Abstract
Mycoplasma synoviae depends on its adhesin VlhA to mediate cytadherence to sialylated host cell receptors. Allelic variants of VlhA arise through recombination between an assemblage of promoterless vlhA pseudogenes and a single transcription promoter site, creating lineages of M. synoviae that each express a different vlhA allele. The predicted full-length VlhA sequences adjacent to the promoter of nine lineages of M. synoviae varying in avidity of cytadherence were aligned with that of the reference strain MS53 and with a 60-a.a. hemagglutinating VlhA C-terminal fragment from a Tunisian lineage of strain WVU1853T. Seven different sequence variants of an imperfectly conserved, single-copy, 12-a.a. candidate cytadherence motif were evident amid the flanking variable residues of the 11 total sequences examined. The motif was predicted to adopt a short hairpin structure in a low-complexity region near the C-terminus of VlhA. Biotinylated synthetic oligopeptides representing four selected variants of the 12-a.a. motif, with the whole synthesized 60-a.a. fragment as a positive control, differed (P<0.01) in the extent they bound to chicken erythrocyte membranes. All bound to a greater extent (P<0.01) than scrambled or irrelevant VlhA domain negative control peptides did. Experimentally introduced branched-chain amino acid (BCAA) substitutions Val3Ile and Leu7Ile did not significantly alter binding, whereas fold-destabilizing substitutions Thr4Gly and Ala9Gly tended to reduce it (P<0.05). Binding was also reduced to background levels (P<0.01) when the peptides were exposed to desialylated membranes, or were pre-saturated with free sialic acid before exposure to untreated membranes. From this evidence we conclude that the motif P-X-(BCAA)-X-F-X-(BCAA)-X-A-K-X-G binds sialic acid and likely mediates VlhA-dependent M. synoviae attachment to host cells. This conserved mechanism retains the potential for fine-scale rheostasis in binding avidity, which could be a general characteristic of pathogens that depend on analogous systems of antigenically variable adhesins. The motif may be useful to identify previously unrecognized adhesins.
Introduction
The bacterial pathogen Mycoplasma synoviae is associated with a broad spectrum of clinical manifestations ranging from inapparent infection to systemic disease of poultry. Infection is most commonly associated with inflammatory lesions of the joints, respiratory and/or reproductive tract and results in reduced feed conversion and poor egg quality. Less commonly, M. synoviae can be found infecting additional tissues in galliform birds (e.g. spleen, liver, central nervous system, skeletal muscle, and eye) [1]–[4] and respiratory tissues or synovial membranes of distantly related avian species such as ducks, geese, pigeons, and sparrows [5].
Attachment to sialylated receptors on host cells is mediated by the M. synoviae variable lipoprotein hemagglutinin VlhA [6]–[7]. Previous analyses indicated that the vlhA gene family has been laterally transferred between M. synoviae and Mycoplasma gallisepticum possibly during coinfection of a shared avian host [8]–[9]. In M. synoviae, antigenic variants of this adhesin result from unidirectional recombination between a single expression site and a large reservoir of vlhA pseudogenes [10]. In contrast, altered expression in M. gallisepticum stems from the expansion and contraction of a poly-GAA repeat upstream of the promoters of each copy of vlhA [11]. The selective pressure of specific host immune responses to these antigens is thought to drive diversity in vlhA allele expression [10]–[13]. Despite the critical importance of cytadherence to the establishment and maintenance of infection, discrete VlhA types were demonstrated to have significantly different avidities for host cell binding, which can be quantified by agglutination of erythrocytes [14]. M. synoviae's capacity for cytadherence maps surprisingly to a hypervariable C-terminal domain of VlhA called MSPA [15]–[16]. The precise means of attachment and how this capacity is retained despite such extensive sequence polymorphism and allele switching are not known. We sought to identify and characterize the specific motif that mediates adhesion of VlhA proteins to host cells.
Materials and Methods
Identification and Structural Modeling of the Putative Hemagglutination Motif (PHM)
The predicted full-length VlhA sequences adjacent to the single transcription promoter of nine lineages of M. synoviae varying in avidity of cytadherence (F10-2AS, FMT, K4907, K5016, K5395, MS117, MS173, MS178, and a>30X-passaged Florida lineage of strain WVU1853T) [14] were aligned with that of the reference strain MS53 [8] and with a 60-a.a. hemagglutinating VlhA C-terminal fragment from a ca. 12X-passaged Tunisian lineage of strain WVU1853T [15] by using ClustalΩ [17]. The multiple alignment was manually inspected for conserved motifs, evident as contiguous residues inferred to be under stabilizing selection (ω<1) by using Bayesian models of sequence evolution in the Selecton v2.4 software suite [18]. The secondary structures of full-length VlhA, MSPA and its C-terminal 60 residues, and of the putative hemagglutination motifs (PHMs) described were modeled using the Phyre2 suite of template-directed and ab initio protein structure prediction algorithms (http://www.sbg.bio.ic.ac.uk/phyre2) [19]. The effects of individual amino acid substitutions on peptide structural stability were predicted by applying the Site Directed Mutator algorithm (http://mordred.bioc.cam.ac.uk/~sdm/sdm.php) [20] to the.pdb files generated by Phyre2. Substitutions having stability scores (ΔΔG) between −0.5 and 0.5 were predicted to be neutral, whereas those <−2 or>2 were predicted to be highly destabilizing. The potential to bind sialic acid (KEGG Compound C00270; PubChem.sdf 445063) or any other ligand in the KEGG Compound database was predicted by applying the eFindSite ligand binding site prediction algorithm (http://brylinski.cct.lsu.edu/) [21]–[22] also to the.pdb files generated by Phyre2.
Quantitative Binding of PHM Peptides
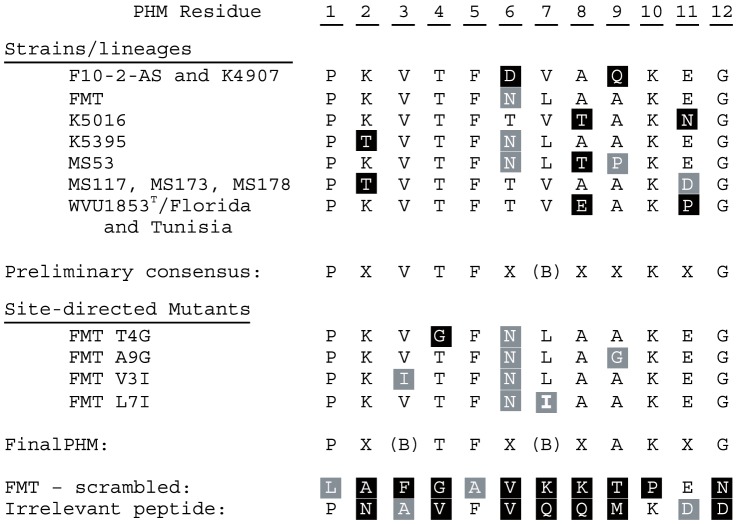
Twelve-a.a. peptides representing five variants of the PHM from strains FMT, K5016, K5395, MS53 and WVU1853T, plus the whole 60-a.a. hemagglutinating fragment of the Tunisian lineage of strain WVU1853T, were synthesized, biotinylated and lyophilized (Biomatik, Wilmington, DE). Purity of each lyophilized preparation was confirmed by HPLC to be 90–92% full-length peptide. Those strains were chosen because FMT, K5016, K5395 and the Florida lineage of WVU1853T spanned a>20-fold range in quantitative hemagglutination phenotypes, and the entire vlhA locus sequence of the reference strain MS53 has been published. [8], [14]. Peptides having single directed mutations introduced at the conserved residues 3 or 4, or non-conserved residues 7 or 9, were also synthesized using the strain FMT motif PKVTFNLAAKEG as a parent. FMT was chosen as the parent motif because it had only one difference (Thr6Asn) from the most commonly observed amino acid at each residue (Figure 1). The functionally synonymous substitutions Val3Ile and Leu7Ile (BLOSUM62 [23] scores>0) were predicted to be inconsequential, while non-synonymous Thr4Gly and Ala9Gly (BLOSUM62 scores ≤0) were predicted to affect PHM structure and/or function.
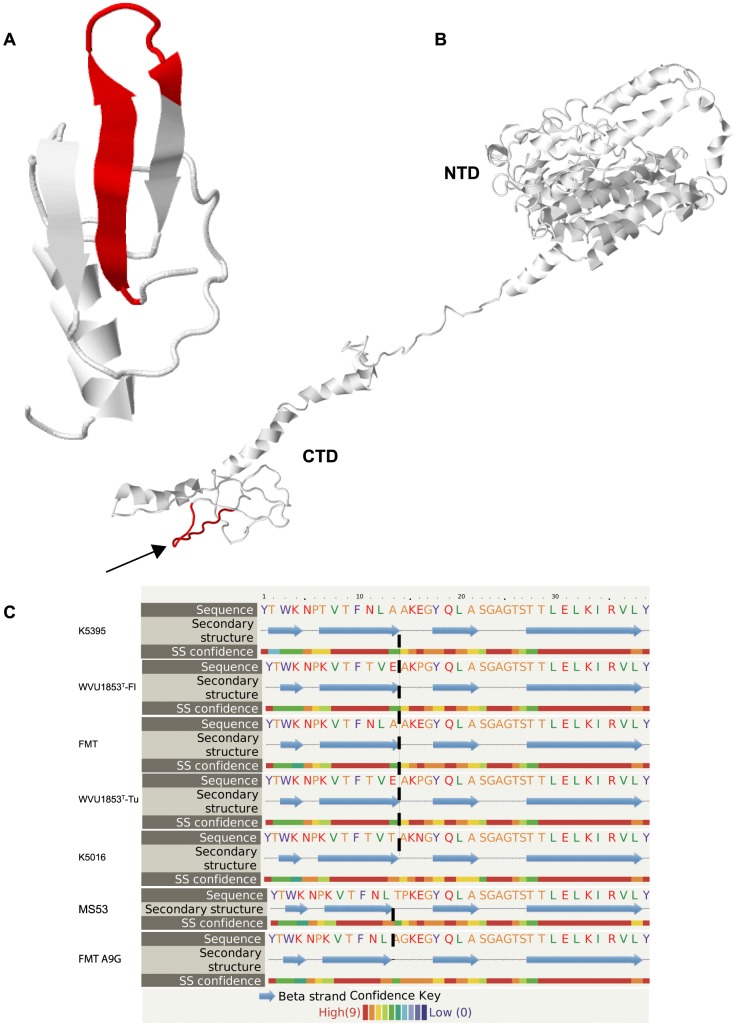
Figure 1. Aligned PHM and control peptide sequences.
The putative hemagglutination motif (PHM) was deduced by aligning the adhesin protein VlhA allele present at the expression site of ten specimens of M. synoviae with a 60-a.a. hemagglutinating VlhA C-terminal fragment from the Tunisian lineage of strain WVU1853T, then inspecting the alignment for contiguous residues inferred to be under stabilizing selection. Peptides representing five variants of the PHM, including strains having a>20-fold range in quantitative hemagglutination phenotypes [14], were synthesized. Directed mutations were introduced at selected residues relative to the PHM from strain FMT, which had only one difference (Thr6Asn) from the most common amino acid at each residue. The mutations Val3Ile and Leu7Ile were predicted to be inconsequential, while Thr4Gly and Ala9Gly were predicted to affect PHM structure and/or function. Negative control peptides used in erythrocyte membrane-binding assays are also shown. Functionally non-synonymous differences relative to the most common amino acid at each residue are shaded in black, synonymous differences are shaded in gray, and identical residues are not shaded. (B) = branched chain amino acid.
The capacity of the peptides to bind to native or desialylated chicken erythrocyte membranes was assessed quantitatively in an ELISA format. Microtiter plates were coated with 5% v/v suspensions of chicken erythrocytes (Lampire Biologicals, Pipersville, PA) diluted 1∶3 in 0.5 M sodium bicarbonate lysis buffer, pH 10.0, to a total volume of 300 µL per well. Desialylated membranes were prepared by pre-treatment of the erythrocytes with 10 U/ml of sialidase purified from Clostridium perfringens (Sigma-Aldrich, St. Louis, MO) for 1 hr at 37°C. Following coating for 12 hr at 4°C, cellular debris including hemoglobin was removed by washing each well 3× with 300 µL of PBS, pH 7.4, and sealed plates were blocked 1 hr at 37°C with 300 µL per well of 5% v/v fetal bovine serum in PBS.
After washing the membrane-coated and blocked wells 3× with 300 µL of PBS, 50 µg of biotinylated peptide solubilized in 50 µL of water was added to each of duplicate wells and allowed to bind for 1 hr at 37°C. After washing each well 3× with 300 µL of PBS, bound peptides were detected using horseradish peroxidase-conjugated streptavidin (2 µg/mL, Sigma-Aldrich, St. Louis, MO) and the chromogenic substrate 3,3′,5,5′-tetramethylbenzidine (Thermo Fisher Scientific, Waltham, MA) with an acid stop followed by spectrophotometric analysis (λ = 450 nm). The hemagglutinating 60-mer of the Tunisian lineage of strain WVU1853T served as the positive control peptide, and negative controls were a scrambled version of the PHM from strain FMT (LAFGAVKKTPEN) and an irrelevant peptide (PNAVFVQQMKDD) from a distant site in the expressed VlhA of the Florida lineage of strain WVU1853T (GenBank AEA01932.1). The effect of pre-saturation with ligand was tested by first incubating the peptides in 250 mg/ml N-acetylneuraminic acid (Sigma-Aldrich, St. Louis, MO) in water without pH adjustment at a peptide: ligand molar ratio of 1∶2×104 for 1 hr at 37°C.
Statistical Procedures
The effect of peptide sequence on extent of adherence to membranes (n = 3 independent replications of each treatment combination, with duplicate measurements of each peptide within replicate) was analyzed by ANOVA, with Tukey-Kramer Honestly Significant Difference (HSD) post-hoc comparisons used to group the means when the main effect was significant (P<0.05 or less). The effects of membrane pre-treatment with sialidase and peptide pre-saturation with sialic acid were analyzed by ANOVA, with HSD or Dunnett's post-hoc comparisons to the corresponding native specimens when the main effect was significant. Statistical analyses were performed using Origin 9 (OriginLab, Northampton, MA) software.
Motif Distribution in M. synoviae and M. gallisepticum
M. synoviae strain MS53 vlhA pseudogene sequences and M. gallisepticum strains R, F, WI01, NY01, NC06, CA06, VA94, NC95, NC08, and NC96 were obtained from GenBank (accession numbers NC_007294.1, NC_004829.2, NC_017503.1, NC_018410.1, NC_018409.1, NC_018411.1, NC_018412.1, NC_018406.1, NC_018407.1, NC_018413.1, and NC_018408.1, respectively). Occurrences of PHM-encoding sequences were totaled and normalized to the total length of vlhA-encoding sequence in each strain. Each member of the vlhA pseudogene reservoir of M. synoviae strain MS53 was used to construct a neighbor-joining tree (bootstrap n = 100) using ClustalW2 [24]. The designated outgroup was vlhA 4.02 from M. gallisepticum.
Results
Identification of the PHM
When the full-length expressed VlhA protein MSPA sequences of nine strains of M. synoviae that vary in avidity of cytadherence were aligned with MSPA of the reference strain MS53 [8] and a 60-a.a. hemagglutinating peptide derived from the C-terminus of MSPA expressed by the Tunisian lineage of strain WVU1853T [15], an imperfectly conserved 12-a.a. motif was evident in all sequences (Figure 1). A total of seven different PHM sequence variants were evident among the 11 total sequences aligned. Strains FMT, K5016, K5395 and MS53 all had unique PHM sequences; the sequences in strains F10-2-AS and K4907 were identical; the sequences in Florida and Tunisian lineages of WVU1853T were identical; and the sequences in Argentine strains MS117, MS173 and MS178 were all identical. Six of twelve residues in the PHM were perfectly conserved across strains, two (residues 6 and 7) were conserved in polarity and hydrophobicity, respectively, and four were variable. Polar Asn6 or Asp6 were invariably paired with Leu7, while Thr6 was invariably paired with Val7. The motif was predicted to adopt a short hairpin secondary structure of two anti-parallel beta strands, separated by a disordered loop of four or five residues, in a region of low structural complexity (regional structure prediction confidence <70%) near the C-terminus of MSPA (Figure 2a, b). Fifty-three percent of residues in the full-length VlhA were modeled at>90% confidence [19], with the regions of greatest confidence being similar to the streptococcal adhesin emb (99.8% confidence) and the staphylococcal extracellular matrix-binding protein ebhA (99.4%). The degree of structural complexity in the C-terminus of MSPA was otherwise too low for the algorithms to predict binding of any specific ligand.
Figure 2. PHM structural predictions.
(A) The putative hemagglutination motif (PHM; red) was predicted to adopt a hairpin structure of two anti-parallel β strands separated by a short disordered loop. (B) The motif (red, indicated by arrow) mapped to a low-complexity region near the carboxyterminal domain (CTD) of the M. synoviae adhesin protein VlhA cleavage product MSPA, shown here in the structure predicted for the Tunisian lineage of strain WVU1853T. The N-terminal domain (NTD) of MSPA was predicted to have much greater 3-dimensional complexity. (C) The length of the disordered loop was predicted to be longer in PHM peptides that bound to avian erythrocyte membranes (representing Florida and Tunisian lineages of strain WVU1853T and strains FMT, K5016 and K5395) than in the reduced-binding peptide mutant FMT-Ala9Gly and the non-binding peptide representing strain MS53.
Synthetic biotinylated peptides representing the full-length 60-a.a. hemagglutinating fragment and four strain variants of its candidate 12-a.a. cytadherence motif (Figure 1) bound to chicken erythrocyte membranes in an ELISA format and could be detected by probing with horseradish peroxidase-conjugated streptavidin. Four of the peptides bound to membranes to a significantly greater extent (P<0.05) than scrambled or irrelevant control peptides did, but a peptide representing the corresponding motif from strain MS53 did not bind to membranes to any extent greater than background (Figure 3). Single neutral substitutions (predicted ΔΔG = −0.25) experimentally introduced at conserved residue 3 (Val3Ile) or non-conserved residue 7 (Leu7Ile) did not alter binding to membranes with respect to the extent of binding by the parent motif of strain FMT, whereas the experimental destabilizing substitution Thr4Gly (predicted ΔΔG = −2.31) tended to reduce binding (Figure 3). The motif of strain MS53 differs naturally from all others by Ala9Pro (BLOSUM62 = −1; predicted ΔΔG = −2.22), and the even more destabilizing substitution Ala9Gly (predicted ΔΔG = −3.88) nearly abolished binding when introduced into the parent motif of strain FMT (P<0.05; Figure 3). These effects correlated with a predicted change in length of the disordered loop in the hairpin secondary structure of the motif (Figure 2c).
Figure 3. Erythrocyte membrane binding by PHM peptides.
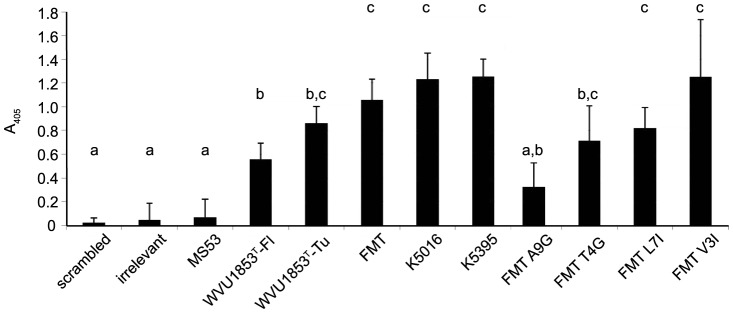
Bars depict mean ± standard error of the amount of synthetic peptide bound to avian erythrocyte membranes in an ELISA format (n = 3 independent replicates, with duplicate measurements of each peptide within replicate). The peptides represented variants of the putative hemagglutination motif (PHM) at the VlhA expression site of M. synoviae strains MS53, WVU1853T (Florida and Tunisian lineages), FMT, K5016 and K5395, which spanned a>20-fold range in quantitative hemagglutination phenotypes [14]. The positive control was the Tunisian lineage of strain WVU1853T, and negative controls were scrambled strain FMT peptide and an irrelevant peptide from a distant site in VlhA from the Florida lineage of strain WVU1853T. Different letters above the bar indicate means that differ (P<0.05 or less) by Tukey-Kramer Honestly Significant Difference test. As predicted, the directed substitution Ala9Gly significantly reduced binding versus the parent peptide from strain FMT, and Thr4Gly tended to reduce binding, whereas Val3Ile and Leu7Ile did not significantly alter binding.
Binding of the peptides to desialylated membranes was significantly reduced (P<0.01) relative to untreated membranes for all peptides except those representing strain MS53 and the scrambled and irrelevant controls (Figure 4a). When pre-incubated with free sialic acid, all peptides except the one representing strain MS53 and the scrambled and irrelevant controls had significantly diminished (P<0.01) capacity for membrane binding (Figure 4b). From this evidence we conclude that the composite amino acid motif P-X-(BCAA)-X-F-X-(BCAA)-X-A-K-X-G binds sialic acid and likely mediates VlhA-dependent M. synoviae attachment to sialylated receptors on the surface of avian erythrocytes.
Figure 4. Effects of sialylation and desialylation on PHM peptide binding.
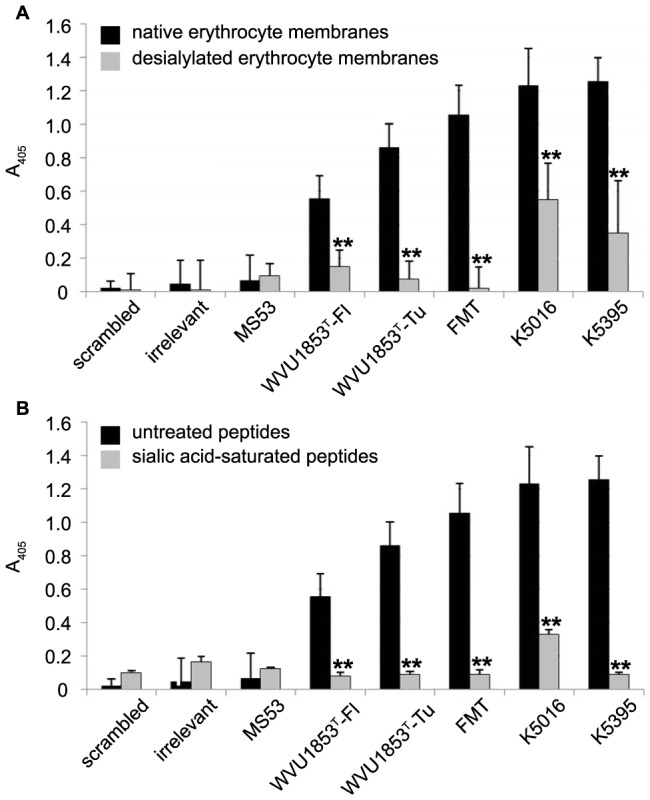
Bars depict mean ± standard error of the amount of synthetic peptide bound to avian erythrocyte membranes in an ELISA format (n = 3 independent replicates, with duplicate measurements of each peptide within replicate). (A) Desialylation of erythrocyte membranes significantly reduced PHM peptide binding relative to native membranes (** = P<0.01) for all strains of M. synoviae except MS53, which bound to native or desialylated erythrocyte membranes at background levels. (B) Presaturation of PHM peptides with free sialic acid before exposure to native erythrocyte membranes significantly reduced binding relative to untreated peptides (** = P<0.01) for all strains except MS53, on which sialic acid had no effect.
PHM Distribution among M. synoviae Strain MS53 vlhA Pseudogenes and Mycoplasma gallisepticum vlhA Homologs
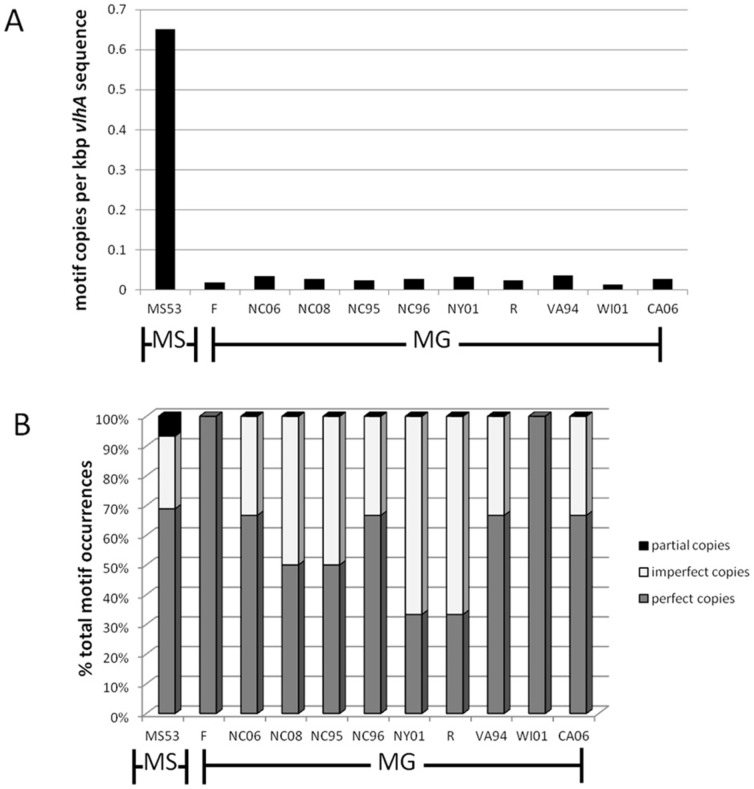
Candidate PHM sequences occurred in 45 of the 70 putative vlhA pseudogenes of M. synoviae strain MS53 [8], 39% of the time with no deviation from the consensus among the alleles expressed by the strains examined, 20% with a single deviation, and 17% with two deviations from consensus. Phylogenetic clustering of vlhA pseudogenes containing intact copies of the PHM did not correlate with their syntenic order in the strain MS53 genome (Figure S1). The PHM occurred at least 18-fold more frequently in strain MS53 (0.65 motifs/kb of vlhA sequence) than in the genomes of any of 10 strains of M. gallisepticum (0.014–0.037 motifs/kb of vlhA sequence), a species known to employ a different primary cytadherence mechanism [25] (Figure 5a). The rate of occurrence of imperfect PHMs was comparable between the two species (Figure 5b).
Figure 5. PHM distribution in vlhA genes and pseudogenes in M. synoviae and M. gallisepticum.
(A) PHM-encoding sequence as a function of total kbp of vlhA sequence is elevated 18-fold in M. synoviae (MS) reference strain MS53, the only strain for which the entire vlhA locus sequence has been published, relative to 10 fully-sequenced strains of M. gallisepticum (MG), evidence that it is far more common in M. synoviae. (B) The relative proportions of perfect and imperfect PHM copies were comparable between strains of M. synoviae and M. gallisepticum.
Discussion
One of the defining moments of many infections is the attachment of a disease-causing agent to its host. Understanding how the parasitic bacterial species M. synoviae colonizes a host cell's surface is paramount to understanding how to prevent infection. It is known that the protein family VlhA is responsible for attachment by M. synoviae, but the functional motifs of the adhesin and the molecular basis for rheostasis in binding avidity have not been characterized. Proteins in this family from multiple strains of M. synoviae have been identified as having a role in the attachment to host blood cells [7], [15]. Khiari et al. [15] mapped the capacity for attachment to the carboxyterminus of VlhA, and we utilized that finding to identify a specific motif sufficient to mediate VlhA binding to sialylated host cells.
Sequence conservation across adherent strains enabled the identification of a 12-residue putative hemagglutination motif that could be characterized further. This motif was predicted to have remarkably little structural complexity, in contrast to the complex topology of sialic acid ligand-binding domains of other microbes [26]–[27]. While residues at PHM positions 3 and 7 were conserved, substitution with similar residues having BLOSUM62 scores>0 did not alter function. The conserved Thr residue at position 4 could be changed to the dissimilar residue Gly (BLOSUM62 = −3) without loss of function. It is thus likely that the binding mechanism will tolerate synonymous substitutions at positions 3 and 7, and nonsynonymous substitutions at position 4. Residue 9 was a conserved Ala in all adherent strains. Strain MS53, which has an unknown attachment phenotype but is an attenuated strain, had the nonsynonmymous substitution Ala9Pro (BLOSUM62 = −1). Changing the strain FMT peptide to Gly9 (BLOSUM62 = 0) significantly diminished binding, and the strain MS53 peptide was non-adherent. Taken together, these results indicate that Ala9 is critical to PHM domain function. Our results indicate that the composite amino acid motif P-X-(BCAA)-X-F-X-(BCAA)-X-A-K-X-G mediates MSPA binding to avian erythrocytes. The potential to accommodate all amino acids with BLOSUM62 scores>0 at PHM positions 3 and 7 (i.e., Ala, Met, Thr and Met, Phe, respectively) rather than restricting the parameters to branched-chain amino acids (Ile, Leu, Val) merits further analysis.
Previous studies indicated that whole M. synoviae cells interact with sialylated host cell receptors in order to facilitate attachment. Extrapolation from PHM peptide-binding to whole cell attachment necessarily requires demonstration of peptide-sialic acid interactions. Desialylation of avian erythrocytes prior to antigen preparation resulted in significant losses of binding capacity for all PHM peptides except the scrambled and irrelevant controls and strain MS53, for which desialylation had no effect on binding. In a reciprocal experiment, pre-adsorption of peptides with free sialic acid prior to exposure to intact erythrocyte antigen similarly diminished binding capacity for all PHM peptides except the scrambled and irrelevant controls and strain MS53. These results indicate a specific interaction between sialic acid and the PHM and support the hypothesis that the PHM domain mediates attachment of whole M. synoviae cells to host sialoreceptors.
The occurrance of PHM domains was not uniform among the pseudogenes of M. synoviae strain MS53, the only strain for which the entire pseudogene reservoir has been sequenced [8]. A majority (69%) of pseudogenes had perfect or near-perfect PHMs, while 31% had no discernible PHMs. To provide some context for the distribution of PHM domains in the sample of VlhA sequences existing within M. synoviae strain MS53, we examined the frequency and distribution in an alternative sample of VlhA sequences that exist distributed across multiple strains of M. gallisepticum. In contrast to the 45 copies in M. synoviae strain MS53, sequenced M. gallisepticum strains ranged from having just a single copy of vlhA encoding a PHM domain (strains WI01 and F) up to a maximum of only 3 copies (strains R, NY01, NC06, CA06, VA94, NC95, and NC96). Normalization to the total amount of vlhA sequence within species confirmed that M. synoviae has a greatly elevated instance of PHM-encoding sequence relative to M. gallisepticum, and that the low frequency of PHM is consistent across strains of M. gallisepticum. The multiple independent cytadherence mechanisms of M. gallisepticum [28]–[32] may allow the decay of PHM domains within VlhA proteins, while selective pressure to retain the functional motif in the homologous proteins in M. synoviae is substantially greater due to the absence of other mechanisms of cytadherence.
This work describes a novel functional motif associated with adherence to sialic acid, and its distribution across vlhA pseudogenes. This very specific protein fragment pattern may be a target to design novel drug therapies or vaccines to alleviate or prevent infection due to M. synoviae as well as other pathogens that use similar mechanisms to attach to their hosts, and allows for the identification of currently unrecognized microbial adhesins targeting sialoreceptors.
Supporting Information
Distribution and relatedness of PHM-encoding pseudogenes. PHM-encoding pseudogenes (shaded) did not cluster together as a separate group from non-encoding pseudogenes. Relatedness of pseudogenes did not reflect gene synteny.
(TIF)
Acknowledgments
We thank Edan Tulman (University of Connecticut) for helpful discussions regarding vlhA loci in M. gallisepticum. This work was supported by the Robert M. Fisher Foundation (MM).
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Robert M. Fisher Foundation (MM). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Stipkovits L, Kempf I (1996) Mycoplasmoses in poultry. Rev Sci Tech 15(4): 1495–525. [DOI] [PubMed] [Google Scholar]
- 2. Sentíes-Cué G, Shivaprasad HL, Chin RP (2005) Systemic Mycoplasma synoviae infection in broiler chickens. Avian Pathol 34(2): 137–42. [DOI] [PubMed] [Google Scholar]
- 3. Chin RP, Meteyer CU, Yamamoto R, Shivaprasad HL, Klein PN (1991) Isolation of Mycoplasma synoviae from the brains of commercial meat turkeys with meningeal vasculitis. Avian Dis 35(3): 631–7. [PubMed] [Google Scholar]
- 4. Lockaby SB, Hoerr FJ, Lauerman LH, Kleven SH (1998) Pathogenicity of Mycoplasma synoviae in broiler chickens. Vet Pathol 35(3): 178–90. [DOI] [PubMed] [Google Scholar]
- 5.Brown DR, May M, Bradbury JM, Balish MF, Calcutt MJ, et al. (2010) Genus I. Mycoplasma In: Krieg NR, Ludwig W, Brown DR, Whitman WB, Hedlund BP, Paster BJ, Staley JT, et al.., editors. Bergey's Manual of Systematic Bacteriology Volume 4. Springer, Inc.: New York, NY. [Google Scholar]
- 6. Manchee R, Taylor-Robinson D (1969) Utilization of neuraminic acid receptors by mycoplasmas. J Bacteriol 98(3): 914–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Noormohammadi A, Markham P, Duffy M, Whithear K, Browning G (1998) Multigene families encoding the major hemagglutinins in phylogenetically distinct mycoplasmas. Infect Immun 66(7): 3470–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vasconcelos A, Ferreira H, Bizarro C, Bonatto S, Carvalho M, et al. (2005) Swine and poultry pathogens: the complete genome sequences of two strains of Mycoplasma hyopneumoniae and a strain of Mycoplasma synoviae . J Bacteriol 187(16): 5568–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Szczepanek SM, Tulman ER, Gorton TS, Liao X, Lu Z, et al. (2010) Comparative genomic analyses of attenuated strains of Mycoplasma gallisepticum . Infect Immun 78(4): 1760–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Noormohammadi A, Markham P, Kanci A, Whithear K, Browning G (2000) A novel mechanism for control of antigenic variation in the haemagglutinin gene family of mycoplasma synoviae. Mol Microbiol 35(4): 911–23. [DOI] [PubMed] [Google Scholar]
- 11. Glew MD, Baseggio N, Markham PF, Browning GF, Walker ID (1998) Expression of the pMGA genes of Mycoplasma gallisepticum is controlled by variation in the GAA trinucleotide repeat lengths within the 5' noncoding regions. Infect Immun 66(12): 5833–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Citti C, Browning GF, Rosengarten R (2005) Phenotypic diversity and cell invasion in host subversion by pathogenic mycoplasmas. In: Blanchard A, Browning GF, editors. Mycoplasmas Molecular Biology Pathogenicity and Strategies for Control. Horizon Bioscience: Norfolk, UK. [Google Scholar]
- 13.Zimmerman C-U (2014). Current insights into phase and antigenic variation in mycoplasmas. In: Browning GF, Citti C, editors. Mollicutes Molecular Biology and Pathogenesis. Caister Academic Press: Norfolk, UK. [Google Scholar]
- 14. May M, Brown DR (2011) Diversity of expressed vlhA adhesin sequences and intermediate hemagglutination phenotypes in Mycoplasma synoviae . J Bacteriol 193(9): 2116–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Khiari AB, Guériri I, Mohammed RB, Mardassi BB (2010) Characterization of a variant vlhA gene of Mycoplasma synoviae, strain WVU 1853, with a highly divergent haemagglutinin region. BMC Microbiol 10: 6 10.1186/1471-2180-10-6]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Noormohammadi A, Markham P, Whithear K, Walker I, Gurevich V, et al. (1997) Mycoplasma synoviae has two distinct phase-variable major membrane antigens, one of which is a putative hemagglutinin. Infect Immun 65(7): 2542–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, et al. (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol 7: 539 10.1038/msb.2011.75]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. May M, Brown DR (2009) Diversifying and stabilizing selection of sialidase and N-acetylneuraminate catabolism in Mycoplasma synoviae . J Bacteriol 191(11): 3588–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kelley LA, Sternberg MJ (2009) Protein structure prediction on the Web: a case study using the Phyre server. Nat Protoc 4(3): 363–71. [DOI] [PubMed] [Google Scholar]
- 20. Worth CL, Preissner R, Blundell TL (2011) SDM–a server for predicting effects of mutations on protein stability and malfunction. Nucleic Acids Res 39 (Web Server issue) W215–22 10.1093/nar/gkr363]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brylinski M, Feinstein WP (2013) eFindSite: improved prediction of ligand binding sites in protein models using meta-threading, machine learning and auxiliary ligands. J Comput Aided Mol Des 27(6): 551–67. [DOI] [PubMed] [Google Scholar]
- 22. Feinstein W, Brylinski M (2014) eFindSite: Enhanced fingerprint-based virtual screening against predicted ligand binding sites in protein models. Mol Inform 33(2): 15 10.1002/minf.201300143]. [DOI] [PubMed] [Google Scholar]
- 23. Henikoff S, Henikoff JG (1992) Amino acid substitution matrices from protein blocks. Proc Natl Acad Sci U S A 89(22): 10915–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Larkin MA, Blackshields G, Brown NP, Chenna R, McGettigan PA, et al. (2007) Clustal W and Clustal X version 2.0. Bioinformatics 23(21): 2947–8. [DOI] [PubMed] [Google Scholar]
- 25. Papazisi L, Frasca S, Gladd M, Liao X, Yogev D, Geary SJ (2002) GapA and CrmA coexpression is essential for Mycoplasma gallisepticum cytadherence and virulence. Infect Immun 70(12): 6839–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tharakaraman K, Jayaraman A, Raman R, Viswanathan K, Stebbins NW, et al. (2013) Glycan receptor binding of the influenza A virus H7N9 hemagglutinin. Cell 153(7): 1486–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pang SS, Nguyen ST, Perry AJ, Day CJ, Panjikar S, et al. (2014) The three-dimensional structure of the extracellular adhesion domain of the sialic acid-binding adhesin SabA from Helicobacter pylori . J Biol Chem 289(10): 6332–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Boguslavsky S, Menaker D, Lysnyansky I, Liu T, Levisohn S, et al. (2000) Molecular characterization of the Mycoplasma gallisepticum pvpA gene which encodes a putative variable cytadhesin protein. Infect Immun 68(7): 3956–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Forsyth MH, Tourtellotte ME, Geary SJ (1992) Localization of an immunodominant 64 kDa lipoprotein (LP 64) in the membrane of Mycoplasma gallisepticum and its role in cytadherence. Mol Microbiol 6(15): 2099–106. [DOI] [PubMed] [Google Scholar]
- 30. Goh MS, Gorton TS, Forsyth MH, Troy KE, Geary SJ (1998) Molecular and biochemical analysis of a 105 kDa Mycoplasma gallisepticum cytadhesin (GapA). Microbiology 144 (11): 2971–8. [DOI] [PubMed] [Google Scholar]
- 31. Jenkins C, Geary SJ, Gladd M, Djordjevic SP (2007) The Mycoplasma gallisepticum OsmC-like protein MG1142 resides on the cell surface and binds heparin. Microbiology 153(5): 1455–63. [DOI] [PubMed] [Google Scholar]
- 32. May M, Papazisi L, Gorton TS, Geary SJ (2006) Identification of fibronectin-binding proteins in Mycoplasma gallisepticum strain R. Infect Immun 74(3): 1777–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution and relatedness of PHM-encoding pseudogenes. PHM-encoding pseudogenes (shaded) did not cluster together as a separate group from non-encoding pseudogenes. Relatedness of pseudogenes did not reflect gene synteny.
(TIF)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.