 12, were used LEDs instead of thermal sources (section 2) and an incoherent heating beam illuminating the whole field of view.
A Köhler illumination was used to ensure an homogeneous illumination of the sample. In this experiment two LEDs Thorlabs (M625L3 and M780L2) were used. The spectra were centered at 625 nm and 780 nm. With this custom setup, lateral resolutions of about 1.3 µm and 1.6 µm respectively and axial resolutions of 6 µm and 5 µm respectively were achieved with a sensitivity of more than 60 dB (for a single image acquisition).
In this study two methods were used in order to detect gold core-shell nanoparticles. In section 3, direct backscattering detection from a single particle was achieved in phantoms by taking advantage of the huge scattering cross-section near the plasmonic resonance. Nevertheless in biological tissues, due to the presence of speckle in the OCT signals, single particles signal detection failed. This is the reason why we have used, in section 4, photothermal signals resulting from the local heating of the gold nanoparticles.
3. Full Field detection of single gold core-shell nanoparticle in scattering media using two-wavelength FF-OCT system
In this first method, the resonant behavior of the backscattering cross-section of gold nanoshell particles is used to extract their signal from two FF-OCT images taken at two different wavelengths (625 nm and 780 nm) using two LEDs sources that are shown on Fig. 1.
According to the Mie theory, non-resonant small and large (compared to the illumination wavelength) particles have a backscattering cross-section that evolves as a decreasing or as a quasi-constant function of the wavelength respectively [22]. Near the plasmonic resonance, the gold nanoshells exhibit a strong increase in their backscattering signals. As the FF-OCT signal is directly related to the backscattering intensity, by taking two FF-OCT images of the same area near the plasmonic resonance of the gold nanoshells - one image at the plasmonic resonance (780 nm) and one off the plasmonic resonance (625 nm) - one should be able to clearly distinguish the signal coming from the gold nanoshells.
This method was first applied to our test sample. The sample were an agarose gel (Agarose – Sigma-Aldrich) with ZnO particles (ZnO – 2005532 Sigma-Aldrich) and gold nanoshells (Nanospectra Biosciences ). ZnO particles with a diameter between 1 and 5 µm were selected to induce large non-resonant optical scattering and to measure the spectral selectivity of the backscattered signal of the gold nanoshells. The concentrations used are 20 mg/g for ZnO particles and 4 × 108 np/ml for gold nanoshells, leading to a mean free path of about 150 µm. Because of the setup low coherence and high numerical aperture, each coherence volume only contains one particle (ZnO or gold particle). From these deterministic signals, it was fairly easy to observe the strong enhancement of the backscattering signals from nanoshells when switching the probe beam from off-resonance (at 625 nm) to resonance (at 780 nm) and to localize them.
The final objective of our work was to assist bio-medical diagnosis by providing a new contrast agent to FF-OCT technique. Therefore, our method was applied to biological tissue.
It is well known that OCT signals in tissues show a random, speckle like, behavior. Indeed, even in the very small coherence volume used, there is a random distribution of backscatterers, so what is measured is usually a speckle pattern. If two images are taken at two different wavelengths, the speckle patterns will be decorrelated and the major variation of the FF-OCT signal will come from the fluctuations of speckle patterns. A larger signal than the fluctuations of the FF-OCT signals is therefore mandatory in order to apply the two wavelengths technique.
At this point we should underline that the OCT experiments using nanoparticles as contrast agents [9–13] did not intend to detect single nanoparticle as e.g. in OCM [16] or FF-OCT. A solution to this problem should be to smooth the speckle pattern for example by taking more images at different wavelengths. In this case the speckle fluctuations could be reduced to the square root of the number of uncorrelated acquisitions, whereas the deterministic nanoparticle signal would show an amplitude that follows the spectral backscattered dependence. Despite of its complexity, this solution would lead to use out of resonance wavelengths and to lose the benefit of the plasmon resonance effects.
We have found a simpler and more efficient way of detecting and localizing the nanoshells in biological tissues by actually detecting thermal variation of the FF-OCT signals induced by the heating of the gold nanoshells.
4. Photothermal detection
We have seen in the previous sections that the presence of plasmonic nanoparticles is easily revealed in phantoms because of their backscattering properties that clearly vary with the wavelength. Unfortunately the OCT images in highly scattering tissues carry a random speckle structure that masks the signals associated to individual particles. In order to overcome this difficulty, the FF-OCT signal was used as a probe of the thermal effects induced by a light source that matches the maximum absorption signal linked to the plasmon resonance. It is indeed well known that the core-shell structure exhibits a pronounced increase in its absorption cross-section at wavelengths that can be predetermined by the thickness of the gold coating [23]. In this study, the maximum absorption was located around 780 nm and a LED (Thorlabs M780L2) was used for heating purpose as can be seen on Fig. 1.
We must mention that OCT has already been used for detection of gold nanoparticles using OCT or dark field OCM [14–19]. A modulated heating beam, at the plasmon resonance, creates a locally modulated temperature field and thus a modulated refractive index one. Hence, light backscattered by the heated particles exhibits a modulated phase that is detected with an OCT probe coupled to a direct or lock-in detection. In these experiments, the particles are located in a clear liquid or in living cells, at moderate depths where heating beam is still dominantly ballistic, and not in highly scattering tissue as in our study.
Moreover, in all the OCT experiments [14–19] described so far, the heating and the probe beams are set in a confocal geometry. This has the advantage of maximizing the power density in the heated volume; nevertheless, the spread of the heating beam due to scattering is noticeable after a few hundred micrometers.
In our setup (Fig. 1), the field of view is homogeneously illuminated and no special alignment of the probe and heating beams is required.
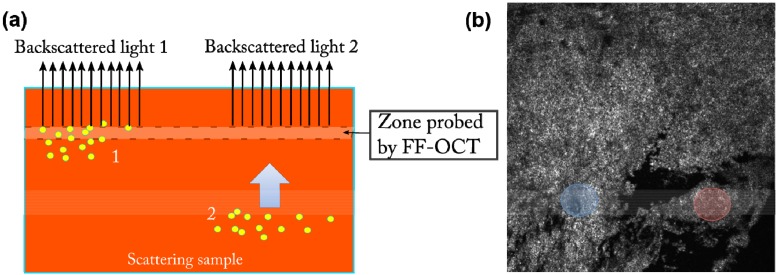
In our experiments we use the photothermal responses, not only to get a contrast that is specifically linked to the absorption spectrum of the nanoparticles, but also to take advantage of the measurement of the time delay associated with the propagation of the thermal wave over the distance between the heated zone and the probed one. Although thermal waves studies have routinely used these phenomena using a surface probe [24], to our knowledge, this is the first use of this kind of approach with a tomographic probe such as FF-OCT. Figure 2(a) shows a schematic of the sample configuration and the signal generation. The gold nanoparticles (yellow) are heated by an external illumination flux. The heat generated in region 1 reaches the probe much faster than the one generated in region 2 that must diffuse to the probed area (blue arrow) before being detected.
Fig. 2.
(a) The gold nanoparticles (yellow) are heated by an external illumination flux. The heat generated in region 1 reaches the probe much faster than the one generated in region 2 that must diffuse to the probed area (blue arrow) before being detected. (b) Full field OCT image of a mouse skin (field of view 0.8x0.8 mm2). Time dependent photothermal signals corresponding to the blue and red zones are displayed in Fig. 3(a) and 3(b) respectively.
In order to evaluate the kind of signals likely to be detected after a step illumination, a numerical calculation based on the analytical solution of the heat equation [25] was performed. The thermal diffusivity of the tissue is typically 10−3 cm2/s, the camera sampling rate is 20 ms and a few seconds of images at this frequency was recorded.
If the probe is close to the local heat source, a very fast increase of the signal is expected, whereas when the distance is higher than 50 μm, a delay larger than 20 ms can be observed. Obviously with faster cameras, much smaller distances could be measured.
The experiments were carried out on nude mouse skin tissue with an intra-dermal injection. The nanoparticles concentration was 2.5x109 np/ml. Figure 2(b) shows a FF-OCT image taken about 20 µm below the surface. We have marked in blue and red two regions that we have probed using the response to the photothermal excitation.
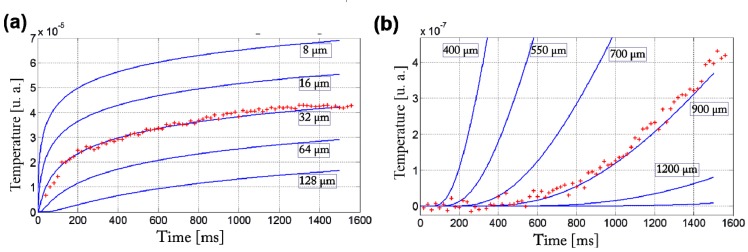
The results of the time response of the two regions enhanced in blue and red in Fig. 2(b) are shown in Fig. 3(a) and 3(b) respectively (dots). We can see the very different behaviors of the two zones: the curve (a) corresponding to the blue zone increases very rapidly meaning that the probed region is close to the heated area; the curve (b) corresponding to the red zone exhibits a clear delay and a progressive increase of the signal.
Fig. 3.
The time response of the two regions enhanced in blue and red in Fig. 2(b) are shown in figure (a) and (b) respectively (dots). The various curves represent the phase difference integrated along a path of a few mm for various particle-probe distances, as a function of time given by a step excitation in the heat diffusion model.
In order to quantify, the experimental curves (dots) have been displayed alongside the simulations of the optical response induced by a step heating for various particle-probes distances (blue lines). Although we cannot localize the heat sources with the same precision as the sectioning ability of the FF-OCT probe, this evaluation gave confidence in the interpretation of the experimental data.
4. Conclusion
In this article, we show that detecting single gold nanoshells from their backscattered light is challenging in highly scattering biological media. Although gold nanoshells scattering cross-section is much larger on resonance, their FF-OCT signal are of the order of biological sample signal and dependent on the speckle distribution. We present an alternative method, using FF-OCT, to axially localize the position of the nanoparticles, taking advantage of the photothermal effect. Using the time response variation to a heating step function, we measure the axial distance from the nanoparticles to the probed area with a precision of a few microns.
Acknowledgments
The authors would like to thank Emmanuel Bossy for fruitful discussions in particular on the photothermic diffusion model. We thank to Ana Maria Rosu and Maya Juenet for reviewing the English language for this manuscript. This work was supported by AXA Research Fund, ESPCI-Charpak, INSERM, National Research Agency project GoldenEye (ANR-10-INTB-1003-01) LABEX WIFI (Laboratory of Excellence ANR-10-LABX-24) within the French Program “Investments for the Future” under reference ANR-10- IDEX-0001-02 PSL*.
References and links
- 1.Akhter S., Ahmad M. Z., Ahmad F. J., Storm G., Kok R. J., “Gold nanoparticles in theranostic oncology: current state-of-the-art,” Expert Opin. Drug Deliv. 9(10), 1225–1243 (2012). 10.1517/17425247.2012.716824 [DOI] [PubMed] [Google Scholar]
- 2.You J., Zhang R., Xiong C., Zhong M., Melancon M., Gupta S., Nick A. M., Sood A. K., Li C., “Effective photothermal chemotherapy using doxorubicin-loaded gold nanospheres that target EphB4 receptors in tumors,” Cancer Res. 72(18), 4777–4786 (2012). 10.1158/0008-5472.CAN-12-1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ghosh R., Singh L. C., Shohet J. M., Gunaratne P. H., “A gold nanoparticle platform for the delivery of functional microRNAs into cancer cells,” Biomaterials 34(3), 807–816 (2013). 10.1016/j.biomaterials.2012.10.023 [DOI] [PubMed] [Google Scholar]
- 4. http://www.clinicaltrials.gov
- 5. http://news.rice.edu/2012/11/02/nanoshell-therapy-to-be-tested-in-lung-cancer-clinical-trial/
- 6.Lin A. W., “Optically tunable nanoparticle contrast agents for early cancer detection: model-based analysis of gold nanoshells,” J. Biomed. Opt. 10(6), 064035 (2005). 10.1117/1.2141825 [DOI] [PubMed] [Google Scholar]
- 7.Zhang H. F., Maslov K., Stoica G., Wang L. V., “Functional photoacoustic microscopy for high-resolution and noninvasive in vivo imaging,” Nat. Biotechnol. 24(7), 848–851 (2006). 10.1038/nbt1220 [DOI] [PubMed] [Google Scholar]
- 8.Zhang Q., Iwakuma N., Sharma P., Moudgil B. M., Wu C., McNeill J., Jiang H., Grobmyer S. R., “Gold nanoparticles as a contrast agent for in vivo tumor imaging with photoacoustic tomography,” Nanotechnology 20(39), 395102 (2009). 10.1088/0957-4484/20/39/395102 [DOI] [PubMed] [Google Scholar]
- 9.Oldenburg A. L., Hansen M. N., Zweifel D. A., Wei A., Boppart S. A., “Plasmon-resonant gold nanorods as low backscattering albedo contrast agents for optical coherence tomography,” Opt. Express 14(15), 6724–6738 (2006). 10.1364/OE.14.006724 [DOI] [PubMed] [Google Scholar]
- 10.Troutman T. S., Barton J. K., Romanowski M., “Optical coherence tomography with plasmon resonant nanorods of gold,” Opt. Lett. 32(11), 1438–1440 (2007). 10.1364/OL.32.001438 [DOI] [PubMed] [Google Scholar]
- 11.Gobin A. M., Lee M. H., Halas N. J., James W. D., Drezek R. A., West J. L., “Near-infrared resonant nanoshells for combined optical imaging and photothermal cancer therapy,” Nano Lett. 7(7), 1929–1934 (2007). 10.1021/nl070610y [DOI] [PubMed] [Google Scholar]
- 12.Zagaynova E. V., Shirmanova M. V., Kirillin M. Y., Khlebtsov B. N., Orlova A. G., Balalaeva I. V., Sirotkina M. A., Bugrova M. L., Agrba P. D., Kamensky V. A., “Contrasting properties of gold nanoparticles for optical coherence tomography: phantom, in vivo studies and Monte Carlo simulation,” Phys. Med. Biol. 53(18), 4995–5009 (2008). 10.1088/0031-9155/53/18/010 [DOI] [PubMed] [Google Scholar]
- 13.Jung Y., Guan G., Wei C. W., Reif R., Gao X., O’Donnell M., Wang R. K., “Multifunctional nanoprobe to enhance the utility of optical based imaging techniques,” J. Biomed. Opt. 17(1), 016015 (2012). 10.1117/1.JBO.17.1.016015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhou C., Tsai T. H., Adler D. C., Lee H. C., Cohen D. W., Mondelblatt A., Wang Y., Connolly J. L., Fujimoto J. G., “Photothermal optical coherence tomography in ex vivo human breast tissues using gold nanoshells,” Opt. Lett. 35(5), 700–702 (2010). 10.1364/OL.35.000700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adler D. C., Huang S.-W., Huber R., Fujimoto J. G., “Photothermal detection of gold nanoparticles using phase-sensitive optical coherence tomography,” Opt. Express 16(7), 4376–4393 (2008). 10.1364/OE.16.004376 [DOI] [PubMed] [Google Scholar]
- 16.Pache C., Bocchio N. L., Bouwens A., Villiger M., Berclaz C., Goulley J., Gibson M. I., Santschi C., Lasser T., “Fast three-dimensional imaging of gold nanoparticles in living cells with photothermal optical lock-in Optical Coherence Microscopy,” Opt. Express 20(19), 21385–21399 (2012). 10.1364/OE.20.021385 [DOI] [PubMed] [Google Scholar]
- 17.Chi T. T., Tu Y. C., Li M. J., Chu C. K., Chang Y. W., Yu C. K., Kiang Y. W., Yang C. C., “Photothermal optical coherence tomography based on the localized surface plasmon resonance of Au nanoring,” Opt. Express 22(10), 11754–11769 (2014). 10.1364/OE.22.011754 [DOI] [PubMed] [Google Scholar]
- 18.Jung Y., Reif R., Zeng Y., Wang R. K., “Three-dimensional high-resolution imaging of gold nanorods uptake in sentinel lymph nodes,” Nano Lett. 11(7), 2938–2943 (2011). 10.1021/nl2014394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tucker-Schwartz J. M., Meyer T. A., Patil C. A., Duvall C. L., Skala M. C., “In vivo photothermal optical coherence tomography of gold nanorod contrast agents,” Biomed. Opt. Express 3(11), 2881–2895 (2012). 10.1364/BOE.3.002881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jain P. K., Lee K. S., El-Sayed I. H., El-Sayed M. A., “Calculated absorption and scattering properties of gold nanoparticles of different size, shape, and composition: applications in biological imaging and biomedicine,” J. Phys. Chem. B 110(14), 7238–7248 (2006). 10.1021/jp057170o [DOI] [PubMed] [Google Scholar]
- 21.Dubois A., Grieve K., Moneron G., Lecaque R., Vabre L., Boccara C., “Ultrahigh-resolution full-field optical coherence tomography,” Appl. Opt. 43(14), 2874–2883 (2004). 10.1364/AO.43.002874 [DOI] [PubMed] [Google Scholar]
- 22.Schmitt J. M., Kumar G., “Optical scattering properties of soft tissue: a discrete particle model,” Appl. Opt. 37(13), 2788–2797 (1998). 10.1364/AO.37.002788 [DOI] [PubMed] [Google Scholar]
- 23.Loo C., Lin A., Hirsch L., Lee M.-H., Barton J., Halas N., West J., Drezek R., “Nanoshell-Enabled Photonics-Based Imaging and Therapy of Cancer,” Technol. Cancer Res. Treat. 3(1), 33–40 (2004). [DOI] [PubMed] [Google Scholar]
- 24.Chahed L., Thèye M. L., Fournier D., Roger J. P., Boccara A. C., Li Y. M., Turner W. A., Paul W., “Surface effects in hydrogenated amorphous silicon studied by photothermal-deflection experiments,” Phys. Rev. B Condens. Matter 43(18), 14488–14497 (1991). 10.1103/PhysRevB.43.14488 [DOI] [PubMed] [Google Scholar]
- 25.Bossy E., Institut Langevin, ESPCI, 1 rue Jussieu, F-75005, Paris, France (personal communication, 2014).