Abstract
Objective
This study aimed to elucidate the natural history of intraductal papillary mucinous neoplasm (IPMN) of the pancreas with mural nodules (MNs) in branch duct IPMN (BD-IPMN).
Methods
Among the 402 registered patients with BD-IPMN on long-term follow-up at 10 institutions in Japan, 53 patients with MNs of less than 10 mm in height detected by endosonography were included in this study. The morphological changes of the BD-IPMN in these patients and histologic findings of the resected specimen were investigated.
Results
The median height of the MNs at the initial diagnosis was 3 mm (range, 1–8 mm), and 12 (23%) of the 53 patients showed an increase in the height of the MNs during follow-up (mean duration, 42 months). Six patients underwent surgery because of an increase in the height of MNs, yielding high-grade dysplasia in 1 patient and low-grade dysplasia in 5 patients. No patients developed invasive carcinoma derived from IPMN, and distinct pancreatic ductal adenocarcinoma developed in 1 (2%) patient. The incidence of the development of malignancy in BD-IPMNs, including distinct pancreatic ductal adenocarcinoma, was similar to that of those without MNs.
Conclusions
In patients who have BD-IPMN with MNs of less than 10 mm in height, observation instead of immediate resection is considered to be possible.
Key Words/Abbreviations: intraductal papillary mucinous neoplasm, natural history, follow-up, endoscopic ultrasonography, pancreatic ductal adenocarcinoma, BD-IPMN - branch duct intraductal papillary mucinous neoplasm, CT - computed tomography, ERCP - endoscopic retrograde cholangiopancreatography, EUS - endoscopic ultrasonography, IPMN - intraductal papillary mucinous neoplasm, MD-IPMN - main duct intraductal papillary mucinous neoplasm, MN - mural nodule, MPD - main pancreatic duct, MRCP - magnetic resonance cholangiopancreatography, PDAC - pancreatic ductal adenocarcinoma, US - ultrasonography
According to the international consensus guidelines 20121 for the management of intraductal papillary mucinous neoplasms (IPMNs) and mucinous cystic neoplasms of the pancreas, main duct IPMN (MD-IPMN) and branch duct IPMN (BD-IPMN) are significantly different with regard to the prevalence of carcinoma, and therefore, the classification has prognostic implications. When MD-IPMN is diagnosed in a patient, surgical treatment should be considered. In BD-IPMN, however, it is important to differentiate low-grade dysplasia from high-grade dysplasia (carcinoma in situ) or ordinary invasive pancreatic ductal adenocarcinoma (PDAC) to avoid excessive surgery.
The presence of mural nodules (MNs) has reportedly been the most important factor for predicting malignancy and determining the indication for surgery of BD-IPMN. However, there is a paucity of data on the morphological and histologic changes in patients with BD-IPMN with MNs during follow-up. The aim of this study was to evaluate long-term follow-up results of patients with BD-IPMN who had MNs on initial imaging in a retrospective multicenter series for better management of patients with BD-IPMN.
MATERIALS AND METHODS
Patients
The working group of the Japan Pancreas Society for the investigation of the natural history of IPMN,2 5 university hospitals and 5 tertiary referral institutions, collected information on 417 follow-up patients for more than 1 year who had undergone endoscopic ultrasonography (EUS) at the time of initial diagnosis during the period from November 1993 to February 2008. Those patients who had been followed up due to an inoperable PDAC derived from IPMN were excluded.
The indications for follow-up were based on the suggestions described in the international consensus guidelines 20063; these are as follows: BD-IPMNs with no symptoms such as abdominal pain, jaundice, or pancreatitis, MNs of less than 10 mm in height, cyst size of less than 3 cm, and main pancreatic duct (MPD) dilation of less than 10 mm. Ten patients with an initial cyst size of 3 cm and 5 patients with that of more than 3 cm were included.
Among the 417 follow-up patients, 15 were excluded from the analysis because they did not satisfy the inclusion criteria, which are as follows: follow-up periods of less than 1 year in 4 patients, MPD dilation of more than 10 mm in 5 patients, histologically diagnosed as non-IPMN in 3 patients, MN height of more than 10 mm in 1 patient, and incomplete data in 2 patients. Accordingly, 402 patients with BD-IPMN without MNs of 10 mm or greater in height on EUS at the time of initial diagnosis who had been followed up by several surveillance imagings for 1 year or more were eligible for this study.2 Finally, a total of 53 patients with BD-IPMN with MNs of less than 10 mm in height who underwent EUS, ultrasonography (US), and/or computed tomography (CT) at least twice, including the initial EUS during follow-up, were included.
Definitions
A diagnosis of IPMN was made by imaging when a dilated MPD or a cystically dilated branch duct was recognized in association with secretion of mucin from the major or minor papilla or mobile filling defects in the pancreatic duct on endoscopic retrograde cholangiopancreatography (ERCP) or when multilocular cystic lesions were recognized on EUS, magnetic resonance cholangiopancreatography (MRCP), and/or CT. Branch duct IPMN was defined as a condition in which the main lesion was a cystically dilated branch duct with an MPD diameter of less than 10 mm. The size of the dilated branch duct was measured en bloc in patients with multilocular cysts. The presence or absence of MNs in cystic branches was determined based on morphological features on EUS at the initial diagnosis. Color Doppler imaging or contrast-enhanced EUS was not applied in most of the cases because of the dominant use of a mechanical radial scanner. The change in the height of MNs was assessed essentially by follow-up EUS at registration, as available. In patients who underwent surgery after follow-up, the diagnosis of IPMN was confirmed histologically. Pathologic results were determined by the World Health Organization criteria published in 20104; these are as follows: low-grade dysplasia (“intraductal papillary mucinous adenoma”), intermediate-grade dysplasia (“IPMN with moderate dysplasia”), high-grade dysplasia (“intraductal papillary mucinous carcinoma, noninvasive,” “carcinoma in situ”), and PDAC. The highest pathologic grade was adapted when there were multifocal lesions.
Pancreatic ductal adenocarcinoma was divided into 2 types, as reported by Yamaguchi et al,5 1 derived from IPMN (“IPMN with an associated invasive carcinoma”) showing a histologic transition between IPMN and invasive carcinoma and the other concomitant with IPMN in which invasive carcinoma developed at a site in the pancreas different from that of the IPMN, according to the radiologic images and macroscopic or microscopic findings.
Methods
In patients with evident MNs in the cystic lumen, the height of the most prominent MNs was measured by EUS. During the follow-up period, strict monitoring of BD-IPMNs was performed by EUS, US, MRCP, and/or CT at intervals of 3 to 6 months. The modality used for the monitoring of BD-IPMNs was at the discretion of each institution and on a case-by-case basis, not following a unified protocol.
The maximum diameter of cystically dilated branch ducts and MPDs was measured by EUS in combination with US, CT, and/or MRCP, as available. Morphological findings at the initial examination, including the height of MNs, size of cystic branch, diameter of MPD, and presence of multifocal lesions, were collected. The frequency of enlargement of the cystically dilated branch, progression of MPD dilation, and an increase in the height of MNs were investigated using the follow-up data. Then, the characteristics of patients with BD-IPMN showing an increase in the height of MNs during follow-up were evaluated and compared with those patients without such an increase. In patients who had undergone surgery with morphological progression during follow-up, histologic findings of the resected specimens were evaluated, and the incidence and background of the development of invasive carcinoma associated with those lesions during follow-up were investigated.
These characteristics and morphological changes in the patients with IPMN with MNs were compared with those of the patients who did not show MNs on EUS at the time of initial diagnosis. The incidences of the development of PDAC derived from IPMN and PDAC concomitant with IPMN during follow-up were compared as well. Furthermore, the factors predictive of PDAC concomitant with IPMN were investigated.
Statistical Analysis
The average age, maximum size of cystic branch, maximum diameter of the MPD, and maximum height of MNs at the initial examination were compared using Student t test. The differences in the incidence of sex, enlargement of cystic branch, progression of MPD dilation, progression of MNs height, and multifocal lesions were examined with the χ2 test or Fisher exact test. P value of less than 0.05 was considered significant. The predictive factors for PDAC concomitant with IPMN were investigated by univariate analysis. The calculations were carried out using SPSS II for Windows (release 16.0; SPSS, Chicago, Ill).
RESULTS
Follow-up Results
The mean follow-up period of the 53 patients with BD-IPMN with MNs was 42.4 (SD, 22.2) months (range, 12–196 months). There were 28 men and 25 women, with a mean age of 66.1 (SD, 8.1) years (range, 44–83 years).
At the time of the initial diagnosis, all 53 patients underwent EUS. Computed tomography, US, MRCP, and ERCP were also carried out in 32, 30, 26, and 40 patients, respectively. At registration, EUS, CT, US, MRCP, and ERCP were carried out in 43, 25, 15, 27, and 20 patients, respectively. The mean height of MNs among the 53 patients at the start of follow-up was 3.2 (SD, 1.6) mm (median, 3 mm; range, 1–8 mm), and MN heights of less than 5 mm and those 5 to 10 mm were found in 41 (77.4%) patients and 12 (22.6%) patients, respectively. The mean maximum size of the cystically dilated branch and the mean diameter of the MPD were 2.2 (SD, 0.9) cm (range, 0.8–4.2 cm) and 3.9 (SD, 1.4) mm (range, 1–9 mm), respectively. The cystically dilated branch with the main MN was located in the head of the pancreas in 32 patients, in the body in 16 patients, and in the tail in 5 patients. Ten patients underwent surgery because of an increase in the height of the MNs (n = 6), enlargement of the dilated branches (n = 1), development of concomitant PDAC (n = 1), emergence of symptoms (n = 1), and patient’s request (n = 1).
Changes in the Size of Cystic Branches
During follow-up, enlargement of the cystic branch was identified in 4 (7.5%) of the 53 patients, none of whom showed an increase in the size of the MNs (Fig. 1). One patient, a 68-year-old man, underwent surgery because of an increase in the size of the cystic branch from 3 to 4.5 cm in 37 months, whereas the height of MN (3 mm) remained unchanged during follow-up. The histologic examination of the resected specimen verified low-grade dysplasia.
FIGURE 1.
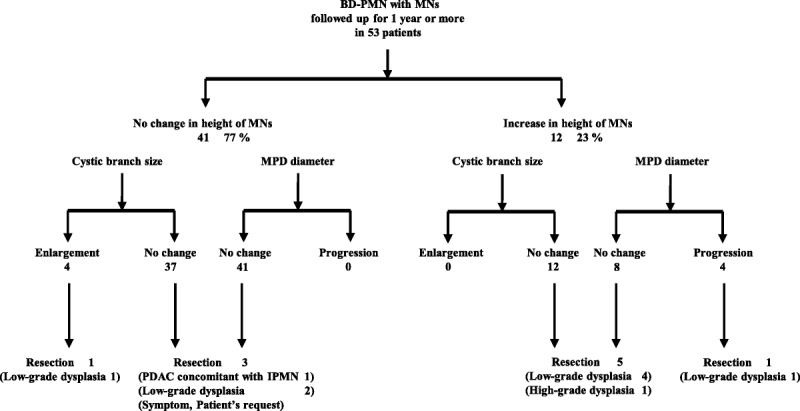
Changes in MNs, cyst size, and MPD diameter during follow-up.
Changes in the Size of MPD
Of the 53 patients, 4 (8%) showed progression of MPD dilation during the follow-up (Fig. 1). All of these 4 patients exhibited an increase in the size of the MNs as well; these are as follows: from 3 to 6 mm, from 8 to 13 mm, from 4 to 8 mm, and from 5 to 8 mm, respectively. One patient underwent resection, leading to a pathologic diagnosis of low-grade dysplasia (patient 7; Table 1). The other patients are now under follow-up.
TABLE 1.
Patients With BD-IPMN Showing an Increase in Height of MNs During Follow-up (n = 12)
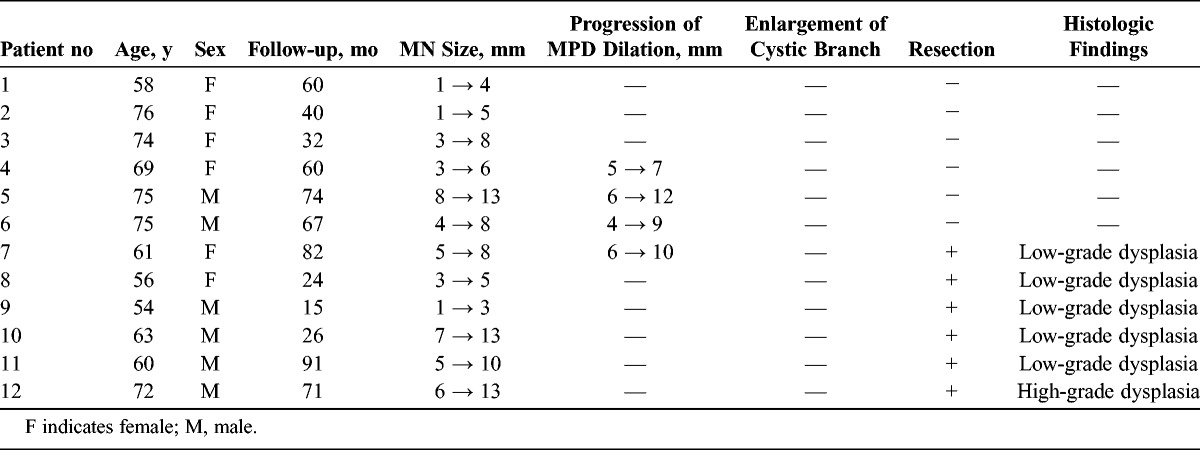
Changes in the Height of MNs
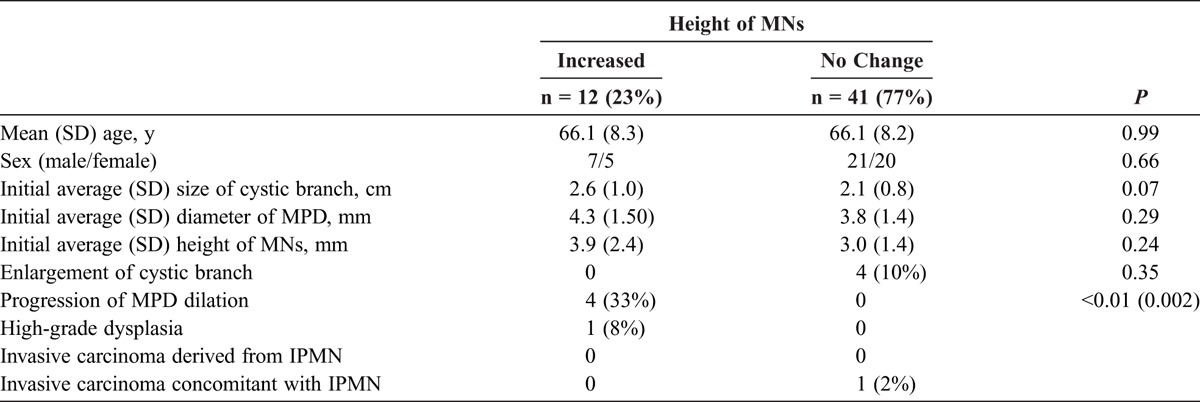
Of the 53 patients, 12 (23%) showed an increase in the height of the MNs during follow-up (Fig. 1). In those 12 patients, the mean size of the cystic branch, the mean diameter of the MPD, and the mean height of the MNs at the initial examination were not significantly different from those in the group without an increase in the size of the MNs during follow-up (Table 2). Furthermore, none of these 12 patients showed an enlargement of the cystic branch during follow-up. The frequency of progression of MPD dilatation during follow-up was significantly higher in the group with an increase in the height of MNs than those in the group without (33% vs 0%, P = 0.002). Furthermore, there was no significant difference in the frequency of enlargement of cystic branch between the groups (0% vs 10%, P = 0.35).
TABLE 2.
Comparison of Characteristics Between the Patients With BD-IPMN With and Without an Increase in Height of MNs During Follow-up (n = 53)
Six of the 12 patients showing an increase in the height of MNs underwent surgery. Histologic examination of the resected specimens verified high-grade dysplasia in 1 patient and low-grade dysplasia in 5 patients. None of them showed development of PDAC (Table 1). Among the 6 other patients who did not undergo surgery, 1 patient with MNs of 13 mm in height refused surgery and the remaining 5 patients who had MNs of less than 10 mm in height are under follow-up.
Of the 41 patients without an increase in the height of MNs, 4 underwent surgery, 1 of whom had a new appearance of a solid mass in a different portion in the pancreas. Histologic examination of the resected specimen revealed the mass to be a PDAC concomitant with IPMN and the IPMN itself was a low-grade dysplasia (Fig. 1). In the remaining 3 patients, pathologic diagnosis was all low-grade dysplasia.
Development of Malignancy Among Surgical Cases
To summarize the 10 surgical cases, 1 (2%) patient developed PDAC concomitant with IPMN without enlargement of the cystic branch, an increase in the height of MNs, or progression of MPD dilation.
In the remaining 9 patients, 1 (2%) had high-grade dysplasia and the others had low-grade dysplasia. The patient with high-grade dysplasia showed an increase in the height of the MNs from 6 to 13 mm in 71 months without enlargement of the cystic branch or progression of MPD dilation (Fig. 1).
Comparison of Morphological Changes and Histologic Findings Between Patients With BD-IPMN With and Without MNs
Size of Cystic Branches
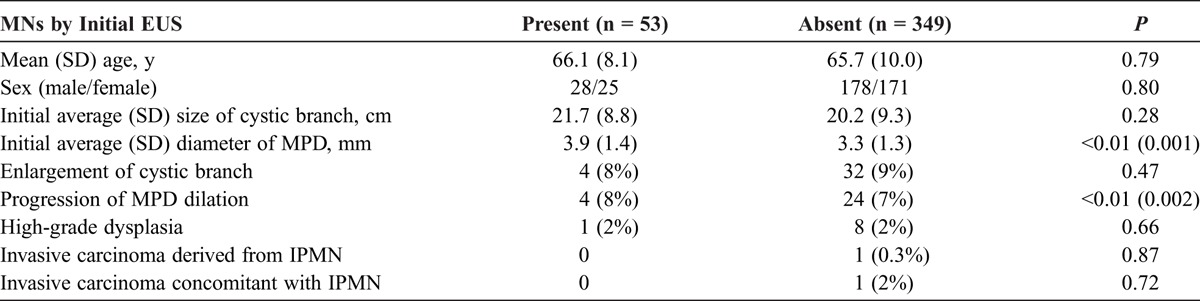
The comparison of morphological changes and histologic findings between patients with BD-IPMN with and without MNs is shown in Table 3. At the time of the initial diagnosis, the mean maximum size of the cystically dilated branch in the patients with and without the MNs was 2.2 (SD, 0.9) cm and 2.0 (SD, 0.9) cm, respectively. There was no significant difference between the 2 groups (P = 0.28). Furthermore, there was no significant difference in the incidence of enlargement of cystic branch during follow-up between the groups (8% vs 9%, P = 0.47).
TABLE 3.
Comparison of BD-IPMNs With and Without MNs by EUS at the Initial Examination (n = 402)
Diameter of MPD
The initial diameter of the MPD in the patients with MNs was significantly greater than that in those without (3.9 [SD, 1.4] mm vs 3.3 [SD, 1.3] mm, P = 0.001). On the other hand, there was no significant difference in the frequency of progression of MPD dilation during follow-up between the groups (8% vs 7%, P = 0.52).
Development of Malignancy
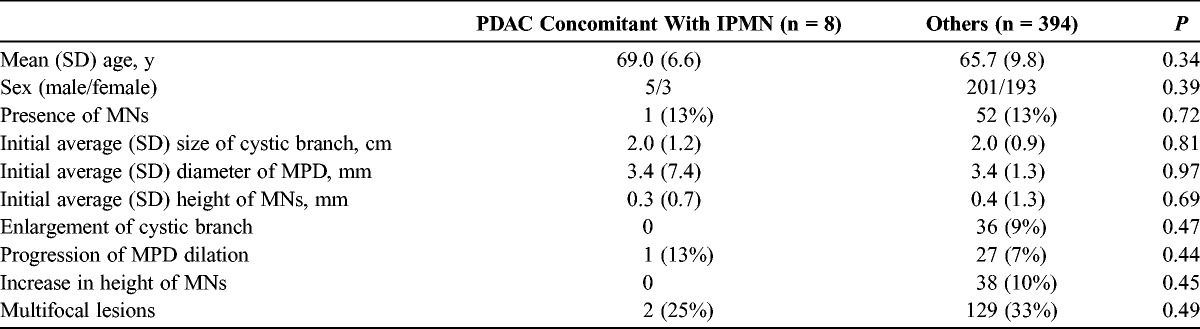
The number of patients who underwent surgery in each group with and without MNs was 10 of the 53 patients and 29 of the 349 patients, respectively. Among the 53 patients with MNs, the PDAC derived from IPMN developed in 0 patient, the PDAC concomitant with IPMN in 1 (1.9%) patient, and high-grade dysplasia in 1 (1.9%) patient. The corresponding numbers in the group without MNs including 3 patients with unresected PDAC concomitant with IPMN were 1 (0.3%), 7 (2.0%), and 8 (2.3%). There were no significant differences in the development of malignancy—high-grade dysplasia (P = 0.66), PDAC derived from IPMN (P = 0.87), and PDAC concomitant with IPMN (P = 0.72)—between the 2 groups.
Factors Predictive of Development of Malignancy in BD-IPMNs During Follow-up
One patient with PDAC derived from IPMN did not show the emergence of MNs, enlargement of cystic branch, increase in the height of MNs, or multifocal lesions. However, MPD dilation progressed during the follow-up. In other patients with BD-IPMN without PDAC derived from IPMN, the frequency of progression of MPD dilation was 7%.
The factors predictive of PDAC concomitant with IPMN in the follow-up patients were investigated by univariate analysis. There was no significant difference in each morphological feature between the patients with PDAC concomitant with IPMN and those without (Table 4).
TABLE 4.
Risk Factors of PDAC Concomitant With IPMN During Follow-up by Univariate Analysis n = 402
DISCUSSION
The international consensus guidelines recommend that patients with BD-IPMNs who have MNs should basically consider surgery if clinically appropriate. The subjects of the present study, however, were patients who had been observed because the height of MNs was less than 10 mm. In these patients, strict monitoring of BD-IPMNs had been performed at short intervals, which enabled investigation of the natural history of BD-IPMNs with MNs of less than 10 mm in height. Among these patients, 23% showed an increase in the height of the MNs during follow-up for over 40 months. In this group, there were no patients who showed enlargement of the cystic branch; however, all 4 patients who showed progression of MPD dilation exhibited an increase in the size of the MNs. The frequency of progression of MPD dilatation during follow-up was significantly higher in the group with an increase in the height of MNs than in the group without.
In the follow-up patients who had BD-IPMN with MNs, none developed PDAC derived from IPMN. The incidence of the development of malignancy in BD-IPMNs including a distinct PDAC was similar to that of those without MNs reported in the literature. Therefore, in those who have a BD-IPMN with MNs of less than 10 mm in height, observation instead of immediate resection is considered to be possible.
It is well known that IPMNs are characterized by slow progression and a favorable prognosis in contrast to ordinary PDAC, which is recognized as being very invasive.6–11 Histologic studies of resected IPMNs have revealed that most IPMNs are dysplasia without parenchymal invasion such as high-grade dysplasia (carcinoma in situ), intermediate-grade dysplasia (borderline, moderate dysplasia), and low-grade dysplasia.4 On the other hand, the presence of PDAC derived from IPMN showing parenchymal invasion has also been recognized, colloid carcinoma and tubular adenocarcinoma being its predominant histologic cell types. Therefore, the indications for surgery and determination of operative procedures based on the biologic behavior of this tumor are currently of great concern.
There are 2 opinions as to the indications for surgery in IPMN. One is that all patients with IPMN, including those with low-grade dysplasia, should undergo resection. This idea is based on the possible existence of an adenoma-carcinoma sequence in the evolution of this type of neoplasm and is also supported by the observations of oncogene activation. Yanagisawa et al12 reported that the same point mutation was detected both in the area of carcinoma and in coexisting adenoma components. Furthermore, duct-ectatic mucinous cystic neoplasms accompany K-ras point mutation similar to typical exocrine pancreatic carcinomas.
On the other hand, with the increase in clinical knowledge on the progression of IPMNs, the demand for establishing surgical indications that take the biologic behavior of such neoplasms into consideration is increasing.13–16 There are some groups who recommend surgery only in cases of high-grade dysplasia or invasive carcinoma, avoiding excessive surgery for benign conditions.
Main duct IPMN and BD-IPMN are significantly different with regard to the prevalence of carcinoma,13–16 and therefore, the classification has prognostic implications. In the review by Tanaka et al,1 the frequency of invasive carcinoma in MD-IPMN and in BD-IPMNs have a mean of 43% (range, 11%–81%) and 18% (range, 1%–37%), respectively.
According to the new international consensus guidelines 2012 for the management of IPMNs,1 when MD-IPMN is diagnosed in a patient with an IPMN, surgical treatment is strongly recommended. In BD-IPMN, however, the likelihood of invasive carcinoma is substantially less compared with that in MD-IPMNs. Thus, the differentiation of low-grade dysplasia from high-grade dysplasia or PDAC derived from IPMN would enable us to avoid excessive surgery.
Among the subjects of the present study on BD-IPMN, surgical resection was indicated according mainly to the previous international guidelines 2006,3 which are as follows: the appearance of symptoms attributable to IPMN (eg, pancreatitis), a cyst size greater than 30 mm, and dilation of the MPD (>6 mm).16–19 The usefulness of the previous consensus criteria for resection has been validated by many reports.20–24 According to the international consensus guidelines 2012, because a cyst size of more than 3 cm is a weaker indicator of malignancy than the presence of MNs and positive cytology, a BD-IPMN of more than 3 cm in size without MNs or positive cytology can be observed without immediate resection, particularly in elderly patients.
Some researchers consider the measurement of the maximum height of the MNs in BD-IPMNs to be effective for the differentiation between high-grade dysplasia and low-grade dysplasia and have suggested the height of the papillary protrusion of 3 to 10 mm as a cutoff value for determining the indication for surgical treatment.25,26 In our retrospective study on the relationship between the height of MNs on EUS and histologic findings,26 most patients in whom the maximum height of the MNs was more than 10 mm experienced high-grade dysplasia (86%). Furthermore, among those who underwent surgery due to the presence of MNs, no patients with MNs of 5 to10 mm in maximum height as shown by EUS developed PDAC derived from the BD-IPMN. Considering the biologic behavior of this neoplasm, performing surgery only in cases of BD-IPMN with a maximum height of MNs of more than 10 mm is likely to be justified.
Unfortunately, these retrospective studies entailed selection bias, that is, only patients with BD-IPMN who had been considered to have indications for surgery due to the presence of MNs and had undergone surgery were included. To verify the appropriateness of surgical indications based on the maximum height of MNs, a better understanding of the developmental course and the process of invasion in IPMN is necessary. The investigation of the morphological and histologic changes in patients with BD-IPMN who have undergone follow-up studies before resection is thus indispensable.
In 2011, the same working group of the Japan Pancreas Society2 reported long-term follow-up results of 349 patients who had no MNs on EUS at initial diagnosis. The results showed that the PDAC derived from IPMN and the distinct PDAC developed in 0.3% of the patients and 2.0% of the patients, respectively. In contrast, among the patients with MNs in the present multicenter study, PDAC derived from IPMN and PDAC concomitant with IPMN developed in 0% of the patients and 2% of the patients, respectively; that is, the incidence of the development of invasive carcinoma in BD-IPMNs with MNs was similar to that of those without MNs.
As previously reported, there may possibly be 2 developmental patterns of PDAC derived from IPMN, 1 with an increase in the height of MNs of more than 10 mm10 and the other at a site that is rather flat.26 Therefore, periodical surveillance is mandatory in BD-IPMNs regardless of the presence/absence and height of MNs.
Concerning the histologic type, most of the patients with PDAC had tubular adenocarcinomas showing few papillary growths, whereas approximately 30% of the patients with PDAC derived from IPMN had colloid carcinomas with high papillary protrusions. Colloid carcinoma derived from intestinal type27 is deemed to show more expansive and slower progression compared with other invasive carcinomas.11 Therefore, PDAC derived from IPMN shows a different invasive behavior from ordinary PDAC. Yamaguchi et al5 reported that the median survival time of 122 patients with PDAC derived from IPMN was 46 months, which was significantly longer than what was reported (12 months) in 7605 patients with ordinary PDAC.
Another problematic issue in patients with BD-IPMNs is that a distinct PDAC may develop in patients with IPMN, either synchronously or metachronously. Ohtsuka et al28 reported that the incidence of synchronous and metachronous multifocal occurrence of IPMNs in the remnant pancreas during follow-up evaluation after pancreatectomy for IPMNs was 20% and that of distinct PDAC was 9.9%. Izawa et al29 stated the possibility of multicentric development of cancer in IPMN, based on the observation that hyperplasia developed multifocally in different branch ducts with a different frequency of K-ras point mutation. In this study, we could not detect any significant predictive factors for the development of PDAC concomitant with IPMN.
The present study has several limitations. First, because of the retrospective nature of this study, the modality used for monitoring of BD-IPMN at intervals of 3 to 6 months was at the discretion of each institution, not following a unified protocol. Second, the presence of MNs was determined based solely on morphological features on EUS without color Doppler imaging, which may have resulted in inclusion of mucus nodules. However, the requirement in the inclusion criteria to have undergone EUS, US, and/or CT at least twice is thought to have minimized this risk. Third, the number of patients with MNs included in this study was not large because the presence of MNs is considered to be the most important factor for the surgical indication of BD-IPMN regardless of its height. However, the subjects were patients who had been followed up despite having MNs, and the number is the largest in the literature to date owing to multicenter cooperation. Fourth, patients with BD-IPMN who underwent surgery and histologic examination of resected specimens included not only patients showing an increase in the MN height to more than 10 mm but also those with no change or a change within 10 mm in height of MNs during follow-up. Furthermore, there is no way to investigate the incidence of high-grade dysplasia in the 43 patients who had not had surgical resection.
In summary, no PDAC derived from BD-IPMN developed in patients with MNs of less than 10 mm in height during follow-up for over 40 months. Furthermore, the incidence of the development of malignancy in BD-IPMNs including a distinct PDAC was similar to that of those without MNs. In patients who have BD-IPMN with MNs of less than 10 mm in height, observation instead of immediate resection is considered to be possible.
Footnotes
The Working Group for the Natural History of Intraductal Papillary Mucinous Neoplasm of the Japan Pancreas Society includes all authors of this article.
The authors declare no conflict of interest.
REFERENCES
- 1. Tanaka M, Fernández-del Castillo C, Adsay V, et al. International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology. 2012; 12: 183– 197 [DOI] [PubMed] [Google Scholar]
- 2. Maguchi H, Tanno S, Mizuno N, et al. Natural history of branch duct intraductal papillary mucinous neoplasms of the pancreas: a multicenter study in Japan. Pancreas. 2011; 40: 364– 370 [DOI] [PubMed] [Google Scholar]
- 3. Tanaka M, Chari S, Adsay V, et al. International consensus guidelines for management of intraductal papillary mucinous neoplasms and mucinous cystic neoplasms of the pancreas. Pancreatology. 2006; 6: 17– 32 [DOI] [PubMed] [Google Scholar]
- 4. Adsay NV, Fukushima N, Furukawa T, et al. Intraductal neoplasms of the pancreas. In: Bosman FT, Carneiro F, Hruban RH, et al. eds. WHO Classification of Tumors of the Digestive System. Lyon, France: IARC Press; 2010: 304– 313 [Google Scholar]
- 5. Yamaguchi K, Kanemitsu S, Hatori T, et al. Pancreatic ductal adenocarcinoma derived from IPMN and pancreatic ductal adenocarcinoma concomitant with IPMN. Pancreas. 2011; 40: 571– 580 [DOI] [PubMed] [Google Scholar]
- 6. Ohashi K, Murakami Y, Maruyama M, et al. Four cases of mucous secreting pancreatic cancer. Prog Dig Endosc. 1982; 20: 348– 351 (in Japanese) [Google Scholar]
- 7. Loftus EV, Jr, Olivares-Pakzad BA, Batts KP, et al. Intraductal papillary mucinous tumors of the pancreas: clinicopathologic features, outcome, and nomenclature. Members of the Pancreas Clinic, and Pancreatic Surgeons of Mayo Clinic. Gastroenterology. 1996; 110: 1909– 1918 [DOI] [PubMed] [Google Scholar]
- 8. Obara T, Maguchi H, Saitoh Y, et al. Mucin-producing tumor of the pancreas: natural history and serial pancreatogram changes. Am J Gastroenterol. 1993; 88: 564– 568 [PubMed] [Google Scholar]
- 9. Cellier C, Cuillerier E, Palazzo L, et al. Intraductal papillary and mucinous tumors of the pancreas: accuracy of preoperative computed tomography, endoscopic retrograde pancreatography and endoscopic ultrasonography, and long-term outcome in a large surgical series. Gastrointest Endosc. 1998; 47: 42– 49 [DOI] [PubMed] [Google Scholar]
- 10. Kobayashi G, Fujita N, Noda Y, et al. Ultrasonographic findings and natural history of intraductal papillary-mucinous neoplasms of the pancreas. J Med Ultrasonics. 2008; 35: 85– 96 [DOI] [PubMed] [Google Scholar]
- 11. Kobayashi G, Fujita N, Noda Y, et al. Intraductal papillary mucinous neoplasms of the pancreas showing fistula formation into other organs. J Gastroenterol. 2010; 45: 1080– 1089 [DOI] [PubMed] [Google Scholar]
- 12. Yanagisawa A, Kato Y, Ohtake K, et al. c-Ki-ras point mutations in ductectatic-type mucinous cystic neoplasms of the pancreas. Jpn J Cancer Res. 1991; 82: 1057– 1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kimura W, Sasahira N, Yoshikawa T, et al. Duct-ectatic type of mucin producing tumor of the pancreas—new concept of pancreatic neoplasia. Hepatogastroenterology. 1996; 43: 692– 709 [PubMed] [Google Scholar]
- 14. Kobari M, Egawa S, Shibuya K, et al. Intraductal papillary mucinous tumors of the pancreas comprise 2 clinical subtypes: differences in clinical characteristics and surgical management. Arch Surg. 1999; 134: 1131– 1136 [DOI] [PubMed] [Google Scholar]
- 15. Yamaguchi K, Ogawa Y, Chijiiwa K, et al. Mucin-hypersecreting tumors of the pancreas: assessing the grade of malignancy preoperatively. Am J Surg. 1996; 171: 427– 431 [DOI] [PubMed] [Google Scholar]
- 16. Irie H, Yoshimitsu K, Aibe H, et al. Natural history of pancreatic intraductal papillary mucinous tumor of branch duct type: follow-up study by magnetic resonance cholangiopancreatography. J Comput Assist Tomogr. 2004; 28: 117– 122 [DOI] [PubMed] [Google Scholar]
- 17. Wakabayashi T, Kawaura Y, Morimoto H, et al. Clinical management of intraductal papillary mucinous tumors of the pancreas based on imaging findings. Pancreas. 2001; 22: 370– 377 [DOI] [PubMed] [Google Scholar]
- 18. Yamaguchi K, Sugitani A, Chijiiwa K, et al. Intraductal papillary-mucinous tumor of the pancreas: assessing the grade of malignancy from natural history. Am Surg. 2001; 67: 400– 406 [PubMed] [Google Scholar]
- 19. Kobayashi G, Fujita N, Noda Y, et al. Lateral spread along the main pancreatic duct in branch-duct intraductal papillary-mucinous neoplasms of the pancreas: usefulness of intraductal ultrasonography for its evaluation. Dig Endsosc. 2011; 23: 62– 68 [DOI] [PubMed] [Google Scholar]
- 20. Mimura T, Masuda A, Matsumoto I, et al. Predictors of malignant intraductal papillary mucinous neoplasm of the pancreas. J Clin Gastroenterol. 2010; 44: e224– e229 [DOI] [PubMed] [Google Scholar]
- 21. Sadakari Y, Ienaga J, Kobayashi K, et al. Cyst size indicates malignant transformation in branch duct intraductal papillary mucinous neoplasm of the pancreas without mural nodules. Pancreas. 2010; 39: 232– 236 [DOI] [PubMed] [Google Scholar]
- 22. Kanno A, Satoh K, Hirota M, et al. Prediction of invasive carcinoma in branch type intraductal papillary mucinous neoplasms of the pancreas. J Gastroenterol. 2010; 45: 952– 959 [DOI] [PubMed] [Google Scholar]
- 23. Nagai K, Doi R, Ito T, et al. Single-institution validation of the international consensus guidelines for treatment of branch duct intraductal papillary mucinous neoplasms of the pancreas. J Hepatobiliary Pancreat Surg. 2009; 16: 353– 358 [DOI] [PubMed] [Google Scholar]
- 24. Sai JK, Suyama M, Kubokawa Y, et al. Pancreatic-duct-lavage cytology in candidates for surgical resection of branch-duct intraductal papillary mucinous neoplasm of the pancreas: should the International Consensus Guidelines be revised? Gastrointest Endosc. 2009; 69: 434– 440 [DOI] [PubMed] [Google Scholar]
- 25. Sugiyama M, Atomi Y. Intraductal papillary mucinous tumors of the pancreas: imaging studies and treatment strategies. Ann Surg. 1998; 228: 685– 691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kobayashi G, Fujita N, Noda Y, et al. Mode of progression of intraductal papillary-mucinous tumor of the pancreas: analysis of patients with follow-up by EUS. J Gastroenterol. 2005; 40: 744– 751 [DOI] [PubMed] [Google Scholar]
- 27. Furukawa T, Klöppel G, Volkan Adsay N, et al. Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch. 2005; 447: 794– 799 [DOI] [PubMed] [Google Scholar]
- 28. Ohtsuka T, Kono H, Tanabe R, et al. Follow-up study after resection of intraductal papillary mucinous neoplasm of the pancreas; special references to the multifocal lesions and development of ductal carcinoma in the remnant pancreas. Am J Surg. 2011; 204: 44– 48 [DOI] [PubMed] [Google Scholar]
- 29. Izawa T, Obara T, Tanno S, et al. Clonality and field cancerization in intraductal papillary-mucinous tumors of the pancreas. Cancer. 2001; 92: 1807– 1817 [DOI] [PubMed] [Google Scholar]


