Abstract
Background
Patients with diabetes are in extract higher risk for fatal cardiovascular events.
Objective
To evaluate major predictors of mortality in subjects with type 2 diabetes.
Methods
A cohort of 323 individuals with type 2 diabetes from several regions of Brazil was followed for a long period. Baseline electrocardiograms, clinical and laboratory data obtained were used to determine hazard ratios (HR) and confidence interval (CI) related to cardiovascular and total mortality.
Results
After 9.2 years of follow-up (median), 33 subjects died (17 from cardiovascular causes). Cardiovascular mortality was associated with male gender; smoking; prior myocardial infarction; long QTc interval; left ventricular hypertrophy; and eGFR <60 mL/min. These factors, in addition to obesity, were predictors of total mortality. Cardiovascular mortality was adjusted for age and gender, but remained associated with: smoking (HR = 3.8; 95% CI 1.3-11.8; p = 0.019); prior myocardial infarction (HR = 8.5; 95% CI 1.8-39.9; p = 0.007); eGFR < 60 mL/min (HR = 9.5; 95% CI 2.7-33.7; p = 0.001); long QTc interval (HR = 5.1; 95% CI 1.7-15.2; p = 0.004); and left ventricular hypertrophy (HR = 3.5; 95% CI 1.3-9.7; p = 0.002). Total mortality was associated with obesity (HR = 2.3; 95% CI 1.1-5.1; p = 0.030); smoking (HR = 2.5; 95% CI 1.0-6.1; p = 0.046); prior myocardial infarction (HR = 3.1; 95% CI 1.4-6.1; p = 0.005), and long QTc interval (HR = 3.1; 95% CI 1.4-6.1; p = 0.017).
Conclusions
Biomarkers of simple measurement, particularly those related to target-organ lesions, were predictors of mortality in subjects with type 2 diabetes.
Keywords: Diabetes Mellitus, Type 2 / mortality; Epidemiology; Diabetes Mellitus, Type 2 / complications
Introduction
The clinical manifestations of atherosclerosis are significantly more prevalent in patients with diabetes mellitus (DM)1. In these patients, cardiovascular event-free survival is greatly reduced, leading to premature loss of work capacity in middle-aged individuals and incapacity to lead a more active life among the elderly. However, although associated with a higher event rate, the traditional risk factors for coronary heart disease, such as hypertension, smoking and high cholesterol, can only partly explain this excess cardiovascular risk in this population.
The World Health Organization estimated that there were 30 million adults with diabetes mellitus worldwide in 1985, with an estimated population of 300 million individuals with DM in 2030 and major socio-economic impact, especially in developing countries such as Brazil, where the epidemiological force seems to be of greater intensity2. Many of these individuals (four in five individuals) live in low-income countries, according to a report from the International Diabetes Federation of 20123. Brazil currently occupies the uncomfortable fourth position among countries with the highest prevalence, with approximately 13.4 million affected individuals, corresponding to 6.5% of the adult population.
In the beginning of the century, regarding mortality rate, 5.2% of all deaths worldwide were attributed to diabetes, confirming that the disease is the fifth leading cause of death, with an estimate that approximately 4.6 million adults died from this cause in 2011 worldwide, representing 8.2% of overall mortality4.
In Brazil it is estimated that diabetes is underreported on death certificates, probably because the affected individuals, most often die due to ischemic complications or kidney disease, and not as a consequence of direct metabolic complications of diabetes, such as ketoacidosis or hypoglycemia.
Diabetic patients have comorbidities such as obesity, arterial hypertension and dyslipidemia, which contribute to the worsening of cardiovascular risk, indicating a 40% rate of hypertension at the diagnosis of diabetes5. Recommendations from several guidelines advise stricter lipid targets, considering this population at a greater risk6,7.
Thus, our study aimed to assess the main predictors of mortality in patients with diabetes mellitus.
Methods
Study population
The study included patients with type 2 diabetes, as defined by the American Diabetes Association (ADA)8. Patients were included based on the established diagnosis of myocardial infarction, but clinically stable at baseline (1/3 of the sample) or without diagnosis of previous myocardial infarction, as well as no evidence of other prior clinical manifestations of atherosclerosis, such as cerebrovascular accident, peripheral obstructive vascular disease or other manifestations of coronary heart disease (2/3 of patients). These patients came from 10 research centers in nine cities of the North (Belém-PA); South (Curitiba-PR); Southeast (São Paulo, Santos, Campinas and São José do Rio Preto); Midwest (Goiás) and Northeast (Fortaleza-CE) regions of Brazil and were consecutively included in the study. Patients with signs or symptoms of heart failure or scheduled percutaneous or surgical myocardial revascularization were excluded.
Of the subjects initially included in 10 research centers, complete data were obtained from 323 individuals by the end of the study, after a median follow-up of 9.2 years and were used in this study. The characteristics of these subjects were similar to that of the initial cohort, with the exception of body mass index, fasting glucose and HDL-C.
The study protocol was approved by the central and local ethics committees and the signed informed consent form was obtained from all participants or their legal guardians.
The data collected at baseline included medical history, physical examination, 12 lead-resting electrocardiogram (ECG) and blood samples after a 12-hour fasting. Outcomes were reported annually during the follow-up. In case of death or patient's incapacity to come to the research center, a family member was contacted. Only deaths clearly confirmed by hospital records or witnessed as attributed to cardiovascular causes or CVA were reported as cardiovascular mortality. The study endpoints were assessed by two experienced investigators. The primary outcomes analyzed in the study were cardiovascular and all-cause mortality. Secondary outcomes included fatal and nonfatal cardiovascular events (myocardial infarction, CVA, hospitalization for unstable angina or myocardial revascularization).
Laboratory assessment
Laboratory tests were performed on samples obtained at baseline and used after adequate bio-freezer storage under ultra-low temperature (-80°C), being measured in the Central Laboratory of Hospital da Universidade Federal de São Paulo (Unifesp).
Glucose was measured in serum by the enzymatic colorimetric method, after fasting for 12 hours, in mg/dL. Components of the lipid profile were analyzed by the enzymatic colorimetric method in serum for total cholesterol, HDL-C, VLDL and triglycerides in mg/dL. LDL-C was estimated by Friedewald's equation, for triglycerides < 400 mg/dL9. Ultrasensitive C-reactive protein (hsCRP) was measured by the immunoturbidimetric method (Vitros ® 5600, Ortho Clinical Diagnostics, Johnson & Johnson) in mg/L. The analysis of homocysteine was performed by the two-point kinetic method (Fusion®, Clinical Diagnosis) and expressed in micromoles/L.
The analysis of troponin I (ultrasensitive) was performed by immunoassay method (chemiluminescence) and expressed in ng / mL (Vitros ® 5600, Ortho Clinical Diagnostics, Johnson & Johnson). Serum creatinine was analyzed by colorimetric kinetics (Opera ® Bayer) and expressed in mg/dL.
The glomerular filtration rate was estimated (estimated Glomerular Filtration Rate - eGFR) using three different equations: Cockcroft-Gault10, Modification of Diet in Renal Disease (MDRD)11 and Chronic Kidney Disease-Epidemiology Collaboration (CKD-EPI)12 and expressed in mL/min per 1.73 m2 . The MDRD and CKD-EPI formulas were used due to assumption of great miscegenation among the Brazilian population, considering the incorporation of the racial component in these equations.
Patients with glomerular filtration rate ≥ 90 mL / min per 1.73 m2 or between 60-89 mL / min per 1.73 m2 were considered normal or having mild reduction in renal function, respectively. CKD was defined as eGFR ≤ 60 mL / min per 1.73 m2 , and the patients were classified as having moderately decreased kidney function (stage III) when eGFR was 30-59 mL / min per 1.73 m2 and severe (stage IV) when eGFR was 30 mL / min per 1.73 m2 . Rates of 15 mL/min per 1.73 m2 were considered as kidney failure, according to the National Kidney Foundation classification13.
Electrocardiographic variables
The 12-lead resting ECG was performed in certified equipment calibrated for 1.0 mV/cm, with the patient in the supine position, at a standard velocity of 25 mm / s and interpreted by experienced cardiologists, who were blinded to the baseline characteristics and outcomes of participants. Axis and QRS duration, the amplitude of R waves in leads D1, aVL, V5 and V6, the amplitude of the S wave in V1, V2 and V3 and the strain pattern were quantified, as well as the largest amplitude of R and S waves in the horizontal plane leads. The strain pattern is defined as convex ST segment depression with asymmetric T-wave inversion (opposite to the QRS complex) in leads V5 and V6.
Bazett's formula was used to correct the QT interval, measured in milliseconds (ms) from the start of the Q wave to the end of the T wave, using the following equation: QTc = QT/RR ½ 14.
The Perugia score was used for the evaluation of ventricular hypertrophy, as determined by the presence of one or more of the following findings: Cornell criteria, considering values ≥ 20 and 24 mm as limits for women and men, respectively; Romhilt-Estes score; presence of strain pattern15.
Statistical Analysis
Data are shown as mean and standard deviation for normally distributed variables or as medians and interquartile ranges for quantitative variables with non-normal distribution. Categorical variables were expressed as number (n) and percentage (%); continuous variables were compared by analysis of variance for repeated data followed by Tukey-Kramer contrast test, when appropriate. Variables with non-Gaussian distribution were compared by Wilcoxon test.
The logistic regression analyses were performed for all potential predictor variables of interest, with the values shown as hazard ratios (HR) within the 95% confidence interval (95% CI) and shown Kaplan-Meier curves. The potential predictors were allocated simultaneously and Cox regression was performed for the significant ones. A significance level of 5% was used in all tests. In the multivariate analysis for cardiovascular and total mortality outcomes, adjustments were performed for age and gender for the significant variables in the univariate analysis. All tests were performed using the statistical software program (SPSS) version 17.0 (SPSS Inc. Chicago, IL, USA).
Results
Demographics and population characteristics at baseline
Data were collected between March 2001 and December 2011, with a median follow-up of 9.2 years. The mean age of participants was 60 years (at the start), and 59% were females. The study population included individuals with the following racial characteristics: Caucasian (69.2%), African descendants (23.0%, with 6% of blacks and 17% of mixed race), Asians (6.3%) and Native Brazilians (1.5%). The study included patients with type 2 diabetes mellitus, both in the primary prevention of cardiovascular disease (63%), as after documented previous myocardial infarction (37%). The epidemiological, clinical and laboratory characteristics are shown in Table 1.
Table 1.
Epidemiological, clinical and laboratory characteristics of patients at baseline
| Characteristics and variables | Studied cohort (n = 323) | Initial cohort (n = 434) | p value |
|---|---|---|---|
| Epidemiological data | |||
| Age (years)msd | 60(52-66) | 60 (53-66) | 1.00 |
| Male gendern | 132 (41) | 179 (41) | 1.00 |
| Smokingn | 24 (7) | 30 (7) | 0.89 |
| Medical history | |||
| Arterial hypertensionn | 276 (85) | 354(82) | 0.70 |
| DM duration (years)msd | 6(2-11) | 6(3-11) | 0.99 |
| Family history (CF)n | 90 (28) | 110 (25) | 0.57 |
| Previous myocardial infarctionn | 108 (33) | 162 (37) | 0.35 |
| BMI (kg/m2)m | 25.6 ± 4.4 | 29.1 ± 4.9 | < 0.001 |
| Overweight/obesityn | 172 (53) | 347(80) | 0.0007 |
| Laboratory data | |||
| Glycemia (mg/d)md | 146 (120-192) | 154 (123-202) | 0.008 |
| Total cholesterol (mg/dL)m | 196 ± 43 | 198 ± 49 | 0.56 |
| HDL-C (mg/dL)m | 31 ± 11 | 36 ± 9.6 | < 0.001 |
| LDL-C (mg/dL)m | 124 ± 39 | 123 ± 38 | 0.72 |
| Triglycerides (mg/dL)msd | 174 (120-226) | 173 (111-248) | 0.57 |
| SBP (mmHg)m | 135 ± 21 | 138 ± 21 | 0.051 |
| DBP (mmHg)m | 84 ± 11 | 84 ± 13 | 1.00 |
| Used medications | |||
| Hypoglycemiant agentsn | 323 (100) | 434 (100) | 1.00 |
| Insulin therapyn | 59 (18) | 80 (18) | 1.00 |
| Lipid-lowering therapyn | 119 (37) | 140 (34) | 0.38 |
| Antihypertensive therapyn | 261 (81) | 343(80) | 0.87 |
Data are expressed as mean and standard deviation (msd), as median and interquartile range (m), and absolute number and percentage (n). DM: diabetes mellitus; CF: coronary failure; BMI: body mass index; DBP: diastolic blood pressure; SBP: systolic blood pressure.
Medians and interquartile ranges were also obtained for the following laboratory variables: homocysteine (10.1 [7.0 to 14.2] mM); hsC-reactive protein (2.2 [1.0-5.0] mg/L); highly sensitive troponin (50 [20-170] pg/mL); creatinine (0.83 [0.64 to 1.02] mg/dL).
For glomerular filtration rates, the following medians and interquartile ranges (mL/min/1, 73 m2) were obtained: Cockcroft-Gault: (91 [69-121]); MDRD: ([87 [71-113]); CKD-EPI: (90 [74-106]).
Table 2 classifies patients according to estimated glomerular filtration rate (eGFR) in functional stages, according to the creatinine clearance, using the CKD-EPI equation13.
Table 2.
Patient staging according to glomerular filtration rate
| Functional stage (impairment) | eGFR mL/min per 1.73 m2 | Patients by CKD-EPI (%) |
|---|---|---|
| I (normal) | ≥ 90 mL/min por 1,73 m2 | 51.60 |
| II (mild) | 60-89 mL/min por 1,73 m2 | 34.40 |
| III (moderate) | 30-59 mL/min por 1,73 m2 | 12.10 |
| IV (severe) | 15-29 mL/min por 1,73 m2 | 1.30 |
| V (failure) | < 15 mL/min por 1,73 m2 | 0.60 |
CKD-EPI: Chronic Kidney Disease-Epidemiology; eGFR; estimated Glomerular Filtration Rate.
Electrocardiogram
All patients were submitted to a 12-lead electrocardiogram. Baseline characteristics of ECGs in the study and the initial cohort population were similar in all variables.
At baseline, the analysis of electrocardiographic tracings showed the presence of sinus rhythm in 99%. The presence of left ventricular hypertrophy (LVH) by Perugia criteria at ECG was observed in 28% of patients, whereas prolonged QT interval was observed in 17%, as shown in Table 3. Other characteristics, such as atrial fibrillation and artificial rhythm (pacemaker) showed low incidence (< 1%).
Table 3.
Electrocardiographic characteristics of patients at baseline
| Studied population | Initial population | |
|---|---|---|
| Electrocardiographic variables | (n = 323) | (n = 434) |
| Sinus rhythm | 321 (99) | 430 (99) |
| First degree AV block | 7 (2) | 8(1.8) |
| Left bundle branch block | 14 (4) | 9 (5) |
| Ventricular ectopia | 8 (2.5) | 10 (2.3) |
| Left ventricular hypertrophy | 87(28) | 126 (29) |
| Long QT interval * | 54 (17) | 69 (15.8) |
Data are expressed as number (n) and percentage (%).
QTc: cutoff value of 450 and 470 ms for men and women, respectively.
Outcomes
During the 10-year study follow-up, 94 fatal and nonfatal events were reported, including 33 deaths (17 from cardiovascular causes). In relation to overall mortality, the mean age of the subjects and the mean duration of exposure to diabetes (from diagnosis to the reported event) were 70.6 and 13.7 years, respectively. In relation to cardiovascular mortality, the mean age of patients and the mean duration of exposure to diabetes (from reported diagnosis to date of the event) were 72.2 and 13.41 years respectively. Data on biological markers associated with fatal and nonfatal cardiovascular outcomes, as well as mortality from all causes, are shown in Table 4.
Table 4.
Association of biological markers with fatal and nonfatal cardiovascular outcomes, and for mortality from all causes
| Events | Mortality | |||
|---|---|---|---|---|
| Variables | Cardiovascular | Coronary | Cardiovascular | Total |
| Male gender | 2.5 (1.6-4.1) | 3.1 (1.8-5.4) | 4.8 (1.6-14.8) | 2.0 (1.0-4.0) |
| p < 0.001 | p < 0.001 | p = 0.006 | p = 0.04 | |
| Obesity | 1.1 (0.7-1.7) | 0.8 (0.5-1.4) | 2.9 (0.9-8.9) | 2.5 (1.1-5.3) |
| p = 0.66 | p = 0.51 | p = 0.06 | p = 0.021 | |
| Smoking | 1.6 (0.8-3.3) | 1.2 (0.5-2.9) | 4.2 (1.4-12.9) | 3.2 (1.3-7.7) |
| p = 0.21 | p = 0.74 | p = 0.012 | p = 0.010 | |
| Previous infarction | 4.9 (3.0-8.0) | 6.0 (3.4-10.6) | 15.8 (3.6-69.2) | 4.3 (2.1-9.0) |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| Age > 65 years | 1.3 (0.8-0.3) | 1.1 (0.6-1.9) | 5.1 (1.9-13.9) | 3.4 (1.7-6.8) |
| p = 0.26 | p = 0.74 | p = 0.001 | p < 0.001 | |
| eGFR < 60mL/min | ||||
| Cockroft-Gault | 2.2 (1.2-4.2) | 3.1 (1.5-6.2) | 6.3 (1.8-21.7) | 3.8 (1.5-9.7) |
| p = 0.02 | p = 0.001 | p = 0.004 | p = 0.005 | |
| MDRD | 4.5 (2.4-8.6) | 4.9 (2.4-9.9) | 9.2 (2.6-32.8) | 4.7 (1.9-11.6) |
| p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | |
| CKD-EPI | 4.2 (2.2-8.1) | 4.5(2.2-9.1) | 9.7 (2.7-34.4) | 4.9 (2.0-12.2) |
| p < 0.001 | p < 0.001 | p < 0.001 | p = 0.001 | |
| Long QTc | 2.6 (1.4-5.0) | 2.8 (1.4-5.5) | 5.2 (1.7-15.6) | 3.0 (1.2-7.6) |
| p = 0.03 | p = 0.002 | p = 0.003 | p = 0.017 | |
| LVH | 2.0 (1.2-3.1) | 1.9(1.1-3.3) | 5.0 (1.8-13.5) | 2.0 (1.0-4.0) |
| p = 0.004 | p = 0.001 | p = 0.002 | p = 0.047 | |
| Ventricular ectopia | 3.9 (1.6-9.7) | 3.4 (1.2-9.4) | 2.4 (0.3-18.4) | 2.7(0.6-11.0) |
| p = 0.003 | p = 0.019 | p = 0.38 | p = 0.17 | |
Non-adjusted data and analyzed using Cox proportional regression model. Hazard ratios (HR), 95% CI and p values are shown.
eGFR: Estimated Glomerular Filtration Rate by Cockroft-Gault formula, MDRD formula and by the CKD-EPI equation; LVH: left ventricular hypertrophy.
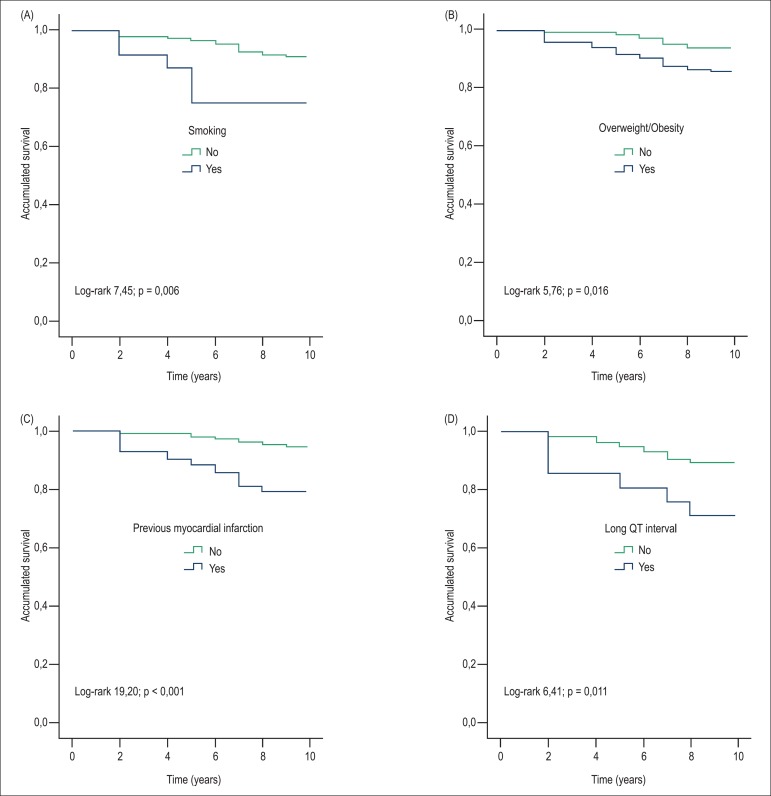
In the multivariate analysis adjusted for gender and age, cardiovascular mortality was associated with smoking (HR = 3.8, 95% CI = 1.3 to 11.8, p = 0.019), prior myocardial infarction (HR = 8.5, 95% CI = 1.8 to 39.9, p = 0.007), glomerular filtration rate < 60 mL/min/1.73 m2 (HR = 9.5, 95% CI = 2.7 -33.7, p = 0.001), long QT syndrome (HR = 5.1, 95% CI = 1.7 to 15.2, p = 0.004) and when positive, according to electrocardiographic criteria, with left ventricular hypertrophy (HR = 3.5, 95% CI = 1.3 to 9.7, p = 0.002). Applying the same adjustments, mortality from all causes was associated with overweight and obesity (HR = 2.3, 95% CI = 1.1 to 5.1, p = 0.030), smoking (HR = 2.5 95% CI = 1.0 to 6.1; p = 0.046), previous infarction ((HR = 3.1; 95% CI = 1.4-6.1; p = 0.005), and long QT interval (HR = 3.1; 95% CI = 1.4-6.1; with p = 0.017), as seen in the Kaplan-Meier curves (Figure 1).
Figure 1.
Kaplan-Meier curves for mortality from all causes. A) Smoking. B) Overweight / obesity. C) Prior myocardial infarction. D) Long QT Interval.
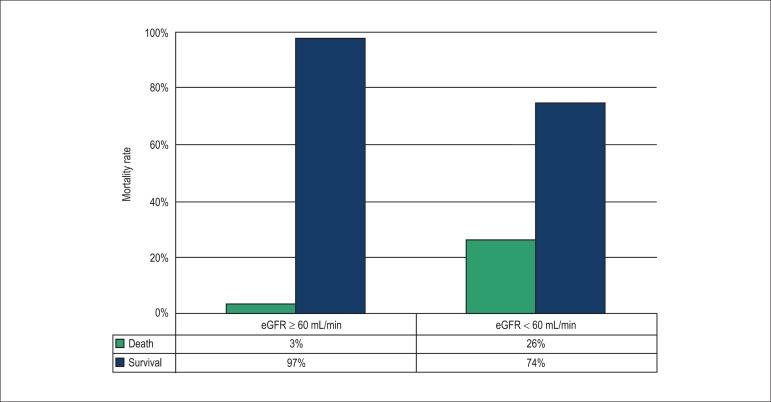
Moreover, according to the Cockcroft-Gault, MDRD and CKD-EPI equations, values < 60 mL/min/1.73 m2 were independent predictors of mortality from all causes. Interestingly, when using the formulas proposed by the CKD-EPI, we observed that those with eGFR ≥ 60 mL/min, only 3% died versus 26% of patients with eGFR < 60 mL/min (Figure 2).
Figure 2.
Association between mortality and impaired renal function.
Discussion
In our cohort of individuals with diabetes, some easy-to-determine biomarkers, especially those expressing target-organ lesions, allowed the identification of a subgroup at higher risk of death. In fact, we showed that the long QTc interval, the presence of LVH by ECG criteria, previous myocardial infarction and impaired renal function (glomerular filtration rate < 60 mL/min per 1.73 m2) were significant predictors of mortality. Moreover, the classical risk factors such as old age, male gender, obesity and smoking were also associated with higher rates for this outcome.
Some tested biomarkers, such as troponin, C-reactive protein and homocysteine were not associated with mortality, a fact probably explained by the low levels found for these variables in this cohort. However, according to literature data, the association with higher rates of cardiovascular outcomes of relevance has yet to be wel-established16-18.
Our results are in agreement with those reported in studies on the contribution of ECG abnormalities, including long QT interval, for prediction of mortality in individuals with DM19,20. In individuals with diabetes, hypoglycemia may be followed by sympathoadrenal stimulation with subsequent increase in QT interval, thus predisposing to severe and complex arrhythmias (torsades de pointes).
Hypoglycemia causes a cascade of pathological effects that can induce both oxidative stress and the onset of cardiac arrhythmias, which undoubtedly contribute to higher rates of death from cardiovascular causes. Literature has demonstrated that plasma concentration of endothelin 1 (ET-1), a potent endogenous vasoconstrictor, increases sharply after hypoglycemia episodes21.
Hypoglycemia can increase circulating levels of C-reactive protein through the mobilization and activation of neutrophils and platelet activation22,23. The possibility of hypokalemia, a common finding in patients with diabetes, may also predispose to changes in QT interval. Therefore, we believe that the analysis of QT interval should be incorporated into routine clinical practice, especially in individuals with diabetes. Its application allows better accuracy in relation to risk stratification, contributing to the selection of individuals at higher risk.
Left ventricular hypertrophy is another parameter that seems to be associated with cardiovascular mortality. In the Framingham study, electrocardiographic evidence of LVH was associated with a two-fold increase in mortality compared to that resulting from hypertension alone24. However, this marker was less often reported among diabetic populations, although the association of LVH with cardiovascular risk in normal or hypertensive populations is already well-established25.
Hemodynamic stress, as well as humoral and genetic processes, plays a crucial role in LVH onset26-28. Although some studies associated age with this onset, we can also enumerate other factors that contribute to the increase in left ventricular mass, especially during the aging process, such as increased pressure levels, the progressive increase in peripheral arterial resistance and the gradual replacement of myocytes by connective tissue. Individuals with LVH have a higher incidence of complex ventricular arrhythmias, as well as a higher incidence of atrial fibrillation; this may be the association between LVH and the occurrence of cerebrovascular accidents (CVAs). In the classic Framingham study, the odds ratio after adjusting for other variables for transient ischemic attacks and CVA was 1.2-1.8 for each quartile of increase in left ventricular mass29.
Prior myocardial infarction has been associated with higher rates of recurrent events and mortality when compared with non-diabetic individuals30. The incidence of infarction in patients with hypertension and LVH is higher due to the increased oxygen consumption and also the disproportionate growth of the myocardial mass in relation to the capillary network, resulting in relative ischemia31.
In a multicenter national study, from which part of our study population originated, the GOLD (Genetics, Outcomes and Lipids in type 2 Diabetes) study assessed the influence of risk factors and several polymorphisms in genes related to lipoprotein metabolism with potential dyslipidemia worsening, in the occurrence of myocardial infarction in patients with type 2 DM. This case-control study showed that male gender, presence of LVH at the ECG, smoking and D9N polymorphism of the gene encoding LPL (lipoprotein lipase) were independent predictors of the risk of myocardial infarction in this population32.
The analyses of the results of several studies, especially in diabetic patients, confirmed the usefulness of estimated glomerular filtration rate as a predictive value for cardiovascular disease and mortality33.
Chronic kidney disease (CKD) has been recognized as a risk factor for all-cause cardiovascular mortality34. Currently, CKD screening is recommended for all patients at high risk, including those with aggravating factors for cardiovascular diseases35. Our findings, in agreement with literature data, draw attention to the fact that chronic kidney disease, especially in individuals with diabetes, should perhaps be added to the list of criteria that define individuals at increased risk for future events.
The use of formulas that estimate glomerular filtration rate, which include ethnic factors, seems to be more appropriate, especially in countries such as Brazil, where high rates of miscegenation are observed. Furthermore, our results are in agreement with the use of the MDRD and CKD-EPI equations as valuable tools to estimate cardiovascular risk in this population.
Recently, two large studies compared the values for the estimated glomerular filtration rate obtained by the MDRD and CKD-EPI equations. Although the MDRD classified a few individuals as having chronic kidney disease, the formula proposed by the CKD-EPI showed good accuracy in predicting mortality or progression to end-stage kidney disease36,37.
Finally, the overweight/obesity variable also was independently associated with total mortality in our series. In fact, obesity is associated with higher levels of inflammatory markers and multiple pathophysiological mechanisms in atherogenesis. Many of them meet the criteria for metabolic syndrome, which is also associated with increased coronary atherosclerosis and increased levels of oxidized LDL38,39.
Our study therefore reinforces the importance of identification of target-organ lesions in the population of individuals with type 2 DM, as we systematically evaluated a composite of individuals of both genders living in different geographical regions of a developing country with continental dimensions, where high rates of miscegenation are present. With the aid of easy to determine and relatively low-cost biological markers, we believe in the broad applicability of these methods in low-income countries, contributing to the adoption of appropriate strategies aiming at a decrease in deaths, particularly early deaths, among the individuals.
Recently, a guideline from the American Heart Association / American College of Cardiology40 considered diabetic patients at different risk levels, also proposing differentiated therapeutic strategies regarding the use of statins of moderate or high intensity. Thus, not all diabetic patients should be classified as high risk, which justifies searching for biomarkers that can identify those of higher cardiovascular risk even in this group of patients.
Study limitations
As in every observational study, it was not possible to have complete control of the variables over time. Although this population was designed to represent a specific group of individuals who live in a large country, the number of monitored patients was small. In comparison with the initial cohort, we observed some differences, such as lower rates of overweight / obesity and fasting glucose in the studied cohort, which constitute potential bias in the sample selection. Glycemic control influences the rate of myocardial infarction and overall mortality41, but this cohort included only data from blood glucose at baseline. However, both populations had similar age and gender distribution, duration of diabetes, hypertension prevalence and blood pressure levels, as well as previous myocardial infarction rates and baseline LDL-cholesterol levels.
Conclusions
The study showed that the use of biological markers of simple measurement and analysis, especially those that indicate target-organs lesions, helps to identify individuals with type 2 diabetes and higher mortality risk.
Acknowledgements
The present study included the participation of other collaborators: Dr. Mario Sérgio Cerci (Universidade Federal do Paraná); Dr. José Carlos Nicolau (Universidade de São Paulo); Dr. Abrahão Afiune Neto (Universidade Federal de Goiás); Dr. Claudine Maria A. Feio (Universidade Federal do Pará), whom we would like to thank for their invaluable participation.
Footnotes
Author contributions
Conception and design of the research: Bianco HT, Izar MC, Fonseca FA; Acquisition of data: Bianco HT, Izar MC, Póvoa RM, Saraiva JF, Forti A, Introcaso L, Yugar-Toledo J, Xavier HT, Faludi AA; Analysis and interpretation of the data, Statistical analysis and Writing of the manuscript: Bianco HT, Fonseca FA; Critical revision of the manuscript for intellectual content: Bianco HT, Izar MC, Póvoa RM, Fonseca FA.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
This study was partially funded by FAPESP and CNPq.
Study Association
This article is part of the thesis of Doctoral submitted by Henrique Tria Bianco, from Universidade Federal de São Paulo (UNIFESP).
References
- 1.Fox CS, Coady S, Sorlie PD, Levy D, Meigs JB, D'Agostino Sr RB, et al. Trends in cardiovascular complications of diabetes. JAMA. 2004;292(20):2495–2499. doi: 10.1001/jama.292.20.2495. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. (WHO) Global status report on noncommunicable disease. 2010. [Accessed on 2013 Jan 10]. Available from: http://www.who.int/nmh/publications/ncd_report_full_en.pdf. [Google Scholar]
- 3.Reddy KS. Cardiovascular disease in non-Western countries. N Engl J Med. 2004;350(24):2438–2440. doi: 10.1056/NEJMp048024. [DOI] [PubMed] [Google Scholar]
- 4.IDF Diabetes Atlas. 5th ed. Brussels (Belgium): 2013. [Google Scholar]
- 5.UK prospective Diabetes Study Group Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ. 1998;317(7160):703–713. [PMC free article] [PubMed] [Google Scholar]
- 6.Xavier HT, Izar MC, Faria Neto JR, Assad MH, Rocha VZ, Sposito AC, et al. Sociedade Brasileira de Cardiologia V Diretriz brasileira de dislipidemia e prevenção da aterosclerose. Arq Bras Cardiol. 2013;101(4) supl 1:1–20. doi: 10.5935/abc.2013S010. [DOI] [PubMed] [Google Scholar]
- 7.Reiner Z, Catapano AL, De Baker G, Grahm I, Taskinen MR, Wiklund O, et al. Guidelines for the management of dyspilidaemias: Task force for the management os dyslipidaemia of the European Society of Cardiology (ESC) and the European Atherosclerosis Society. Eur Heart J. 2011;32(14):1769–1818. doi: 10.1093/eurheartj/ehr158. [DOI] [PubMed] [Google Scholar]
- 8.Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20(7):1183–1197. doi: 10.2337/diacare.20.7.1183. [DOI] [PubMed] [Google Scholar]
- 9.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol I in plasma without use of the preparative ultracentrifuge. Clin Chem. 1972;18(6):499–502. [PubMed] [Google Scholar]
- 10.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 12.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro 3rd AF, Feldman HI, et al. CKD-EPI (Chronic Kidney Disease-Epidemiology Collaboration) A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. Erratum in Ann Intern Med. 2011;155(6):408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.K/DOQI clinical practice guideline for chronic Kidney disease: evaluation, classification and stratification. Am J Kidney Dis. 2002;39(2) Suppl 1:S1–266. [PubMed] [Google Scholar]
- 14.Bazett HC. An analysis of the time-relations of electrocardiograms. Heart. 1920;7:353–370. [Google Scholar]
- 15.Schillaci G, Verdecchia P, Borgioni C, Ciucci A, Guerrieri M, Zampi I, et al. Improved electrocardiographic diagnosis of left ventricular hypertrophy. Am J Cardiol. 1994;74(7):714–719. doi: 10.1016/0002-9149(94)90316-6. [DOI] [PubMed] [Google Scholar]
- 16.Everett BM, Cook NR, Magnone MC, Bobadilla M, Kim E, Rifai N, et al. Sensitive cardiac troponin T assay and the risk of incident cardiovascular disease in women with and without diabetes mellitus: the Women's Health Study. Circulation. 2011;123(24):2811–2818. doi: 10.1161/CIRCULATIONAHA.110.009928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schöttker B, Herder C, Rothenbacher D, Roden M, Kolb H, Müller H, et al. Proinflammatory cytokines, adiponectin, and increased risk of primary cardiovascular events in diabetes patients with or without renal dysfunction: results from the ESTHER study. Diabetes Care. 2013;36(6):1703–1711. doi: 10.2337/dc12-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herder C, Baumert J, Zierer A, Roden M, Meisinger C, Karakas M, et al. Immunological and cardiometabolic risk factors in the prediction of type 2 diabetes and coronary events: MONICA/KORA Augsburg Case-Cohort Study. PLoS ONE. 2011;6(6): doi: 10.1371/journal.pone.0019852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christensen PK, Gall MA, Major-Pedersen A, Sato A, Rossing P, Breum L, et al. QTc interval length and QT dispersion as predictors of mortality in patients with non-insulin-dependent diabetes. Scand J Clin Lab Invest. 2000;60(4):323–332. doi: 10.1080/003655100750046486. [DOI] [PubMed] [Google Scholar]
- 20.Robinson RT, Harris ND, Ireland RH, Lee S, Newman C, Heller SR. Mechanisms of abnormal cardiac repolarization during insulin-induced hypoglycaemia. Diabetes. 2003;52(6):1469–1474. doi: 10.2337/diabetes.52.6.1469. [DOI] [PubMed] [Google Scholar]
- 21.Jin WL, Azuma K, Mita T, Goto H, Kanazawa A, Shimizu T, et al. Repetitive hypoglycaemia increases serum adrenaline and induces monocyte adhesion to the endothelium in rat thoracic aorta. Diabetologia. 2011;54(7):1921–1929. doi: 10.1007/s00125-011-2141-5. [DOI] [PubMed] [Google Scholar]
- 22.Wright RJ, Frier BM. Vascular disease and diabetes: is hypoglycemia an aggravating factor? Diabetes Metab Res Rev. 2008;24(5):353–363. doi: 10.1002/dmrr.865. [DOI] [PubMed] [Google Scholar]
- 23.Graveling AJ, Frier BM. Does hypoglycemia cause cardiovascular events? Br J Diab Vasc Dis. 2011;10(1):5–13. [review] [Google Scholar]
- 24.Kannel WB. Prevalence and natural history of electrocardiographic left ventricular hypertrophy. Am J Med. 1983;75(3A):4–11. doi: 10.1016/0002-9343(83)90111-0. [DOI] [PubMed] [Google Scholar]
- 25.Lonn E, Mathew J, Pogue J, Johnstone D, Danisa K, Bosch J, et al. Heart Outcomes Preventions Evaluations Study Investigators Relationship of electrocardiographic left ventricular hypertrophy to mortality in high risk patients. Eur J Cardiovasc Prev Rehabil. 2003;10(6):420–428. doi: 10.1097/01.hjr.0000106836.97722.cf. [DOI] [PubMed] [Google Scholar]
- 26.Laycock SK, Mc-Murray J, Kane KA, Parratt JR. Effects of chronic norepinephrine administration on cardiac-function in rats. J Cardiovasc Pharmacol. 1995;26(4):584–589. doi: 10.1097/00005344-199510000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Yamazaki T, Komuro I, Yazaka Y. Signaling pathways for cardiac hypertrophy. Cell Signal. 1998;10(10):693–698. doi: 10.1016/s0898-6568(98)00036-9. [DOI] [PubMed] [Google Scholar]
- 28.Hocher B, George I, Rebstock J, Bauch A, Schwarz A, Neumayer HH, et al. Endothelin system-dependent cardiac remodeling in renovascular hypertension. Hypertension. 1999;33(3):816–822. doi: 10.1161/01.hyp.33.3.816. [DOI] [PubMed] [Google Scholar]
- 29.Bikkina M, Levy D, Evans JC, Larson MG, Benjamin EJ, Wolf PA, et al. Left ventricular mass and risk of stroke in a elderly cohort. The Framingham Heart Study. JAMA. 1994;272(1):33–36. [PubMed] [Google Scholar]
- 30.Volpe M, Battistoni A, Tocci G, Rosei EA, Catapano AL, Coppo R, et al. Cardiovascular risk assessment beyond systemic coronary risk estimation: a role for organ damage markers. J Hypertens. 2012;30(6):1056–1064. doi: 10.1097/HJH.0b013e3283525715. [DOI] [PubMed] [Google Scholar]
- 31.Mueller TM, Marcus ML, Kerber RE, Young JA, Barnes RW, Abboud FM. Effect of renal hypertension and left ventricular hypertrophy on the coronary circulation in dogs. Cir Res. 1978;42(4):542–549. doi: 10.1161/01.res.42.4.543. [DOI] [PubMed] [Google Scholar]
- 32.Izar MC, Helfenstein T, Ihara SS, Relvas WG, Santos AO, Fischer SC, et al. Association of lipoprotein lipase D9N polymorphism with myocardial infarction in type 2 diabetes. The genetics, outcomes, and lipids in type 2 Diabetes (GOLD) study. Atherosclerosis. 2008;204(1):165–170. doi: 10.1016/j.atherosclerosis.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, et al. American Heart Association Councils on Kidney in Cardiovascular disease, High Blood Pressure Research. Kidney disease as a risk factor for development of cardiovascular disease: a statement from the American Heart Association Clinical Cardiology, and Epidemiology and Prevention. Circulation. 2003;108(17):2154–2169. doi: 10.1161/01.CIR.0000095676.90936.80. [DOI] [PubMed] [Google Scholar]
- 34.Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, Eckardt KU, et al. Chronic kidney disease as a global public health problem: approaches and initiatives a position statement from Kidney Disease Improving Global Outcomes. Kidney Int. 2007;72(3):247–259. doi: 10.1038/sj.ki.5002343. [DOI] [PubMed] [Google Scholar]
- 35.Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, et al. Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol. 2004;15(5):1307–1315. doi: 10.1097/01.asn.0000123691.46138.e2. [DOI] [PubMed] [Google Scholar]
- 36.Matsushita K, Mahmoodi BK, Woodward M, Emberson JR, Jafar TH, Jee SH, et al. Chronic Kidney Disease Prognosis Consortium: Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA. 2012;307(18):1941–1951. doi: 10.1001/jama.2012.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Afkarian M, Sachs MC, Kestenbaum B, Hirsch IB, Tuttle KR, Himmelfarb J, et al. Kidney disease and increased mortality in type 2 diabetes. J Am Soc Nephron. 2013;24(2):302–308. doi: 10.1681/ASN.2012070718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fonseca HA, Fonseca FA, Monteiro AM, Bianco HT, Boschcov P, Brandão SA, et al. Obesity modulates the immune response to oxidized LDL in hypertensive patients. Cell Biochem Biophys. 2013;67(3):1451–1460. doi: 10.1007/s12013-013-9585-9. [DOI] [PubMed] [Google Scholar]
- 39.Izar MC, Fonseca HA, Pinheiro LF, Monteiro CM, Póvoa RM, Monteiro AM, et al. Adaptive immunity is related to coronary artery disease severity after acute coronary syndrome in subjects with metabolic syndrome. Diab Vasc Dis Res. 2013;10(1):32–39. doi: 10.1177/1479164112443374. [DOI] [PubMed] [Google Scholar]
- 40.Stone NJ, Robinson J, Lichtenstein AH, Merz CN, Blum CB, Eckel RH, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2013 Nov 12; [Epub ahead of print.] [Google Scholar]
- 41.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008;359(5):1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]