Abstract
Despite significant therapeutic advancements, heart failure remains a highly prevalent clinical condition associated with significant morbidity and mortality. In 30%-40% patients, the etiology of heart failure is nonischemic. The implantable cardioverter-defibrillator (ICD) is capable of preventing sudden death and decreasing total mortality in patients with nonischemic heart failure. However, a significant number of patients receiving ICD do not receive any kind of therapy during follow-up. Moreover, considering the situation in Brazil and several other countries, ICD cannot be implanted in all patients with nonischemic heart failure. Therefore, there is an urgent need to identify patients at an increased risk of sudden death because these would benefit more than patients at a lower risk, despite the presence of heart failure in both risk groups. In this study, the authors review the primary available methods for the stratification of the risk of sudden death in patients with nonischemic heart failure.
Keywords: Heart Failure / mortality; Death, Sudden, Cardiac; Defibrillators, Implantable
Introduction
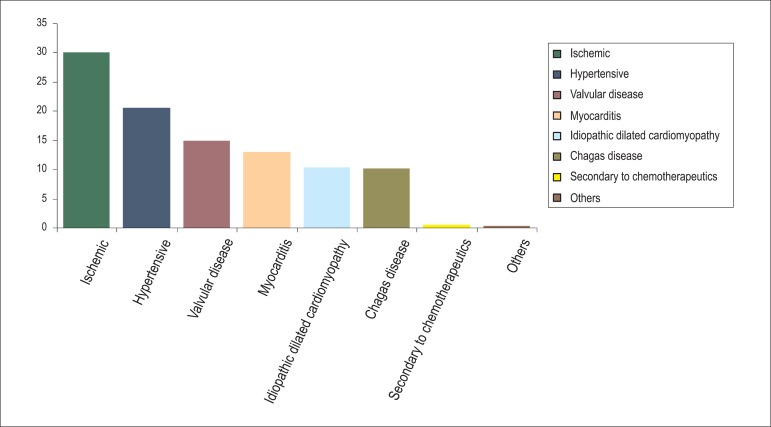
The prevalence and incidence of heart failure (HF) indicate that it is an important public health problem. Data from the city of São Paulo indicate high rates of hospitalization in the elderly population, in addition to high treatment costs and a mortality rate approaching 15%1. In 30%-40% patients with heart failure accompanied by a decreased ejection fraction (EF), the etiology of ventricular dysfunction is nonischemic2,3. Data from the I Brazilian Registry of Heart Failure (BREATHE registry) indicate that HF is nonischemic in 70% hospitalized patients (Graph 1)4. Nonischemic HF (NIHF) is characterized by the absence of major lesions on coronary angiography or by negative findings on imaging studies performed to assess ischemia. Among patients with NIHF, the cause of ventricular dysfunction-designated as idiopathic dilated cardiomyopathy-may be unknown; alternatively, it can be attributed to several causes, including hypertension, exposure to potentially toxic agents (chemotherapeutic drugs and alcohol), Chagas disease, myocarditis, infiltrative disease, peripartum cardiomyopathy, valvular heart disease, and genetic and autoimmune diseases.
Graph 1.
Etiology of HF: BREATHE registry data. HF: heart failure; BREATHE: I Brazilian Registry of Heart Failure.
Sudden cardiac death (SD) is an unexpected natural death from cardiac causes, and it usually occurs within an hour of symptom onset5. Considering that advancements in the treatment of NIHF have brought about a significant decrease in mortality in recent decades, SD remains a significant problem, accounting for approximately 30% deaths6,7. The primary prevention of SD in patients with NIHF involves pharmacological treatment and the use of implantable cardioverter-defibrillators (ICD)8. Randomized clinical trials have demonstrated that the use of beta-blockers and spironolactone significantly decreased SD in this group of patients. The Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT), which included patients with both ischemic and nonischemic HF of New York Heart Association (NYHA) functional class II-III, indicated that ICD decreased SD and total mortality in patients with NIHF9. Moreover, an analysis of all patients with implanted ICD revealed that 33.2% received some level of shock by the defibrillator, 22.4% received appropriate defibrillator shocks, and 10.7% received only inappropriate shocks10. Undoubtedly, the costs and potential complications related ICD implantation demand a better selection of patients who are at an increased risk of SD and would benefit the most from ICD implantation. For ischemic HF, in addition to EF, electrophysiological studies identified groups of patients at an increased risk of SD11, as opposed to NIHF, which still requires a better selection of patients12. Therefore, this study aimed to review the primary available options for the stratification of SD risk in patients with NIHF (Table 1).
Table 1.
Predictors of SD Risk In Patients with NIHF
| Method | Marker/Risk | Comment |
|---|---|---|
| Clinical Evaluation | ||
| NYHA functional class | Associated with different risks of SD | |
| Class III → 59% deaths from SD | ||
| Syncope | In patients with advanced HF (NYHA class III and IV) | |
| Without syncope → 12% SD in 1 year | ||
| Laboratory tests | BNP, uric acid, and hemoglobin | Included in risk prediction scores |
| LVEF | → RR for major arrhythmic events was 2.28 for every 10% decrease in EF | |
| Validated in ICD cohorts and trials | ||
| ECG | Duration of the QRS interval and late potentials | |
| Late potentials with conflicting results | ||
| Holter ECG | ||
| NSVT | Independent marker in a meta-analysis with meta-regression | |
| NSVT + LVEF < 30% → RR of 8.2 for events | ||
| HRV | SDNN | Studies with controversial results |
| TWA | Altered TWA → RR of 2.99 for death or arrhythmia | |
| Studies with conflicting results | ||
| Cardiopulmonary exercise test | Occurrence of periodic breathing → chi-square of 44.7 | Independent marker in a study with ischemic and NIHF patients |
| 123I-MIBG | Altered result → HR of 4.79 for SD | In a study of ischemic and NIHF patients |
| EPS | Positive EPS → HR of 4.19 for ICD therapy | In a study of patients with NIHF |
| Genetic Evaluation | Genotype Arg389Gly of the β1-adrenergic receptor | Mutations and polymorphisms associated with increased risk of SD |
| Cardiac MRI | Fibrosis → HR of 3.2-5.4 for arrhythmic events | Fibrosis increases risk of SD in patients with NIHF |
SD: sudden death; HF: heart failure; NIHF: nonischemic heart failure; NYHA: New York Heart Association; ICD: cardioverter-defibrillator; LVEF: left ventricular ejection fraction; NSVT: nonsustained ventricular tachycardia; MIBG: metaiodobenzylguanidine; MRI: magnetic resonance imaging; TWA: T-wave alternans; ECG: electrocardiogram; HRV: heart rate variability; SD: sudden death; RR: relative risk; SDNN: standard deviation of normal-to-normal R-R intervals; EPS: electrophysiological study; BNP: basal natriuretric peptide; HR: hazard ratio.
Clinical and laboratory evaluation
Routine clinical and laboratory evaluations provide information that can be used for the risk stratification of patients with NIHF. The functional class of patients with HF is related to distinct SD risks. Among patients with NYHA functional class II HF, SD is responsible for 64% deaths, whereas HF progression is responsible for 12% deaths. Among patients with NYHA functional class III HF, SD accounts for 59% deaths and HF progression accounts for 26% (Table 2). Among patients with NYHA functional class IV HF, HF progression accounts for 56% deaths and SD accounts for 33%13. Chagas disease, NYHA functional class III-IV HF, cardiomegaly on chest radiography, ventricular dysfunction on echocardiography, low voltage of the QRS complex on electrocardiography, nonsustained ventricular tachycardia on Holter electrocardiography, and male sex are risk factors for total mortality. However, a specific analysis of SD has not been performed14. Both Brazilian and international guidelines include functional classes among the criteria for ICD implantation for the primary prevention of NIHF12,15.
Table 2.
Functional class and type of death in patients with HF(*)
| NYHA Functional Class | Sudden Death (%) | Death from HF progression (%) | Death from other causes (%) |
|---|---|---|---|
| II | 64 | 12 | 24 |
| III | 59 | 26 | 15 |
| IV | 33 | 56 | 11 |
HF: heart failure; NYHA: New York Heart Association.
Adapted from reference 13
Syncope is considered to be an important risk factor for SD in patients with NIHF. In a cohort study involving 491 patients with severe HF, 51% of whom experienced NIHF, Middlekauff et al. demonstrated that the incidence of SD was 45% among patients with syncope compared with 12% for patients without a history of syncope16. A cohort study of patients with NIHF who received ICD indicated that the administration of appropriate therapies was similar for patients with syncope and those who experienced resuscitated SD17. Phang et al. followed 108 patients with NIHF and syncope and 71 patients with NIHF and sustained ventricular arrhythmia18 and observed that the incidence of ventricular arrhythmia, SD, and total mortality was similar in both groups. The occurrence of syncope is contemplated in the guidelines as an indication for ICD in patients with NIHF12,15. In addition to NYHA functional class and syncope, other clinical factors associated with an increased risk of arrhythmic events include the lack of use of beta-blockers and systolic blood pressure19,20.
Several studies have evaluated the prognostic value of routine screening procedures for the risk stratification of patients with NIHF. Blood tests, including those for hemoglobin, uric acid, and atrial natriuretic peptide (ANP), were identified in isolated studies as predictors of mortality and arrhythmic events20,21. However, considering that these results are still inconsistent, these tests cannot be considered in isolation for risk assessment. Using risk prediction models derived from the Seattle score on the basis of routine clinical and laboratory variables, Levy et al. classified the patients in the SCD-HeFT into 5 risk groups22. In group I patients, ICD significantly decreased the relative risk of SD by 88%, whereas in group V, the decrease was 24%. However, these differences were not significant. With regard to total mortality, the absolute decrease in risk after ICD implantation, in risk quintiles I to V, was 6.6%, 8.8%, 10.6%, 14%, and -4.9%, respectively. However, an analysis including only patients with NIHF was not performed.
Left ventricular ejection fraction
Left ventricular EF (LVEF) can be evaluated using several available methods, the primary one being echocardiography. The decrease in EF is considered to be a major risk factor for SD and total mortality in patients with HF6,23. However, few studies till date have evaluated EF as a risk factor for SD specifically in patients with NIHF. The Marburg Cardiomyopathy Study (MACAS), a prospective cohort study involving 343 patients with NIHF, revealed that in patients with sinus rhythm, the relative risk for major arrhythmic events was 2.28 for every 10% decrease in EF19. In patients with atrial fibrillation, the relative risk was 4.5.
EF ≤ 35% was an inclusion criterion for the SCD-HeFT, a trial that serves as the basis for ICD indications in patients with NIHF9. Both Brazilian and international guidelines consider EF ≤ 35% as a criterion for ICD implantation as the primary preventive measure in patients with NIHF12,15. However, we should note that, although EF is considered a major risk factor for SD, several other events occur in patients with EF > 35%23. In addition, the relative risk of SD is significantly higher in patients with EF ≤ 35% than in those with EF > 35%. However, the absolute number of SD cases is higher among patients with more preserved EF, considering that these patients represent a much larger subgroup. Data from the Maastricht study indicate that, among patients for whom EF was measured before an SD episode, 52% had EF > 30% and 32% had EF > 40%24.
Electrocardiography
The electrocardiogram (ECG) is a simple, readily available tool that provides useful information for the risk stratification of patients with NIHF. The rate of prolongation of the QRS interval in patients with HF ranges from 20% to 50%6. In specific cohort studies involving patients with NIHF, no correlation was found between the presence of bundle branch block and the increased risk of SD19,25. In the Defibrillators in Nonischemic Cardiomyopathy Treatment Evaluation (DEFINITE), a clinical trial involving patients with NIHF, ICD decreased the arrhythmic causes of SD without causing a significant decrease in total mortality, and no correlation was found between the QRS duration and total mortality26. In the SCD-HeFT, an increased benefit from ICD implantation was observed in patients with QRS > 120 ms. However, a specific analysis was not performed for the group with NIHF9. Another study indicated that fragmentation of the QRS complex (default RSR' and a duration of < 120 ms in two contiguous leads) was associated with a higher incidence of arrhythmic events in patients with NIHF27. However, this finding warrants further investigation. In addition, observational studies indicate conflicting results for the association between the measured QT interval and mortality from HF, and a specific analysis was not performed in patients with NIHF23. Furthermore, the dispersion in the QT interval (maximum difference between the QT intervals on a surface ECG) was evaluated in patients with NIHF. Although previous studies have indicated a positive correlation, recent studies have found no association between QT interval dispersion and an increase in major arrhythmic events28.
The high-resolution ECG (HRECG) is a useful tool for the amplification and processing of ECG signals. It evaluates the presence of late potentials at the end of the QRS complex and the duration of the QRS interval. Previous studies on the role of HRECG in the risk stratification of patients with NIHF have revealed conflicting results. The presence of late potentials using HRECG was identified in 27% patients with NIHF and was associated with an increased cardiovascular mortality and arrhythmic events29. However, in other studies, this method could not stratify the risk of arrhythmic events in patients with NIHF, despite similar variations in HRECG19,30.
Holter electrocardiogram (ambulatory electrocardiographic monitoring)
Holter ECG is a widely available diagnostic tool used for risk assessment of patients with NIHF, taking into consideration the presence of nonsustained ventricular tachycardia (NSVT) and the analysis of measures of autonomic activity, including heart rate (HR) variability (HRV) and HR turbulence (HRT).
The incidence of NSVT (Figure 1) in patients with NIHF ranges from 30%-79%; therefore, its use in the risk stratification of arrhythmic events is considered controversial31. In a prospective study involving 179 patients, Iacovielo et al. revealed that the presence of NSVT was associated with a relative risk of 2.96 [95% confidence interval (CI), 1.17-7.49; p = 0.022] for the occurrence of major arrhythmic events32. In the MACAS, the presence of NSVT alone was not significantly correlated with an increased risk of arrhythmic events19. However, a combination of NSVT with EF < 30% was associated with an 8.2-fold increase in the risk of arrhythmic events (95% CI, 3.1-22.6; p = 0.0001). In this same cohort, a subsequent analysis was performed after taking into consideration the duration of and HR in NSVT. The incidence of arrhythmic events was 2% per year in patients without NSVT, 5% per year in patients with NSVT lasting from 5 to 9 beats, and 10% per year in patients with NSVT lasting > 10 beats (Table 3)33. With regard to HR in NSVT, there was no significant difference between groups with and without arrhythmic events. Meta-analysis data indicated that the presence of NSVT was associated with a 3.2-fold increase in the risk of SD (95% CI, 2.12-4.89; p < 0.05)34. In patients with NIHF who were implanted with ICD, the presence of NSVT was associated with a 7.8-times increase in the risk of appropriate therapy for ICD (95%CI, 1.8-33.7; p = 0.006)35. The presence of NSVT or ≥ 10 ventricular extrasystoles per hour on Holter ECG was an inclusion criterion in the DEFINITE26. A combined analysis of these results demonstrates that the detection of NSVT can indicate an increased risk of major ventricular arrhythmias. However, these results do not allow us to justify the clinical decision on ICD implantation on the basis of NSVT detection.
Figure 1.
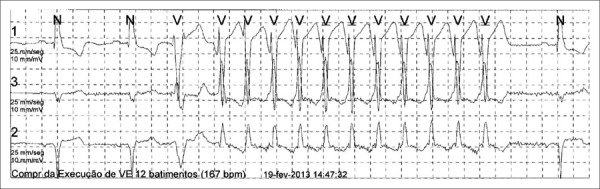
Example of nonsustained ventricular tachycardia (NSVT) on a Holter electrocardiogram.
Table 3.
Incidence of major arrhythmic events and NSVT on 24-h Holter ECG(*)
| NSVT | Major arrhythmic events (% per year)(†) |
|---|---|
| Absence of NSVT | 2 |
| NSVT of 5-9 beats | 5 |
| NSVT of ≥ 10 beats | 10 |
NSVT: nonsustained ventricular tachycardia
ECG: electrocardiogram. Adapted from reference33 .
p < 0.05.
HRV is a measure of autonomic activity that takes into consideration the beat-to-beat variation in the R-R interval. HRV analysis can be performed as a function of time or frequency. In clinical practice, the most commonly used measure is the standard deviation of normal-to-normal R-R intervals (SDNN), which represents the standard deviation in R-R intervals. A decreased HRV is associated with an increased risk of death from HF progression, but not an increased risk of SD36,37. In the MACAS, no association was found between a decreased HRV and an increased risk of arrhythmic events19. Moreover, an analysis of the DEFINITE reveals that patients with preserved HRV exhibit a good prognosis and may not benefit from ICD implantation38.
HRT is a measure of autonomic activity that takes into account variations in R-R intervals that manifest after the occurrence of ventricular extrasystoles. During follow-up of patients from the MACAS, there was no significant correlation between HRT and arrhythmic events, similar to the finding observed in the study by Klingenheben, who evaluated a series of markers of autonomic activity19,39.
T-wave alternans
T-wave alternans (TWA) can be defined as the beat-to-beat alternation in morphology, amplitude, and/or polarity of T-waves. Considering that these changes are measured in microvolts, specific processing programs are available for the detection of specific TWA. The T-Wave Alternans in Patients with Heart Failure (ALPHA) study prospectively monitored 476 patients with NYHA functional class II and III NIHF40. Of these, 44.8% had positive TWA, 34.6% had negative TWA, and 20.6% were undetermined. The primary outcome (cardiac death in addition to serious arrhythmias) occurred in 6.5% patients with altered TWA (positive or indeterminate) and 1.6% patients with normal TWA. According to multivariate analysis, the relative risk was 3.2 (95% CI, 1.12-9.20; p = 0.013). The negative predictive value for this outcome after 18 months was 97.3% (95% CI, 93.3-99.3). However, the positive predictive value was only 9% (95% CI, 5.9-13.0).
In contrast, in the MACAS study, TWA was not able to stratify patients with an increased risk of arrhythmic events19. The event rate was 13% in patients with positive TWA, 10% in those with negative TWA, and 24% in those with indeterminate TWA, without significant differences between groups. In a meta-analysis that evaluated major arrhythmic events combined with death from any cause, the relative risk for altered TWA (abnormal or indeterminate) was 2.99 (95% CI, 1.88- 4.75), with a negative predictive value of 96.2%41. In the assessment of TWA in the SCD-HeFT, no significant differences were observed in major arrhythmic events among patients with normal, altered, or undetermined TWA42. Therefore, these studies with conflicting results and the absence of clinical trials that addressed TWA as an inclusion criteria preclude TWA from being included in the guidelines as a criterion for the selection of patients eligible for ICD implantation.
Cardiopulmonary exercise test
The cardiopulmonary exercise test is recommended for the evaluation and follow-up of patients with HF8. Meta-analysis data indicate that variables derived from cardiopulmonary exercise testing and oxygen consumption (VO2), slope of the ventilatory equivalent for CO2 (VE/VCO2 slope), and the occurrence of periodic breathing, in isolation, indicate an increased risk of combined events, including total mortality, cardiac death, heart transplantation, hospitalization, and the need for ventricular assistive devices43. A national database of patients using beta-blockers indicated that peak VO2 ≤ 10 ml/kg-1/min-1 indicates an increased risk of cardiovascular events, peak VO2 between 10 and 16 ml/kg-1/min-1 represents a moderate risk, and peak VO2 > 16 ml/kg-1/min-1 indicate a lower risk. In addition, a VE/VCO2 slope of > 34 was associated with an increased risk44. Patients with NIHF accounted for 68% of the sample. However, a stratified analysis on the etiology of HF was not performed.
Guazzi et al45 assessed the performance of the cardiopulmonary exercise test variables in relation to the risk of SD. Patients without complications exhibited a peak VO2 of 16.8 ± 4.5 ml/kg-1/min-1, a VE/VCO2 slope of 32.8 ± 6.4, and a 20.3% prevalence of periodic breathing. Patients who developed SD exhibited a peak VO2 of 13.58 ± 3.2 mL/kg/min, a VE/VCO2 slope of 41.5 ± 11.4, and a 100% prevalence of periodic breathing. According to multivariate analysis, the only variable associated with an increased risk of SD was the development of periodic breathing. In this study, patients with NIHF represented 37% of the sample (n = 156). However, a stratified analysis according to the etiology of HF was not performed. These findings warrant further investigations involving a larger number of patients that can analyze the etiology of HF.
Myocardial scintigraphy with iodine-123-metaiodobenzylguanidine
Myocardial scintigraphy with iodine-123-metaiodobenzylguanidine (123I-MIBG) can evaluate the function of the sympathetic nervous system in patients with HF. Recent data indicate that a low late heart-to-mediastinum ratio and an increased washout rate of 123I-MIBG were associated with an increased incidence of cardiovascular events46. In a cohort study with 106 patients, 44% of whom had NIHF, patients with abnormal 123I-MIBG scintigraphy results exhibited a significantly higher risk of SD (hazard ratio, 4.79; 95% CI, 1.55-14.76; p = 0.006)47. In another study that monitored 116 patients with ICD, those with alterations in 123I-MIBG exhibited a higher rate of ICD therapy (52% versus 5%, p < 0.01)48. In both studies, no specific analysis was performed in patients with NIHF. Considering these promising findings, it is estimated that other prospective studies will be conducted with a larger number of patients, with the view to performing a stratified analysis of the etiology of HF, and will enable the inclusion of 123I- MIBG in the guidelines for the risk stratification of patients with NIHF.
Electrophysiological study
In a group of patients with ischemic HF, an electrophysiological study (EPS) with programmed ventricular stimulation was able to identify patients at a higher risk of major arrhythmic events11. However, the results are controversial for patients with NIHF. Poll et al49 conducted EPS involving 20 patients with NIHF and observed the induction of ventricular tachyarrhythmia in 30% patients. Of these, 50% had ventricular tachyarrhythmia during follow-up. Among those with negative EPS, 36% experienced arrhythmias. In the 1990s, Brembilla-Perrot et al50 conducted EPS involving 92 patients and observed the induction of ventricular tachyarrhythmia in only 8%. The incidence of ventricular arrhythmias in this group was 50%; however, it was only 4% in the group with negative EPS findings. In another study involving 34 patients, Grimm et al51 induced ventricular tachyarrhythmia in 38%. The incidence of ventricular arrhythmias in this group was 30% compared with 24% in the group with negative EPS findings. Nonetheless, these studies involved a small number of patients, adopted heterogeneous methodologies, and were conducted before the widespread use of beta-blockers for HF treatment.
Recent studies have re-evaluated EPS conducted in these conditions. In a subanalysis of the DEFINITE EPS findings were positive in 14% patients, and 34% of these patients presented with ventricular arrhythmias52. Among patients with negative EPS findings, the incidence of ventricular arrhythmias was 12%. The positive and negative predictive values of EPS for determining the requirement of ICD therapy were 34% and 88%, respectively. Gatzoulis et al53 prospectively monitored 158 patients with NIHF undergoing EPS for the primary prevention of SD. EPS findings were positive (induction of ventricular tachycardia or ventricular fibrillation) in 44 patients (28%) and negative in 114 (72%). ICD implantation was performed in 41 patients from the positive EPS group and 28 patients from the negative EPS group. However, the total mortality during a follow-up of 46.9 months was not significantly different between patients with positive or negative EPS. In patients implanted with ICD, the rate of administration of therapies for ICD (e.g., shock or anti-tachycardia stimulation) was 73.2% for those with positive EPS and 17.9% for those with negative EPS (p = 0.001). Positive EPS was the only prognostic factor for the administration of ICD therapy (hazard ratio, 4.19; 95% CI, 1.467-11.994; p = 0.007). Considering the overall results of these studies, the guidelines do not recommend the routine performance of EPS for the risk stratification of patients with NIHF12,15. Therefore, future studies using a larger number of patients, optimal pharmacological therapies, and more uniform protocols for ventricular stimulation can better assess the role of EPS in this group of patients.
Genetic evaluation
Several studies have evaluated the association between genetic mutations and the pathophysiology and prognosis of patients with NIHF, particularly those with familial diseases54. Among the best investigated conditions are mutations in the lamin A/C (LMNA) gene. Pasotti et al55 demonstrated that patients with NIHF carrying LMNA mutations have a high incidence of major arrhythmic events (40%-67%), and the risk factors included NYHA functional class, type of mutation, and practice of competitive physical activity. In the study by van Rijsingen et al56, the incidence of major arrhythmic events was 18%, and the risk factors included NSVT, EF, male sex, and type of mutation. Furthermore, mutations in the SCN5A sodium channel gene have been associated with an increased risk of arrhythmic events57. Mutations in the RBM20 gene, which is responsible for regulation of the splicing process in cardiac tissue, were identified in 2.8% patients from a cohort with NIHF and ICD and were not associated with an increased risk of ventricular arrhythmias58. Considering the prevalence and the important prognostic implications, the investigation of mutations in the LMNA gene may be considered in all patients with idiopathic NIHF, particularly when there is significant impairment in the conduction system59. In Brazil, this genetic evaluation is not yet available for routine clinical use.
Moreover, analysis of the presence of genetic polymorphisms has been evaluated as a tool for the risk stratification of patients with NIHF. In a cohort study, the presence of the Gly389 allele in the polymorphism of the β1-adrenergic receptor Arg389Gly was associated with a lower incidence of ventricular arrhythmias60. In addition, a national cohort study including patients with ICD identified a protective effect of the presence of the Gly389 allele61. However, a stratified analysis of patients with NIHF - which corresponded to 41% of the study group - was not performed. The AT1R-1166CC genotype of the polymorphism associated with the renin-angiotensin-aldosterone system was correlated with a two-fold increase in the requirement of ICD therapies in patients with CI62. In this same study, circulating levels of the microRNA miR-155 were associated with an increased risk of ICD therapies. In addition, 23% patients who received ICD therapy presented with NIHF. Therefore, the analysis of genetic polymorphisms is very promising, and future studies may lead to the identification of patients with genetic patterns associated with greater risk and patients who may benefit the most from ICD implantation.
Cardiac magnetic resonance imaging
The presence of myocardial fibrosis is an important arrhythmogenic substrate in patients with NIHF6,63. The detection of myocardial fibrosis using serum markers of collagen metabolism or imaging methods could identify high-risk patients. Kanoupakis et al. demonstrated that patients with NIHF and changes in serum markers of collagen metabolism exhibited a higher rate of appropriate ICD therapies64. Cardiac magnetic resonance imaging (MRI) using the delayed enhancement technique is the primary imaging method used to detect and quantify the extent of myocardial fibrosis. Approximately 30% patients with NIHF exhibited myocardial fibrosis on MRI65 (Figure 2).
Figure 2.
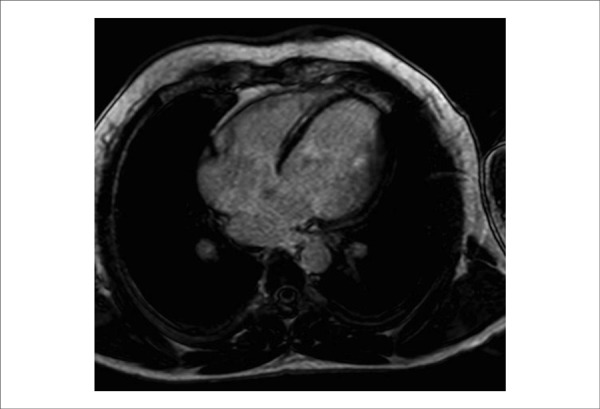
MRI of a patient with cardiac NIHF, 35% EF, and a mesocardiac area of fibrosis in the interventricular septum. MRI: magnetic resonance imaging; EF: ejection fraction.
Several studies have evaluated the relationship between the presence of fibrosis on MRI and the occurrence of major arrhythmic events in patients with NIHF. In a pioneering study, Nazarian et al66 evaluated 26 patients with NIHF subjected to EPS and MRI. The presence of fibrosis covering 26%-75% of the wall thickness was associated with a nine-fold increase in the risk of ventricular arrhythmia according to EPS. In a cohort study with 101 patients, Assomull et al67 revealed that fibrosis was an independent predictor of SD associated with ventricular tachycardia (hazard ratio, 5.2; 95% CI, 1.10-32.2; p = 0.04). In a study by Lehrke et al68 involving 184 patients, the presence of fibrosis was considered to be an independent predictor of the combined events of cardiac death, appropriate ICD therapy, and hospitalization (hazard ratio, 3.4; 95% CI, 1.26-9.00; p = 0.015). In this cohort, a fibrosis rate of > 4.4% of the left ventricular (LV) mass was associated with a worse prognosis, and the prognostic value of the presence of fibrosis was restricted to patients with EF < 30%. However, these findings were not confirmed in the study of Hombach et al69, who monitored 151 patients and found no association between the presence of fibrosis and the combined events of cardiac death and SD. MRI variables associated with the event were cardiac index, index of the end-diastolic volume of the right ventricle, and the presence of QRS > 110 ms and diabetes.
Gulati et al. recently published the results for the largest cohort of patients with NIHF examined on MRI.70 In this study, 472 patients were monitored, with a median follow up of 5.3 years. The primary outcome of total mortality occurred in 26.8% patients, and myocardial fibrosis occurred in 10.6% patients without fibrosis. After multivariate analysis with adjustment for EF and other prognostic factors, the presence of fibrosis represented a hazard ratio of 2.43 (95% CI, 1.50-3.92; p < 0.001), and the extent of fibrosis represented a hazard ratio of 1.11 (95% CI, 1.06-1.16; p < 0.001). Combined events of SD and aborted SD were observed in 29.6% patients with myocardial fibrosis and 7.0% patients without fibrosis. For this event, the presence of fibrosis represented a hazard ratio of 4.61 (95% CI, 2.75-7.74; p < 0.001), and the extent of fibrosis represented a hazard ratio of 1.10 (95% CI, 1.05-1.16; p < 0.001). These results, together with those from previous studies, indicate that MRI can be a useful technique for the risk stratification of patients with NIHF. The usefulness of MRI needs further confirmation through prospective multicenter studies designed specifically for this purpose.
Conclusions
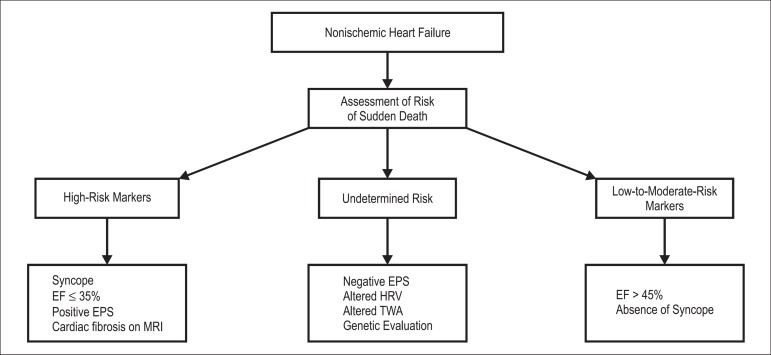
The risk stratification of SD among patients with NIHF remains an important clinical challenge. To make a decision regarding ICD implantation, the most important factors that should be considered are EF, NYHA functional class, and presence of syncope (Figure 3). Several noninvasive tests and invasive EPS have yielded controversial results. In addition, cardiac MRI has shown promising results and should be considered for the risk stratification of patients with NIHF. Prospective multicenter studies evaluating the association between different noninvasive and invasive methods may generate risk scores capable of identifying high-risk patients with SD and those who would benefit the most from ICD implantation with a greater accuracy.
Figure 3.
Risk stratification of sudden death in patients with nonischemic heart failure EF: ejection fraction; EPS: electrophysiological study; MRI: magnetic resonance imaging; HRV: heart rate variability; TWA: T-wave alternans.
Footnotes
Author contributions
Conception and design of the research: Pimentel M, Rohde LE; Acquisition of data: Pimentel M; Writing of the manuscript and Critical revision of the manuscript for intellectual content: Pimentel M, Zimerman LI, Rohde LE.
Potential Conflict of Interest
No potential conflict of interest relevant to this article was reported.
Sources of Funding
There were no external funding sources for this study.
Study Association
This article is part of the thesis of Doctoral submitted by Maurício Pimentel, from Universidade Federal do Rio Grande do Sul.
References
- 1.Godoy HL, Silveira JA, Segalla E, Almeida DR. Hospitalização e mortalidade por insuficiência cardíaca em hospitais públicos no município de São Paulo. Arq Bras Cardiol. 2011;97(5):402–407. doi: 10.1590/s0066-782x2011005000096. [DOI] [PubMed] [Google Scholar]
- 2.Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93(9):1137–1146. doi: 10.1136/hrt.2003.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mann DL. Heart failure: a companion to Braunwald's heart disease. Philadelphia: Saunders; 2011. pp. 363–389. [Google Scholar]
- 4.BREATHE Investigators Rationale and design: BREATHE registry -- I Brazilian Registry of Heart Failure. Arq Bras Cardiol. 2013;100(5):390–394. doi: 10.5935/abc.20130093. [DOI] [PubMed] [Google Scholar]
- 5.Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98(21):2334–2351. doi: 10.1161/01.cir.98.21.2334. [DOI] [PubMed] [Google Scholar]
- 6.Koutalas E, Kanoupakis E, Vardas P. Sudden cardiac death in non-ischemic dilated cardiomyopathy: a critical appraisal of existing and potential risk stratification tools. Int J Cardiol. 2013;167(2):335–341. doi: 10.1016/j.ijcard.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 7.Gaui EN, Klein CH, Oliveira GM. Mortalidade por insuficiência cardíaca: análise ampliada e tendência temporal em três estados do Brasil. Arq Bras Cardiol. 2010;94(1):55–61. doi: 10.1590/s0066-782x2010000100010. [DOI] [PubMed] [Google Scholar]
- 8.Bocchi EA, Marcondes-Braga FG, Bacal F, Ferraz AS, Albuquerque D, Rodrigues DA, et al. Sociedade Brasileira de Cardiologia Atualização da Diretriz Brasileira de Insuficiência Cardíaca Crônica 2012. Arq Bras Cardiol. 2012;98(1) supl. 1:1–33. doi: 10.1590/s0066-782x2012001000001. [DOI] [PubMed] [Google Scholar]
- 9.Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, et al. Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352(3):225–237. doi: 10.1056/NEJMoa043399. Erratum in N Engl J Med. 2005;352(20):2146. [DOI] [PubMed] [Google Scholar]
- 10.Poole JE, Johnson GW, Hellkamp AS, Anderson J, Callans DJ, Raitt MH, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359(10):1009–1017. doi: 10.1056/NEJMoa071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moss AJ, Hall WJ, Cannom DS, Daubert JP, Higgins SL, Klein H, et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. Multicenter Automatic Defibrillator Implantation Trial Investigators. N Engl J Med. 1996;335(26):1933–1940. doi: 10.1056/NEJM199612263352601. [DOI] [PubMed] [Google Scholar]
- 12.Russo AM, Stainback RF, Bailey SR, Epstein AE, Heidenreich PA, Jessup M, et al. ACCF/HRS/AHA/ASE/HFSA/SCAI/SCCT/SCMR 2013 appropriate use criteria for implantable cardioverter-defibrillators and cardiac resynchronization therapy: a report of the American College of Cardiology Foundation appropriate use criteria task force, Heart Rhythm Society, American Heart Association, American Society of Echocardiography, Heart Failure Society of America, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society for Cardiovascular Magnetic Resonance. Heart Rhythm. 2013;10:e11–e58. doi: 10.1016/j.hrthm.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet. 1999;353(9169):2001–2007. [PubMed] [Google Scholar]
- 14.Rassi A, Jr, Rassi A, Little WC, Xavier SS, Rassi SG, Rassi AG, et al. Development and validation of a risk score for predicting death in Chagas' heart disease. N Engl J Med. 2006;355(8):799–808. doi: 10.1056/NEJMoa053241. [DOI] [PubMed] [Google Scholar]
- 15.Martinelli Filho M, Zimerman L, Lorga AM, Vasconcelos JT, Rassi A., Jr Diretrizes brasileiras de dispositivos cardíacos eletrônicos implantáveis. Arq Brasil Cardiol. 2007;89(6):e210–e238. [Google Scholar]
- 16.Middlekauff HR, Stevenson WG, Stevenson LW, Saxon LA. Syncope in advanced heart failure: high risk of sudden death regardless of origin of syncope. J Am Coll Cardiol. 1993;21(1):110–116. doi: 10.1016/0735-1097(93)90724-f. [DOI] [PubMed] [Google Scholar]
- 17.Knight BP, Goyal R, Pelosi F, Flemming M, Horwood L, Morady F, et al. Outcome of patients with nonischemic dilated cardiomyopathy and unexplained syncope treated with an implantable defibrillator. J Am Coll Cardiol. 1999;33(7):1964–1970. doi: 10.1016/s0735-1097(99)00148-5. [DOI] [PubMed] [Google Scholar]
- 18.Phang RS, Kang D, Tighiouart H, Estes NA, 3rd, Link MS. High risk of ventricular arrhythmias in patients with nonischemic dilated cardiomyopathy presenting with syncope. Am J Cardiol. 2006;97(3):416–420. doi: 10.1016/j.amjcard.2005.08.063. [DOI] [PubMed] [Google Scholar]
- 19.Grimm W, Christ M, Bach J, Müller HH, Maisch B. Noninvasive arrhythmia risk stratification in idiopathic dilated cardiomyopathy: results of the Marburg Cardiomyopathy Study. Circulation. 2003;108(23):2883–2891. doi: 10.1161/01.CIR.0000100721.52503.85. [DOI] [PubMed] [Google Scholar]
- 20.Anselmino M, De Ferrari GM, Massa R, Manca L, Tritto M, Molon G, et al. ALPHA Study Group Investigators Predictors of mortality and hospitalization for cardiac causes in patients with heart failure and nonischemic heart disease: a subanalysis of the ALPHA study. Pacing Clin Electrophysiol. 2009;32(Suppl 1):S214–S218. doi: 10.1111/j.1540-8159.2008.02286.x. [DOI] [PubMed] [Google Scholar]
- 21.Scott PA, Barry J, Roberts PR, Morgan JM. Brain natriuretic peptide for the prediction of sudden cardiac death and ventricular arrhythmias: a meta-analysis. Eur J Heart Fail. 2009;11(10):958–966. doi: 10.1093/eurjhf/hfp123. [DOI] [PubMed] [Google Scholar]
- 22.Levy WC, Lee KL, Hellkamp AS, Poole JE, Mozaffarian D, Linker DT, et al. Maximizing survival benefit with primary prevention implantable cardioverter-defibrillator therapy in a heart failure population. Circulation. 2009;120(10):835–842. doi: 10.1161/CIRCULATIONAHA.108.816884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldberger JJ, Cain ME, Hohnloser SH, Kadish AH, Knight BP, Lauer MS, et al. American Heart Association Council on Clinical Cardiology. American Heart Association Council on Epidemiology and Prevention. American College of Cardiology Foundation. Heart Rhythm Society American Heart Association/American College of Cardiology Foundation/Heart Rhythm Society scientific statement on noninvasive risk stratification techniques for identifying patients at risk for sudden cardiac death: a scientific statement from the American Heart Association council on clinical cardiology committee on electrocardiography and arrhythmias and council on epidemiology and prevention. Heart Rhythm. 2008;5(10):e1–21. doi: 10.1016/j.hrthm.2008.05.031. [DOI] [PubMed] [Google Scholar]
- 24.Gorgels AP, Gijsbers C, Vreede-Swagemakers J, Lousberg A, Wellens HJ. Out-of-hospital cardiac arrest - the relevance of heart failure. The Maastricht circulatory arrest registry. Eur Heart J. 2003;24(13):1204–1209. doi: 10.1016/s0195-668x(03)00191-x. [DOI] [PubMed] [Google Scholar]
- 25.Brembilla-Perrot B, Alla F, Suty-Selton C, Huttin O, Blangy H, Sadoul N, et al. Nonischemic dilated cardiomyopathy: results of noninvasive and invasive evaluation in 310 patients and clinical significance of bundle branch block. Pacing Clin Electrophysiol. 2008;31(11):1383–1390. doi: 10.1111/j.1540-8159.2008.01199.x. [DOI] [PubMed] [Google Scholar]
- 26.Kadish A, Dyer A, Daubert JP, Quigg R, Estes NA, Anderson KP, et al. Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators Prophylactic defibrillator implantation in patients with nonischemic dilated cardiomyopathy. N Engl J Med. 2004;350(21):2151–2158. doi: 10.1056/NEJMoa033088. [DOI] [PubMed] [Google Scholar]
- 27.Das MK, Maskoun W, Shen C, Michael MA, Suradi H, Desai M, et al. Fragmented QRS on twelve-lead electrocardiogram predicts arrhythmic events in patients with ischemic and nonischemic cardiomyopathy. Heart Rhythm. 2010;7(1):74–80. doi: 10.1016/j.hrthm.2009.09.065. [DOI] [PubMed] [Google Scholar]
- 28.Gang Y, Ono T, Hnatkova K, Hashimoto K, Camm AJ, Pitt B, et al. ELITE II investigators QT dispersion has no prognostic value in patients with symptomatic heart failure: an ELITE II substudy. Pt 2Pacing Clin Electrophysiol. 2003;26(1):394–400. doi: 10.1046/j.1460-9592.2003.00057.x. [DOI] [PubMed] [Google Scholar]
- 29.Fauchier L, Babuty D, Cosnay P, Poret P, Rouesnel P, Fauchier JP. Long-term prognostic value of time domain analysis of signal-averaged electrocardiography in idiopathic dilated cardiomyopathy. Am J Cardiol. 2000;85(5):618–623. doi: 10.1016/s0002-9149(99)00821-8. [DOI] [PubMed] [Google Scholar]
- 30.Grimm W, Glaveris C, Hoffmann J, Menz V, Müller HH, Hufnagel G, et al. Arrhythmia risk stratification in idiopathic dilated cardiomyopathy based on echocardiography and 12-lead, signal-averaged, and 24-hour holter electrocardiography. Am Heart J. 2000;140(1):43–51. doi: 10.1067/mhj.2000.107178. [DOI] [PubMed] [Google Scholar]
- 31.Okutucu S, Oto A. Risk stratification in nonischemic dilated cardiomyopathy: current perspectives. Cardiol J. 2010;17(3):219–229. [PubMed] [Google Scholar]
- 32.Iacoviello M, Forleo C, Guida P, Romito R, Sorgente A, Sorrentino S, et al. Ventricular repolarization dynamicity provides independent prognostic information toward major arrhythmic events in patients with idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 2007;50(3):225–231. doi: 10.1016/j.jacc.2007.02.071. [DOI] [PubMed] [Google Scholar]
- 33.Grimm W, Christ M, Maisch B. Long runs of non-sustained ventricular tachycardia on 24-hour ambulatory electrocardiogram predict major arrhythmic events in patients with idiopathic dilated cardiomyopathy. Pacing Clin Electrophysiol. 2005;28(Suppl 1):S207–S210. doi: 10.1111/j.1540-8159.2005.00035.x. [DOI] [PubMed] [Google Scholar]
- 34.de Sousa MR, Morillo CA, Rabelo FT, Nogueira Filho AM, Ribeiro AL. Non-sustained ventricular tachycardia as a predictor of sudden cardiac death in patients with left ventricular dysfunction: a meta-analysis. Eur J Heart Fail. 2008;10(10):1007–1014. doi: 10.1016/j.ejheart.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Verma A, Sarak B, Kaplan AJ, Oosthuizen R, Beardsall M, Wulffhart Z, et al. Predictors of appropriate implantable cardioverter defibrillator (ICD) therapy in primary prevention patients with ischemic and nonischemic cardiomyopathy. Pacing Clin Electrophysiol. 2010;33(3):320–329. doi: 10.1111/j.1540-8159.2009.02566.x. [DOI] [PubMed] [Google Scholar]
- 36.Nolan J, Batin PD, Andrews R, Lindsay SJ, Brooksby P, Mullen M, et al. Prospective study of heart rate variability and mortality in chronic heart failure: results of the United Kingdom heart failure evaluation and assessment of risk trial (UK-heart) Circulation. 1998;98(15):1510–1516. doi: 10.1161/01.cir.98.15.1510. [DOI] [PubMed] [Google Scholar]
- 37.Fauchier L, Babuty D, Cosnay P, Autret ML, Fauchier JP. Heart rate variability in idiopathic dilated cardiomyopathy: characteristics and prognostic value. J Am Coll Cardiol. 1997;30(4):1009–1014. doi: 10.1016/s0735-1097(97)00265-9. [DOI] [PubMed] [Google Scholar]
- 38.Rashba EJ, Estes NA, Wang P, Schaechter A, Howard A, Zareba W, et al. Preserved heart rate variability identifies low-risk patients with nonischemic dilated cardiomyopathy: results from the DEFINITE trial. Heart Rhythm. 2006;3(3):281–286. doi: 10.1016/j.hrthm.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 39.Klingenheben T, Ptaszynski P, Hohnloser SH. Heart rate turbulence and other autonomic risk markers for arrhythmia risk stratification in dilated cardiomyopathy. J Electrocardiol. 2008;41(4):306–311. doi: 10.1016/j.jelectrocard.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Salerno-Uriarte JA, De Ferrari GM, Klersy C, Pedretti RF, Tritto M, Sallusti L, et al. ALPHA Study Group Investigators Prognostic value of t-wave alternans in patients with heart failure due to nonischemic cardiomyopathy: results of the ALPHA Study. J Am Coll Cardiol. 2007;50(19):1896–1904. doi: 10.1016/j.jacc.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 41.De Ferrari GM, Sanzo A. T-wave alternans in risk stratification of patients with nonischemic dilated cardiomyopathy: Can it help to better select candidates for ICD implantation? Heart Rhythm. 2009;6(3) Suppl:S29–S35. doi: 10.1016/j.hrthm.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 42.Gold MR, Ip JH, Costantini O, Poole JE, McNulty S, Mark DB, et al. Role of microvolt T-wave alternans in assessment of arrhythmia vulnerability among patients with heart failure and systolic dysfunction: primary results from the T-wave alternans sudden cardiac death in heart failure trial substudy. Circulation. 2008;118(20):2022–2028. doi: 10.1161/CIRCULATIONAHA.107.748962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cahalin LP, Chase P, Arena R, Myers J, Bensimhon D, Peberdy MA, et al. A meta-analysis of the prognostic significance of cardiopulmonary exercise testing in patients with heart failure. Heart Fail Rev. 2013;18(1):79–94. doi: 10.1007/s10741-012-9332-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guimarães GV, Silva MS, d'Avila VM, Ferreira SM, Silva CP, Bocchi EA. VO2 pico e inclinação VE/VCO2 na era dos betabloqueadores na insuficiência cardíaca: uma experiência brasileira. Arq Bras Cardiol. 2008;91(1):39–48. doi: 10.1590/s0066-782x2008001300007. [DOI] [PubMed] [Google Scholar]
- 45.Guazzi M, Raimondo R, Vicenzi M, Arena R, Proserpio C, Sarzi Braga S, et al. Exercise oscillatory ventilation may predict sudden cardiac death in heart failure patients. J Am Coll Cardiol. 2007;50(4):299–308. doi: 10.1016/j.jacc.2007.03.042. [DOI] [PubMed] [Google Scholar]
- 46.Kelesidis I, Travin MI. Use of cardiac radionuclide imaging to identify patients at risk for arrhythmic sudden cardiac death. J Nucl Cardiol. 2012;19:142–152. doi: 10.1007/s12350-011-9482-9. [DOI] [PubMed] [Google Scholar]
- 47.Tamaki S, Yamada T, Okuyama Y, Morita T, Sanada S, Tsukamoto Y, et al. Cardiac iodine-123 metaiodobenzylguanidine imaging predicts sudden cardiac death independently of left ventricular ejection fraction in patients with chronic heart failure and left ventricular systolic dysfunction: results from a comparative study with signal-averaged electrocardiogram, heart rate variability, and QT dispersion. J Am Coll Cardiol. 2009;53(5):426–435. doi: 10.1016/j.jacc.2008.10.025. [DOI] [PubMed] [Google Scholar]
- 48.Boogers MJ, Borleffs CJ, Henneman MM, van Bommel RJ, van Ramshorst J, Boersma E, et al. Cardiac sympathetic denervation assessed with 123-iodine metaiodobenzylguanidine imaging predicts ventricular arrhythmias in implantable cardioverter-defibrillator patients. J Am Coll Cardiol. 2010;55(24):2769–2777. doi: 10.1016/j.jacc.2009.12.066. [DOI] [PubMed] [Google Scholar]
- 49.Poll DS, Marchlinski FE, Buxton AE, Josephson ME. Usefulness of programmed stimulation in idiopathic dilated cardiomyopathy. Am J Cardiol. 1986;58(10):992–997. doi: 10.1016/s0002-9149(86)80025-x. [DOI] [PubMed] [Google Scholar]
- 50.Brembilla-Perrot B, Donetti J, de la Chaise AT, Sadoul N, Aliot E, Juillière Y. Diagnostic value of ventricular stimulation in patients with idiopathic dilated cardiomyopathy. Pt 1Am Heart J. 1991;121(4):1124–1131. doi: 10.1016/0002-8703(91)90672-5. [DOI] [PubMed] [Google Scholar]
- 51.Grimm W, Hoffmann J, Menz V, Luck K, Maisch B. Programmed ventricular stimulation for arrhythmia risk prediction in patients with idiopathic dilated cardiomyopathy and nonsustained ventricular tachycardia. J Am Coll Cardiol. 1998;32(3):739–745. doi: 10.1016/s0735-1097(98)00306-4. [DOI] [PubMed] [Google Scholar]
- 52.Daubert JP, Winters SL, Subacius H, Berger RD, Ellenbogen KA, Taylor SG, et al. Defibrillators In Nonischemic Cardiomyopathy Treatment Evaluation (DEFINITE) Investigators Ventricular arrhythmia inducibility predicts subsequent ICD activation in nonischemic cardiomyopathy patients: a DEFINITE substudy. Pacing Clin Electrophysiol. 2009;32(6):755–761. doi: 10.1111/j.1540-8159.2009.02362.x. [DOI] [PubMed] [Google Scholar]
- 53.Gatzoulis KA, Vouliotis AI, Tsiachris D, Salourou M, Archontakis S, Dilaveris P, et al. Primary prevention of sudden cardiac death in a nonischemic dilated cardiomyopathy population: Reappraisal of the role of programmed ventricular stimulation. Circ Arrhythm Electrophysiol. 2013;6(3):504–512. doi: 10.1161/CIRCEP.113.000216. [DOI] [PubMed] [Google Scholar]
- 54.Piran S, Liu P, Morales A, Hershberger RE. Where genome meets phenome: rationale for integrating genetic and protein biomarkers in the diagnosis and management of dilated cardiomyopathy and heart failure. J Am Coll Cardiol. 2012;60(4):283–289. doi: 10.1016/j.jacc.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 55.Pasotti M, Klersy C, Pilotto A, Marziliano N, Rapezzi C, Serio A, et al. Long-term outcome and risk stratification in dilated cardiolaminopathies. J Am Coll Cardiol. 2008;52(15):1250–1260. doi: 10.1016/j.jacc.2008.06.044. [DOI] [PubMed] [Google Scholar]
- 56.van Rijsingen IA, Arbustini E, Elliott PM, Mogensen J, Hermans-van Ast JF, van der Kooi AJ, et al. Risk factors for malignant ventricular arrhythmias in lamin a/c mutation carriers a European cohort study. J Am Coll Cardiol. 2012;59(5):493–500. doi: 10.1016/j.jacc.2011.08.078. [DOI] [PubMed] [Google Scholar]
- 57.McNair WP, Sinagra G, Taylor MR, Di Lenarda A, Ferguson DA, Salcedo EE, et al. Familial Cardiomyopathy Registry Research Group SCN5A mutations associate with arrhythmic dilated cardiomyopathy and commonly localize to the voltage-sensing mechanism. J Am Coll Cardiol. 2011;57(21):2160–2168. doi: 10.1016/j.jacc.2010.09.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Refaat MM, Lubitz SA, Makino S, Islam Z, Frangiskakis JM, Mehdi H, et al. Genetic variation in the alternative splicing regulator RBM20 is associated with dilated cardiomyopathy. Heart Rhythm. 2012;9(3):390–396. doi: 10.1016/j.hrthm.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hershberger RE, Lindenfeld J, Mestroni L, Seidman CE, Taylor MR, Towbin JA, Heart Failure Society of America Genetic evaluation of cardiomyopathy - a Heart Failure Society of America practice guideline. J Card Fail. 2009;15(2):83–97. doi: 10.1016/j.cardfail.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 60.Iwai C, Akita H, Shiga N, Takai E, Miyamoto Y, Shimizu M, et al. Suppressive effect of the Gly389 allele of the beta1-adrenergic receptor gene on the occurrence of ventricular tachycardia in dilated cardiomyopathy. Circ J. 2002;66(8):723–728. doi: 10.1253/circj.66.723. [DOI] [PubMed] [Google Scholar]
- 61.Chemello D, Rohde LE, Santos KG, Silvello D, Goldraich L, Pimentel M, et al. Genetic polymorphisms of the adrenergic system and implantable cardioverter-defibrillator therapies in patients with heart failure. Europace. 2010;12(5):686–691. doi: 10.1093/europace/euq040. [DOI] [PubMed] [Google Scholar]
- 62.Blanco RR, Austin H, Vest RN, 3rd, Valadri R, Li W, Lassegue B, et al. Angiotensin receptor type 1 single nucleotide polymorphism 1166A/C is associated with malignant arrhythmias and altered circulating miR-155 levels in patients with chronic heart failure. J Card Fail. 2012;18(9):717–723. doi: 10.1016/j.cardfail.2012.06.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Soejima K, Stevenson WG, Sapp JL, Selwyn AP, Couper G, Epstein LM. Endocardial and epicardial radiofrequency ablation of ventricular tachycardia associated with dilated cardiomyopathy: the importance of low-voltage scars. J Am Coll Cardiol. 2004;43(10):1834–1842. doi: 10.1016/j.jacc.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 64.Kanoupakis EM, Manios EG, Kallergis EM, Mavrakis HE, Goudis CA, Saloustros IG, et al. Serum markers of collagen turnover predict future shocks in implantable cardioverter-defibrillator recipients with dilated cardiomyopathy on optimal treatment. J Am Coll Cardiol. 2010;55(24):2753–2759. doi: 10.1016/j.jacc.2010.02.040. [DOI] [PubMed] [Google Scholar]
- 65.McCrohon JA, Moon JC, Prasad SK, McKenna WJ, Lorenz CH, Coats AJ, et al. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance. Circulation. 2003;108(1):54–59. doi: 10.1161/01.CIR.0000078641.19365.4C. [DOI] [PubMed] [Google Scholar]
- 66.Nazarian S, Bluemke DA, Lardo AC, Zviman MM, Watkins SP, Dickfeld TL, et al. Magnetic resonance assessment of the substrate for inducible ventricular tachycardia in nonischemic cardiomyopathy. Circulation. 2005;112(18):2821–2825. doi: 10.1161/CIRCULATIONAHA.105.549659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Assomull RG, Prasad SK, Lyne J, Smith G, Burman ED, Khan M, et al. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48(10):1977–1985. doi: 10.1016/j.jacc.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 68.Lehrke S, Lossnitzer D, Schöb M, Steen H, Merten C, Kemmling H, et al. Use of cardiovascular magnetic resonance for risk stratification in chronic heart failure: prognostic value of late gadolinium enhancement in patients with non-ischaemic dilated cardiomyopathy. Heart. 2011;97(9):727–732. doi: 10.1136/hrt.2010.205542. [DOI] [PubMed] [Google Scholar]
- 69.Hombach V, Merkle N, Torzewski J, Kraus JM, Kunze M, Zimmermann O, et al. Electrocardiographic and cardiac magnetic resonance imaging parameters as predictors of a worse outcome in patients with idiopathic dilated cardiomyopathy. Eur Heart J. 2009;30(16):2011–2018. doi: 10.1093/eurheartj/ehp293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gulati A, Jabbour A, Ismail TF, Guha K, Khwaja J, Raza S, et al. Association of fibrosis with mortality and sudden cardiac death in patients with nonischemic dilated cardiomyopathy. JAMA. 2013;309(9):896–908. doi: 10.1001/jama.2013.1363. Erratum in JAMA. 2013;310(1):99. [DOI] [PubMed] [Google Scholar]