Abstract
Background
Studies on atrial fibrillation (AF) in decompensated heart failure (DHF) are scarce in Brazil.
Objectives
To determine AF prevalence, its types and associated factors in patients hospitalized due to DHF; to assess their thromboembolic risk profile and anticoagulation rate; and to assess the impact of AF on in-hospital mortality and hospital length of stay.
Methods
Retrospective, observational, cross-sectional study of incident cases including 659 consecutive hospitalizations due to DHF, from 01/01/2006 to 12/31/2011. The thromboembolic risk was assessed by using CHADSVASc score. On univariate analysis, the chi-square, Student t and Mann Whitney tests were used. On multivariate analysis, logistic regression was used.
Results
The prevalence of AF was 40%, and the permanent type predominated (73.5%). On multivariate model, AF associated with advanced age (p < 0.0001), non-ischemic etiology (p = 0.02), right ventricular dysfunction (p = 0.03), lower systolic blood pressure (SBP) (p = 0.02), higher ejection fraction (EF) (p < 0.0001) and enlarged left atrium (LA) (p < 0.0001). The median CHADSVASc score was 4, and 90% of the cases had it ≥ 2. The anticoagulation rate was 52.8% on admission and 66.8% on discharge, being lower for higher scores. The group with AF had higher in-hospital mortality (11.0% versus 8.1%, p = 0.21) and longer hospital length of stay (20.5 ± 16 versus 16.3 ± 12, p = 0.001).
Conclusions
Atrial fibrillation is frequent in DHF, the most prevalent type being permanent AF. Atrial fibrillation is associated with more advanced age, non-ischemic etiology, right ventricular dysfunction, lower SBP, higher EF and enlarged LA. Despite the high thromboembolic risk profile, anticoagulation is underutilized. The presence of AF is associated with longer hospital length of stay and high mortality.
Keywords: Atrial Fibrillation; Heart Failure; Ventricular Dysfunction, Left; Inpatients; Hospital Mortality
Introduction
Atrial fibrillation (AF) is a very important health problem. It is the most frequent arrhythmia in patients with chronic heart or lung diseases, affects 1% of the population, and is associated with an increase in the risk for stroke and with a double-fold increase in mortality1,2. Heart failure (HF) is considered a severe public health problem worldwide due to its elevated prevalence, increasing incidence and high costs. In addition, it has a high hospitalization rate, being one of the major causes of morbidity and mortality in industrialized countries3. Decompensated HF (DHF) is the major cause of hospitalization of patients older than 65 years in the Brazilian Unified Health Care System (SUS) and has an elevated in-hospital mortality4. Approximately 50% of the patients are readmitted in six months due to DHF5.
The concomitance of AF and HF can be explained because both share similar risk factors and mechanisms, or because there is a causal relationship between the entities. Considering that the morbidity and mortality attributed to each of those comorbidities are significant, the concomitance of AF and HF identifies individuals at higher mortality risk. Most studies on AF and HF have been conducted with outpatient cohorts, and studies on patients with DHF are rare6,7.
Brazilian data on AF and HF are still scarce, and no Brazilian study has assessed in a representative population the prevalence of associated factors, the thromboembolic risk profile and the influence of AF on in-hospital mortality and on hospital length of stay of patients with DHF.
The present study had the following objectives: to determine the prevalence of AF and its types in patients hospitalized due to DHF at the university-affiliated Clementino Fraga Filho hospital (HU); to define the independent risk factors associated with AF; to analyze the thromboembolic risk profile and anticoagulation rate of those patients; and to assess the impact of AF on in-hospital mortality and on hospital length of stay.
Method
This retrospective, observational, cross-sectional study of incident cases included 659 consecutive hospitalizations due to DHF, from 01/01/2006 to 12/31/2011, at HU. The patients were identified by using the search tool of the HU's electronic medical record, and both electronic and printed medical records were used to identify the variables of interest.
Based on the electronic medical record, all index hospitalizations of DHF cases were assessed, in the search for any supraventricular arrhythmia reported. The medical records with the following terms were selected: atrial fibrillation; atrial flutter; supraventricular tachycardia; atrial tachycardia; atrial rhythm. From that selection, all printed medical records with those terms were obtained, and the presence of AF on electrocardiogram (ECG) was assessed. All ECGs with AF since the beginning of the patient's follow-up at HU were scanned or photographed and analyzed according to the definitions. Other supraventricular tachycardias were not considered. Occasional doubts were clarified with an experienced examiner.
Heart failure was defined according to the criteria of the European Society of Cardiology published in 20058: presence of HF symptoms at rest or during exertion in addition to evidence of cardiac dysfunction at rest, and, in case of doubt, the additional criterion of response to specific treatment for HF was used. On cardiac function assessment, the echocardiography performed at the index-hospitalization (performed in 64% of the cases) or data of previous echocardiographies were considered. Heart failure with preserved ejection fraction (HFPEF) was diagnosed based on those criteria when ejection fraction (EF) was greater than 40%.
The criterion used to diagnose DHF was the appearance of new symptoms or recent worsening of functional class, requiring urgent hospitalization for compensation. Atrial fibrillation was diagnosed based on the definition of the European Society of Cardiology9: irregular rhythm with RR intervals with no repeated pattern; absence of sinus P waves, and possible visualization of atrial electrical activity on some leads, mainly V1, in which case, the atrial cycle length should be variable and shorter than 200 ms.
Atrial fibrillation was classified according to the European Society of Cardiology9 as follows:
- paroxysmal, when AF episodes ended spontaneously, requiring neither drugs nor electrical cardioversion, lasting usually less than seven days, and frequently less than 24 hours, with or without recurrences;
- persistent, when AF episodes did not end spontaneously, requiring pharmacological and/or electrical cardioversion to sinus rhythm reversion;
- permanent, when AF was always present, and attempts to sinus rhythm reversion failed or when arrhythmia reversion was not attempt by any means.
The thromboembolic risk of patients with AF was estimated by use of the CHADSVASc score, which attributes 1 point to the presence of HF or EF < 40%, systemic arterial hypertension (SAH), age between 65 and 74 years, diabetes mellitus (DM), vascular disease, and female sex; and 2 points to age ≥ 75 years, ischemic stroke, transient ischemic attack or thromboembolism.
The outcomes assessed were as follows: in-hospital all-cause mortality and hospital length of stay.
For the purpose of statistical analysis, data were stored in a specific data bank, elaborated in the SPSS software, version 15.0. In descriptive analysis, the categorical variables were described as frequency and percentage, and the continuous variables as mean and standard deviation or median and interquartile interval, according to the distribution pattern. On univariate analysis, to compare categorical variables, the chi-square test was used, and, for continuous variables, Student t test or Mann-Whitney U test, as indicated. On multivariate analysis, logistic regression model was used.
To assess intra- and interobserver reliability in the diagnosis of AF, 100 electrocardiographic tracings were randomly selected and blindly reviewed by the study's author and by a specialist in arrhythmia. Kappa statistics was used to measure agreement between the observers. Those indices were high: intraobserver, 0.96, and interobserver, 0.84.
The significance level adopted was 5%.
The present study was approved by the Committee on Ethics and Research (065/09). Because this was a retrospective study without intervention, informed written consent was not required by that committee.
Results
Table 1 shows the general characteristics of the cases of DHF included in this study, such as the type of HF. Of those cases, 426 (64.6%) underwent echocardiography during hospitalization, and Table 2 shows the results. The presence of AF was assessed in 4,013 ECG, and that arrhythmia was identified during hospitalization in 264 cases (40.1%) as follows: permanent AF, in 194 cases (73.4%); and paroxysmal and persistent AF, in 35 cases each (13.3%).
Table 1.
Demographic, clinical and laboratory characteristics of the hospitalizations due to DHF in this study
| Variables* | Result |
|---|---|
| Age (years)† | 64 (54-75) |
| SBP (mm Hg)† | 120 (100-130) |
| HR (bpm)† | 80 (70-96) |
| Hemoglobin (mg/dL)† | 12.6 (11.2 - 14.0) |
| Urea (admission)† | 53.0 (36.5 - 82) |
| Creatinine (admission)† | 1.2 (0.9 - 1.7) |
| Sodium (mEq/L)† | 137 (134 - 140) |
| Male sex‡ | 54.6 |
| HFPEF‡ | 16.1 |
| Ischemic etiology‡ | 41.0 |
| Previous hospitalization due to DHF‡ | 57.6 |
| DM‡ | 30.9 |
| SAH‡ | 69.7 |
| CKF‡ | 22.8 |
| Previous stroke‡ | 8.2 |
| COPD‡ | 9.3 |
| PVD‡ | 7.6 |
| Smoking habit‡ | 22.9 |
| Pacemaker‡ | 10.0 |
| Resynchronization‡ | 3.9 |
| ICD‡ | 3.6 |
| LBBB‡ | 35.2 |
DHF: decompensated heart failure; SBP: systolic blood pressure; HR: heart rate; HFPEF: heart rate with preserved ejection fraction; DM: diabetes mellitus; SAH: systemic arterial hypertension; CKF: chronic kidney failure; COPD: chronic obstructive pulmonary disease; PVD: peripheral vascular disease; LBBB: left bundle-branch block; ICD: implantable cardioverter defíbrillator.
n of 659 hospitaladmissions
median (IQ)
percentage.
Table 2.
Echocardiographic variables on hospital admission in this study
| Variables* | Result |
|---|---|
| EF (%)† | 33 (26 - 45.5) |
| LVd (mm)† | 61 (53 - 70) |
| LVs (mm)† | 51 (41 - 59) |
| LA (mm)† | 47 (42 - 52) |
| PASP (mm Hg)† | 46 (38 - 55) |
| Normal ventricular function‡ | 69 (17.1) |
| Mild ventricular dysfunction‡ | 20 (5.0) |
| Moderate ventricular dysfunction‡ | 61 (15.1) |
| Severe ventricular dysfunction‡ | 253 (62.8) |
| Right ventricular dysfunction‡ | 185 (50.5) |
| Moderate or severe MR‡ | 240 (60.2) |
EF: ejection fraction; LVd: diastolic left ventricle; LVs: systolic left ventricle; LA: left atrium; PASP: pulmonary artery systolic pressure; MR: mitral regurgitation
n of 426 hospital admissions
Median (IQ)
n (%).
Table 3 shows the comorbidities and demographic, clinical and laboratory variables in the groups with and without AF. Atrial fibrillation was associated with the following: older age; reduced systolic blood pressure (SBP); higher prevalence of non-ischemic and valvar etiologies; previous hospitalization; lower prevalence of DM; previous stroke; smoking habit; HFPEF; less frequent use of resynchronization therapy; and left bundle-branch block (LBBB).Table 4 compares the echocardiographic variables between the groups with and without AF. Atrial fibrillation was associated with the following: more preserved systolic function; lower left ventricular dilatation; larger left atrium; and higher prevalence of right ventricular dysfunction and of moderate to severe mitral regurgitation.
Table 3.
Demographic, clinical, laboratory variables and comorbidities in the groups with and without atrial fibrillation
| Variables | AF* | No AF† | p Value |
|---|---|---|---|
| Age (years)‡ | 66.8 (13.5) | 62.2 (14.2) | < 0.0001 |
| SBP (mm Hg)§ | 110 (100-130) | 120 (106-140) | < 0.0001 |
| HR (bpm)§ | 84 (70-98) | 80 (70-96) | 0.07 |
| Hemoglobin (mg/dL)§ | 12.6 (11.5-14) | 12.5 (11-14) | 0.52 |
| Urea§ | 56 (39-85) | 50.5 (35-80) | 0.12 |
| Creatinine§ | 1.2 (0.9-1.6) | 1.2 (0.9-1.7) | 0.77 |
| Sodium§ | 136 (133-139) | 137 (134-140) | 0.06 |
| Male sex// | 56.4 | 53.4 | 0.45 |
| Ischemic etiology// | 30.6 | 47.8 | < 0.0001 |
| Valvar etiology *4 | 16.2 | 4.8 | < 0.0001 |
| Previous hospitalization due to HF// | 64.5 | 52.6 | 0.004 |
| DM// | 22.1 | 36.8 | < 0.0001 |
| SAH// | 66.6 | 71.8 | 0.17 |
| CKF// | 19.8 | 23.8 | 0.46 |
| Previous stroke// | 11.8 | 5.7 | 0.005 |
| Smoking habit// | 17.2 | 26.8 | 0.004 |
| COPD// | 9.5 | 9.1 | 0.84 |
| PVD// | 8.8 | 6.7 | 0.33 |
| HFPEF// | 16.8 | 9.5 | 0.008 |
| Pacemaker// | 12.6 | 17.6 | 0.16 |
| ICD// | 6.7 | 5.2 | 0.53 |
| Resynchronization// | 1.5 | 5.5 | 0.011 |
| LBBB// | 28.4 | 39.9 | 0.032 |
AF: atrial fibrillation; SBP: systolic blood pressure; HR: heart rate; HF: heart failure; HFPEF: heart rate with preserved ejection fraction; DM: diabetes mellitus; SAH: systemic arterial hypertension; CKF: chronic kidney failure; COPD: chronic obstructive pulmonary disease; PVD: peripheral vascular disease; LBBB: left bundle-branch block; ICD: implantable cardioverter defibrillator.
n of 264 hospital admissions
n of 395 hospital admissions
mean (SD)
median (IQ)
percentage.
Table 4.
Echocardiographic variables in the groups with and without atrial fibrillation
| Variables | AF* | No AF† | p Value |
|---|---|---|---|
| EF (%)‡ | 42.4 (19.5) | 35.1 (15.4) | 0.003 |
| LVd (mm)‡ | 59.3 (12.3) | 62.5 (11.3) | 0.02 |
| LVs (mm)‡ | 46.9 (14.8) | 51.6 (13.1) | 0.004 |
| LA (mm)‡ | 50.9 (9.9) | 45.0 (9.2) | < 0.0001 |
| PASP (mm Hg)‡ | 47.9 (14.8) | 47.9 (15.0) | 0.78 |
| Normal ventricular function§ | 27.3 | 10.8 | |
| Mild ventricular dysfunction§ | 4.5 | 5.2 | |
| Moderate ventricular dysfunction§ | 9.7 | 18.5 | < 0.0001 |
| Severe ventricular dysfunction§ | 58.4 | 65.5 | |
| Right ventricular dysfunction§ | 57.7 | 47.3 | 0.06 |
| Moderate or severe MR§ | 66.9 | 55.9 | 0.03 |
AF: atrial fibrillation; EF: ejection fraction; LVd: diastolic left ventricle; LVs: systolic left ventricle; LA: left atrium; PASP: pulmonary artery systolic pressure; MR: mitral regurgitation
n of 154 hospital admissions
n of 249 hospital admissions
mean (SD)
percentage.
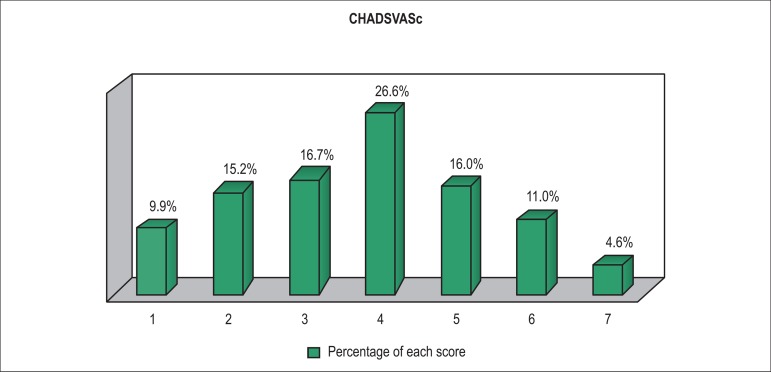
The multivariate model included variables with p < 0.10 in the comparison between the groups with and without AF.Table 5 shows the variables that maintained an independent association with AF after logistic regression. Figure 1 shows the thromboembolic risk profile estimated by use of the CHADSVASc score, whose median in this study was 4, with 90% of the cases having a score ≥ 2.
Table 5.
Logistic regression model: variables associated with atrial fibrillation
| Variables | B | p Value | OR | 95% CI |
|---|---|---|---|---|
| Age | 0.05 | < 0.0001 | 1.053 | 1.03-1.08 |
| Ischemic etiology | -0.77 | 0.023 | 0.46 | 0.24-0.90 |
| Right ventricular dysfunction | 0.76 | 0.03 | 2.13 | 1.09-4.20 |
| SBP | -0.02 | 0.02 | 0.98 | 0.97-1.00 |
| EF | 0.05 | < 0.0001 | 1.05 | 1.03-1.08 |
| LA | 0.10 | < 0.0001 | 1.11 | 1.06-1.16 |
B: regression constant; OR: Odds Ratio; CI: confidence interval; SBP: systolic blood pressure; EF: ejection fraction; LA: left atrium.
Figure 1.
Percentage distribution of hospitalizations of patients with atrial fibrillation according to CHADSVASc scoring.
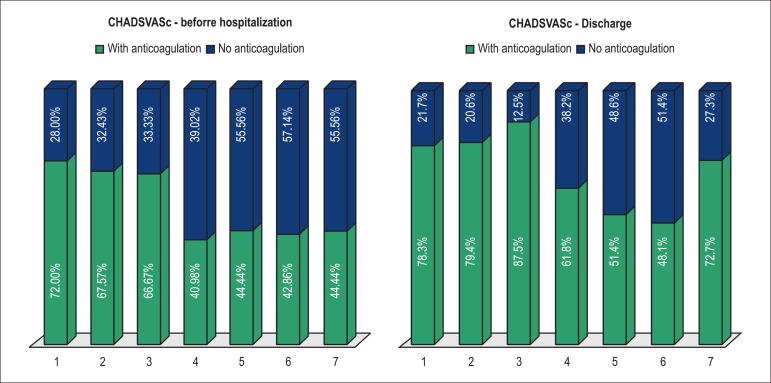
The warfarin sodium use rate on hospital admission was 52.8%, increasing to 66.8% on hospital discharge.Figure 2 shows the anticoagulation rate on hospital admission and on discharge for each CHADSVASc score.
Figure 2.
Percentage of anticoagulation according to the CHADSVASc score on hospital admission and discharge.
Regarding the outcomes, the mean hospital length of stay of patients with DHF was 18.0 ± 14 days. Patients with AF had longer hospital length of stay than those without AF (means of 20.5 ± 16 days and 16.3 ± 12 days, respectively; p = 0.001). The in-hospital mortality of patients with DHF was 9.3%, being 11.0% in those with AF and 8.1% in those without AF (p = 0.21). Mortality did not statistically differ according to the types of AF as follows: paroxysmal, 8.6%; persistent, 17.1%; and permanent, 10.3% (p = 0.44).
Discussion
This is the first Brazilian study to assess several aspects related to AF in patients hospitalized due to DHF. The results confirm a high prevalence of AF in DHF in Brazil, mainly of the permanent type, with a high thromboembolic risk profile and low rate of use of anticoagulants. Atrial fibrillation was associated with several factors, implying longer hospital length of stay and higher mortality, although with no statistical significance.
The present study used the same methodology of international registries of DHF, with consecutive cases, in which each hospitalization is considered one case10. Thus, one patient can contribute with more than one hospitalization. However, the analysis of the general characteristics of the case series shows important differences as compared with international registries, mainly regarding age, which was on average 10 years younger than that reported in the following registries: EHFS, 71.3 years11; ADHERE, 72.5 years10 ; and OPTIMIZE-HF, 78 years12. Another important difference is the much lower prevalence of HFPEF in our study population (16%) as compared with that of international registries (50%)11. In addition to younger age, underdiagnosis of HFPEF in Brazil might be the major cause of that difference. Such data have already been reported in a previous study of our group13, are in accordance with the public health network profile reported in the EPICA study14, and show the importance of Brazilian studies for proper knowledge about the Brazilian reality.
The prevalence of AF was 40% during hospitalization, being in accordance with that reported in the literature as follows: 41% in the EHFS II Registry, and 33% in the ADHERE Registry 15. The study by Latado et al16, assessing patients with DHF admitted to intensive care units, has reported a 22% prevalence of AF, similar to that reported in the study by Villacorta et al 17 (22.3%).
Permanent AF was the most frequent type (73.5%), followed by the paroxysmal and persistent types (at equal proportions). Only a few studies have assessed the type of AF in cases of DHF. Some studies have only reported the occurrence of a new episode of AF or permanent AF during hospitalization. A Polish study with 99 patients published in 200818 described results similar to those of the present study, with permanent AF in 62% of the cases and paroxysmal or persistent (not differentiated) in 38%.
Similarly to ours, some studies have demonstrated an association of AF with more advanced age19-21. That might be explained by the fact that the conditions predisposing to AF are common among the elderly.
Similarly, the association of AF with higher EF and HFPEF found in the present study has been reported in the international literature. In the GWTG-HF21, the echocardiogram performed during hospitalization has also shown greater frequency of HFPEF and higher EF in the group with AF. A Canadian study published in 200622, assessing 2,802 patients with their first episode of DHF, has shown that the group with HFPEF had a significantly higher proportion of AF. Those data are in accordance with those of other clinical trials, such as the CHARM study23.
An enlarged left atrium was strongly associated with AF. That was already expected, because left atrial enlargement is related to atrial cardiac remodeling and to the pathophysiology of AF in patients with HF24. Several studies have shown an association between lower SBP and AF. Our case series had a median SBP of 110 mm Hg versus 120 mm Hg in patients without AF. In the GWTHF21, the result was similar, but with higher absolute levels as compared with those of the present study (143 mm Hg for sinus rhythm versus 135 mm Hg with AF). In the Cardiovascular Research Network, published in 201325, the differences in SBP were smaller, when analyzed into the following three groups: 132 mm Hg for sinus rhythm; 129 mm Hg for previous AF; and 132 mm Hg for new-onset AF. Reduced SBP is an important prognosis predictor in DHF, according to the ADHERE Registry10, and its association with AF emphasizes the potential severity of the patients.
In the present study, the CHADSVASc score of patients with AF showed an elevated thromboembolic risk profile: median of 4, and 90% of the cases with a score ≥ 2. However, the anticoagulation rate prior to hospital discharge was only 66%. Underutilization of anticoagulants in patients with AF and DHF has also been reported in the international literature. The ADHERE Registry Linked to Medicare Claims26 has shown that 79% of hospitalized patients eligible for anticoagulation have been discharged with no anticoagulants, even at a high risk for stroke. Not receiving warfarin sodium was independently associated with a higher risk of death one year after hospital discharge.
The present study showed an additional interesting and paradoxical finding: the use of anticoagulants decreased with a higher thromboembolic risk score. This might reflect the concern with hemorrhagic complications of the anticoagulant therapy, because elevated thromboembolic risk scores, such as CHADSVASc score, are associated with also higher bleeding risk scores, such as HASBLED score. Our data did not allow assessing the bleeding risk profile of the cases with AF by using the HASBLED score, and not even the percentage of patients with absolute contraindications to anticoagulants, so that those results could be better interpreted. Nevertheless, the figures strongly suggest that there is significant underutilization of anticoagulation in those patients, mainly those at higher thromboembolic risk, which might have an important impact on morbidity and mortality after hospital discharge, as shown in the ADHERE Study15.
The higher future availability of new anticoagulants, in addition to better knowledge on those new drugs and experience with them, might help to increase the anticoagulation use rate in those patients, because part of that underutilization might be explained by the difficulties and limitations of traditional coumarin anticoagulation.
The mean hospital length of stay in the present study was very high (18 days), much higher than that obtained in the SUS data bank (DATA-SUS), around 6 days27. A study assessing hospitalizations due to HF in São Paulo from 1992 to 2010 has reported a mean hospital length of stay of 10 days (± 1.0)28. The hospital length of stay of patients with DHF at public and private health care institutions reported in the EPICA project14 was the closest to that found in the present study. At private health care institutions, the mean hospital length of stay was 8 days, while at public health care institutions, that figure increased to 12.6 days. According to international studies, that hospital length of stay tends to be shorter. The analysis of Medicare data29, assessing hospitalizations due to HF, has shown a mean hospital length of stay of 5.5 ± 5.4 days. The ADHERE Registry30 has reported a hospital length of stay of 4.3 days.
The longer hospital length of stay of our case series suggests greater severity of the cases, requiring a longer length of stay for clinical compensation. The difficulty of having access to beds at a tertiary hospital tends to select more severely ill patients. In addition, the functioning characteristics of the university-affiliated hospital might contribute to increase the hospital length of stay of those patients.
In our study, the presence of AF was associated with a significant increase in the hospital length of stay, on average from 14 to 20 days, which is in accordance with other articles published. In GWTG-HF21, the hospital length of stay was significantly longer in patients with AF (mean of 5 days in the presence of AF versus 4 days with sinus rhythm). In the study by Rivero-Ayerza et al 31, patients with AF of recent onset had a longer length of stay at the intensive care unit (2.6 ± 5.3 days) as compared with those with previous AF (1.2 ± 3.5 days) and those without AF (1.5 ± 4.1 days). Similarly to hospitalization due to DHF, in the present study, hospitalizations due to AF were much longer. No Brazilian study has assessed the difference in the hospital length of stay due to DHF between the groups with and without AF.
In the present study, in-hospital mortality was 9.26%, greater than that reported in the international literature, such as the ADHERE Registry (4%), once again suggesting the more severe profile of the patients in this study, as shown by Gripp et al13.
The comparison with Brazilian studies shows similar results. Barreto et al32 have reported an 8.8% in-hospital mortality of patients admitted due to DHF at the Cotoxó hospital, while the EPICA study14 has reported a 9% in-hospital mortality, both very similar to that of the present study.
Those results show that the presence of AF was associated with a 35% higher mortality (from 8.1% to 11%), but without reaching statistical significance, probably because the sample lacks statistical power (type II error). International registries with much larger samples have shown similar results: the EHFS, assessing new-onset AF31, has reported mortality due to DHF of 7% without AF and of 8% with AF, and significant difference only for new-onset AF (12%); the GWTG-HF has reported mortality of 4% in the presence of AF and of 2.6% without AF.
The major limitations of this study result from its retrospective characteristic, depending on information extracted from medical records. Current criteria for the HFPEF diagnosis could not be used, because they were not available in the medical records consulted. Finally, echocardiography was not performed at all hospitalizations, the use of previous tests being required to assess the type of HF.
Conclusion
Atrial fibrillation is very common in DHF, the most prevalent type being permanent AF. Atrial fibrillation is associated with more advanced age, non- ischemic etiology, right ventricular dysfunction, lower SBP, higher EF and enlarged left atrium. Although the thromboembolic risk profile is high, anticoagulation is underutilized and tends to be less frequent in the highest risk scores. The presence of AF is associated with longer hospital length of stay and high in-hospital mortality.
Footnotes
Author contributions
Conception and design of the research: Mendes FSNS, Atié J, Garcia MI, Sousa AS, Feijó LA, Xavier SS; Acquisition of data: Mendes FSNS, Gripp EA, Xavier SS; Analysis and interpretation of the data: Mendes FSNS, Atié J, Gripp EA, Xavier SS; Statistical analysis: Xavier SS; Writing of the manuscript: Mendes FSNS; Critical revision of the manuscript for intellectual content: Atié J, Garcia MI, Sousa AS, Feijó LA, Xavier SS.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
There were no external funding sources for this study.
Study Association
This article is part of the thesis of master submitted by Fernanda de Souza Nogueira Sardinha Mendes, from Universidade Federal do Rio de Janeiro.
References
- 1.Chen LY, Shen WK. Epidemiology of atrial fibrillation: a current perspective. Heart Rhythm. 2007;4(3) Suppl:S1–S6. doi: 10.1016/j.hrthm.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Wolf PA, D'Agostino RB, Silbershatz H, Kannel WB, Levy D. Impact of atrial fibrillation on the risk of death: the Framingham Heart Study. Circulation. 1998;98(10):946–952. doi: 10.1161/01.cir.98.10.946. [DOI] [PubMed] [Google Scholar]
- 3.Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, et al. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119(14):1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 4.Araujo DV, Tavares LR, Veríssimo R, Ferraz MB, Mesquita ET. Custo da insuficiência cardíaca no sistema único de saúde. Arq Bras Cardiol. 2005;84(5):422–427. [PubMed] [Google Scholar]
- 5.Aaronson KD, Cowger J. Heart failure prognostic models: why bother? Circ Heart Fail. 2012;5(1):6–9. doi: 10.1161/CIRCHEARTFAILURE.111.965848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ahmed MI, White M, Ekundayo OJ, Love TE, Aban I, Liu B, et al. A history of atrial fibrillation and outcomes in chronic advanced systolic heart failure: a propensity-matched study. Eur Heart J. 2009;30(16):2029–2037. doi: 10.1093/eurheartj/ehp222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107(23):2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 8.Swedberg K, Cleland J, Dargie H, Drexler H, Follath F, Komajda M, et al. Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): The Task Force for the Diagnosis and Treatment of Chronic Heart Failure of the European Society of Cardiology. Eur Heart J. 2005;26(11):1115–1140. doi: 10.1093/eurheartj/ehi204. [DOI] [PubMed] [Google Scholar]
- 9.Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, et al. European Heart Rhythm AssociationEuropean Association for Cardio-Thoracic Surgery Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC) Europace. 2010;12(10):1360–1420. doi: 10.1093/europace/euq350. [DOI] [PubMed] [Google Scholar]
- 10.Gheorghiade M, Filippatos G. Reassessing treatment of acute heart failure syndromes: the ADHERE Registry. Eur Heart J Suppl. 2005;7(Suppl B):B13–B19. [Google Scholar]
- 11.Cleland JG, Swedberg K, Follath F, Komajda M, Cohen-Solal A, Aguilar JC, et al. Study Group on Diagnosis of the Working Group on Heart Failure of the European Society of Cardiology The EuroHeart Failure survey programme-- a survey on the quality of care among patients with heart failure in Europe. Part 1: patient characteristics and diagnosis. Eur Heart J. 2003;24(5):442–463. doi: 10.1016/s0195-668x(02)00823-0. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez AF, Hammill BG, O'Connor CM, Schulman KA, Curtis LH, Fonarow GC. Clinical effectiveness of beta-blockers in heart failure: findings from the OPTIMIZE-HF (Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with Heart Failure) Registry. J Am Coll Cardiol. 2009;53(2):184–192. doi: 10.1016/j.jacc.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gripp EA, Tedeschi B, Sales AL, Iso MA, Spineti PP, Coloma M, et al. Os resultados dos registros internacionais de insuficiência cardíaca descompensada se aplicam aos pacientes brasileiros. Rev SOCERJ. 2009;22(3):165–169. [Google Scholar]
- 14.Tavares LR, Victer H, Linhares JM, de Barros CM, Oliveira MV, Pacheco LC, et al. Epidemiologia da insuficiência cardíaca descompensada em Niterói - Projeto EPICA - Niterói. Arq Bras Cardiol. 2004;82(2):125–128. 121–124. doi: 10.1590/s0066-782x2004000200003. [DOI] [PubMed] [Google Scholar]
- 15.Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC, ADHERE Scientific Advisory Committee and Investigators Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47(1):76–84. doi: 10.1016/j.jacc.2005.09.022. Erratum in J Am Coll Cardiol. 2006;47(7):1502. [DOI] [PubMed] [Google Scholar]
- 16.Latado AL, Passos LC, Braga JC, Santos A, Guedes R, Moura SS, et al. Predictors of in-hospital lethality in patients with advanced heart failure. Arq Bras Cardiol. 2006;87(2):185–192. doi: 10.1590/s0066-782x2006001500018. [DOI] [PubMed] [Google Scholar]
- 17.Villacorta H, Mesquita ET, Cardoso R, Bonates T, Maia ER, Silva AC, et al. Preditores de sobrevida obtidos na unidade de emergência em pacientes atendidos por insuficiência cardíaca descompensada. Rev Port Cardiol. 2003;22(4):495–507. [PubMed] [Google Scholar]
- 18.Targonski R, Salczynska D, Sadowski J, Cichowski L. Relationship between inflammatory markers and clinical patterns of atrial fibrillation in patients with congestive heart failure. Kardiol Pol. 2008;66(7):729–736. [PubMed] [Google Scholar]
- 19.Ahmed A, Perry GJ. Incident atrial fibrillation and mortality in older adults with heart failure. Eur J Heart Fail. 2005;7(7):1118–1121. doi: 10.1016/j.ejheart.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 20.Mentz RJ, Chung MJ, Gheorghiade M, Pang PS, Kwasny MJ, Ambrosy AP, et al. Atrial fibrillation or flutter on initial electrocardiogram is associated with worse outcomes in patients admitted for worsening heart failure with reduced ejection fraction: findings from the EVEREST Trial. Am Heart J. 2012;164(6):884-92.e2. doi: 10.1016/j.ahj.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 21.Mountantonakis SE, Grau-Sepulveda MV, Bhatt DL, Hernandez AF, Peterson ED, Fonarow GC. Presence of atrial fibrillation is independently associated with adverse outcomes in patients hospitalized with heart failure: an analysis of get with the guidelines-heart failure. Circ Heart Fail. 2012;5(2):191–201. doi: 10.1161/CIRCHEARTFAILURE.111.965681. [DOI] [PubMed] [Google Scholar]
- 22.Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355(3):260–269. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 23.Olsson LG, Swedberg K, Ducharme A, Granger CB, Michelson EL, McMurray JJ, et al. CHARM Investigators Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricular systolic dysfunction: results from the Candesartan in Heart failure-Assessment of Reduction in Mortality and morbidity (CHARM) program. J Am Coll Cardiol. 2006;47(10):1997–2004. doi: 10.1016/j.jacc.2006.01.060. [DOI] [PubMed] [Google Scholar]
- 24.Workman AJ. Cardiac adrenergic control and atrial fibrillation. Naunyn Schmiedebergs Arch Pharmacol. 2010;381(3):235–249. doi: 10.1007/s00210-009-0474-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McManus DD, Hsu G, Sung SH, Saczynski JS, Smith DH, Magid DJ, et al. Atrial fibrillation and outcomes in heart failure with preserved versus reduced left ventricular ejection fraction. J Am Heart Assoc. 2013;2(1): doi: 10.1161/JAHA.112.005694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hess PL, Greiner MA, Fonarow GC, Klaskala W, Mills RM, Setoguchi S, et al. Outcomes associated with warfarin use in older patients with heart failure and atrial fibrillation and a cardiovascular implantable electronic device: findings from the ADHERE registry linked to Medicare claims. Clin Cardiol. 2012;35(11):649–657. doi: 10.1002/clc.22064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ministério da Saúde [Acesso em 2014 fev 10];DATASUS. Disponível em http:www.datasus.gov.br.
- 28.Godoy HL, Silveira JA, Segalla E, Almeida DR. Hospitalization and mortality rates for heart failure in public hospitals in São Paulo. Arq Bras Cardiol. 2011;97(5):402–407. doi: 10.1590/s0066-782x2011005000096. [DOI] [PubMed] [Google Scholar]
- 29.Aranda JM, Jr, Johnson JW, Conti JB. Current trends in heart failure readmission rates: analysis of Medicare data. Clin Cardiol. 2009;32(1):47–52. doi: 10.1002/clc.20453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fonarow GC, Corday E, ADHERE Scientific Advisory Committee Overview of acutely decompensated congestive heart failure (ADHF): a report from the ADHERE registry. Heart Fail Rev. 2004;9(3):179–185. doi: 10.1007/s10741-005-6127-6. [DOI] [PubMed] [Google Scholar]
- 31.Rivero-Ayerza M, Scholte Op Reimer W, Lenzen M, Theuns DA, Jordaens L, Komajda M, et al. New-onset atrial fibrillation is an independent predictor of in-hospital mortality in hospitalized heart failure patients: results of the EuroHeart Failure Survey. Eur Heart J. 2008;29(13):1618–1624. doi: 10.1093/eurheartj/ehn217. [DOI] [PubMed] [Google Scholar]
- 32.Barretto AC, Nobre MR, Wajngarten M, Canesin MF, Ballas D, Serro-Azul JB. Insuficiência cardíaca em grande hospital terciário de São Paulo. Arq Bras Cardiol. 1998;71(1):15–20. doi: 10.1590/s0066-782x1998000700004. [DOI] [PubMed] [Google Scholar]