Abstract
Background
Transcutaneous electrical nerve stimulation (TENS) is a useful modality for pain control. TENS has recently been applied to decrease spasticity. The purpose of this study is to determine whether the addition of TENS to an exercise program reduces spasticity and improves balance and gait in chronic stroke patients.
Material/Methods
This was a single-blinded, multicenter, randomized controlled trial. Thirty-four ambulatory individuals with chronic stroke participated and were randomly allocated to the TENS or Placebo group. The TENS group performed therapeutic exercise with TENS while the placebo (non-stimulation) TENS group performed therapeutic exercise with placebo TENS. Participants in both groups followed the same 30-min exercise regimen 5 times per week for a period of 6 weeks. Spasticity (modified Ashworth scale), static (balance system), and dynamic balance (timed up and go test), and gait ability (gait analyzer) were measured at 1 week before and 1 week after the intervention.
Results
Significant differences were observed between the 2 groups. Spasticity improved by 0.80 points in the TENS group. Anterior-posterior and medial-lateral sway velocity among static balance parameters and dynamic balance showed significant differences between the TENS and Placebo TENS groups (p=.000). Gait speed and cadence were enhanced significantly in the TENS group (p=.000). Step and stride length on the paretic side showed a significant difference in the TENS group (p=.000), while only velocity showed a significant difference in the Placebo TENS group (p=.004).
Conclusions
A combination of therapeutic exercise and TENS may reduce spasticity and improve balance, gait, and functional activity in chronic stroke patients.
MeSH Keywords: Gait, Muscle Spasticity, Postural Balance, Stroke, Transcutaneous Electric Nerve Stimulation
Background
Chronic stroke patients commonly experience decreased balance control, which dramatically impacts their ability to perform activities of daily living (ADLs), or purposeful activities[1], and maintain independent gait [2]. Decreased balance may be due to cognitive changes, decreased muscle power, limited range of motion, abnormal muscle tone, uncoordinated movements, or sensory integration alterations [3].
Spasticity, which is the major cause of decreased balance and gait, has commonly been reported in patients with stroke, multiple sclerosis, spinal cord injury, and traumatic brain injury. Moreover, spasticity causes spastic movement disorder, slowed gait, and disturbances in voluntary movement [4]. In addition, passive and active range of motion, functional ability, and dynamic balance are affected [5]. Passive movement [6], passive stretching [7], prostheses [8], and electrical stimulation [1] have been employed in efforts to decrease spasticity.
Electrical stimulation has been used in the rehabilitation of chronic stroke patients, and functional electrical stimulation by neuromuscular electrical stimulation and transcutaneous electrical nerve stimulation (TENS) have been used for pain and sensory stimulation. TENS and functional electrical stimulation have been shown to increase muscle power and movement function, and decrease spasticity [9]. However, functional electrical stimulation induces more powerful muscle contraction and a wider range of motion than does TENS, and the repetitive movement and sensory information that results from functional electrical stimulation effectively improve gait speed and increase muscle power in chronic stroke patients [9]. Use of TENS for inhibition of spasticity, antagonist stimulation [1], agonist stimulation [2], and dermatome stimulation on spastic muscle [3] has been reported.
The combination of TENS and task-oriented movement was demonstrated to improve functional ability relative to the placebo TENS group [10], and TENS with Bobath treatment resulted in decreased spasticity, compared to the use of Bobath treatment group, have been reported [11]. For chronic hemi-neglect patients, application of TENS on the paretic neck muscle resulted in increased postural control and spatial improvement [12], while normal participants who received TENS showed decreased postural sway when standing [13]. However, previous studies used TENS independently or with an exercise program for a short period of time. Therefore, in this study, we examined the effects of a TENS exercise program on spasticity, balance, and gait in chronic stroke patients to determine whether exercise with TENS increases patients’ exercise function.
Material and Methods
Design
This study was a single-blinded, multicenter, randomized controlled trial. The patients were randomly allocated to either TENS group or Placebo TENS group in a ratio 1:1. All patients had an equal probability of assignment to the groups. External randomization was achieved by Random Allocation Software (Ver. 2.0) [14] in blocks of 4 stratified by 4 hospitals.
This study protocol was approved by the institutional review board of Sahmyook University.
Participants
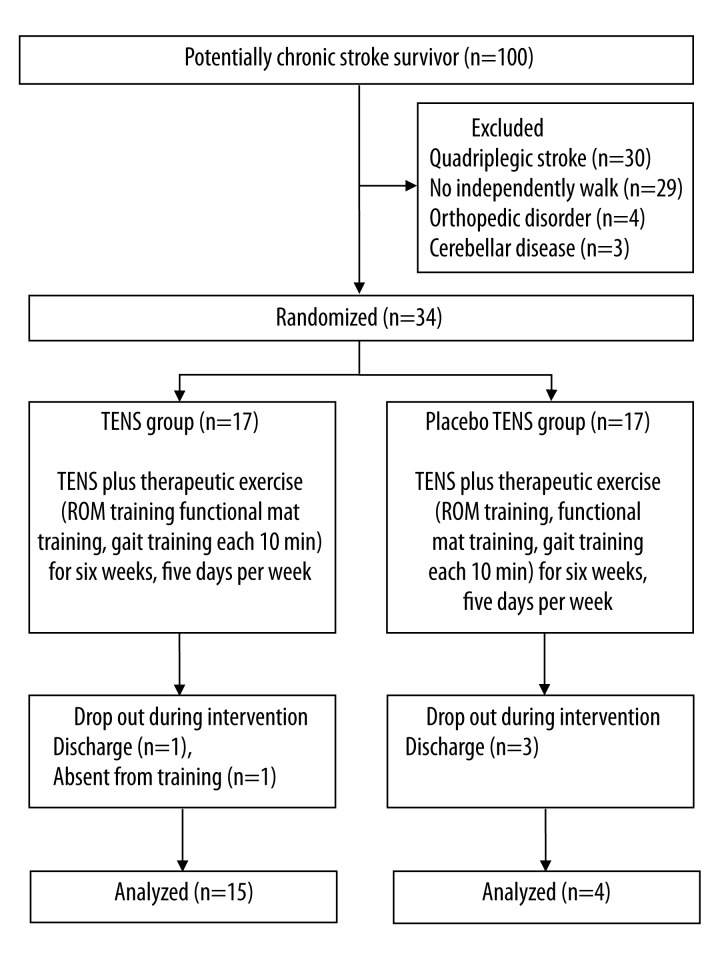
One hundred participants were initially recruited from 4 rehabilitation hospitals in Seoul, South Korea. Participants were included if they had been diagnosed with hemiplegic stroke more than 6 months previously (to exclude natural recovery) and were able to walk 10 m independently. Exclusion criteria included cognitive impairment indicated by scoring higher than 24 on the Mini-Mental State Examination [15], other orthopedic disease, and visual or auditory disorders. Thirty quadriplegic patients, 29 patients who could not walk 10 m independently, 4 patients with orthopedic disorder, and 3 patients with cerebellar disease were excluded from the study. Thirty-four eligible participants provided written informed consent after receiving an explanation of the study (Figure 1).
Figure 1.
Flow diagram of the experimental procedure.
The sample size was calculated using the mean difference in modified Ashworth scale (MAS) for spasticity between the experimental and control groups. Using G-power software (Ver. 3.1) founded on pilot study results, we set the effect size at 0.992. When a 2-tailed test with a test power of β=0.8 and significance level of α=.05 was applied [16]. The calculated sample size was 30. Four additional participants were recruited in anticipation of a dropout rate of approximately 15% during the study.
Intervention
Seventeen participants received TENS plus therapeutic exercise (TENS group), and 17 participants received Placebo TENS plus therapeutic exercise (Placebo TENS group). Participants in the 2 groups engaged in the same 30-min therapeutic exercise 5 days per week for 6 weeks.
Therapeutic exercise
Participants engaged in a 30-min exercise with a physical therapist. The exercise comprised a one-to-one ROM exercise (10 min), a functional mat exercise (10 min), and a gait exercise (10 min), which were each performed at a difficulty level appropriate for the patient. In order to minimize differences between the present and previous interventions, the exercise program was performed according to the pre-set principles, once 1 week before the experiment, and 6 times during the experiment; thus, there were 7 education and practice sessions in total. Education was provided to resolve problems occurring during the exercise program, and to teach performance of exercise program according to the established principles. Participants in both groups performed exercises in the same manner.
TENS plus therapeutic exercise group (TENS group)
Two-channel TENS (TENS-7000, Koalaty Products Inc., USA) was used. TENS electrodes (5 cm2) were placed on the affected lower extremity on the lateral and medial quadriceps and gastrocnemius. A frequency of 100 Hz and a pulse width 200 μs were used. Participant pre-stimulation threshold was measured from 0.01 mA and stimulated by 90% amplitude using the sub-sensory threshold [4]. Stimulation was 30 min, and the patient perceived no sensation. TENS was used with the general exercise program.
Placebo TENS plus therapeutic exercise group (Placebo TENS group)
Two-channel TENS was used in the same manner as in the TENS group. However, stimulation was not applied and patients were informed that the treatment would be imperceptible.
Outcome measures
Modified Ashworth scale (MAS)
The MAS was used to measure spasticity. The MAS is a subjective method used for the measurement of spasticity. The intra-rater credibility was.57 and inter-rater credibility was.62 [5]. To perform the MAS, the patient’s ankle is passively extended from maximal plantar flexion to the painless range and the examiner assigns a score that ranges from 0 to 4 (0, 1, 1+, 2, 3, and 4): 0 indicates normal or very low muscle tone, and 4 indicates that passive extension is not possible. The examiner repeats the measurement 3 times while covering the patient’s calf with 1 hand to ensure that the knee does not bend while the patient is lying supine, and dorsiflexion of the patient’s ankle with the other hand.
Balance
For measurement of static balance, the Good Balance (Metitur Ltd, Finland, 2008) device was used. This device, which comprises a portable triangular foot plate and a scale on the foot plate for foot placement, is widely used for measuring balance in elderly persons and chronic stroke patients [6]. The device also contains a Bluetooth® system. Using the test-retest method, the intra-rater correlation coefficient was above.83, which suggests high credibility. Participants stood with eyes open on the foot plate with their second toe and heel over the scale lines and maintained the position for 30 s; measurements were performed 3 times, maintaining for 30 s with eyes closed. The average of the measurements was used as an indicator of anterior-posterior and medial-lateral postural sway, speed, and speed moment.
The Timed Up and Go (TUG) test was used to assess dynamic balance in the patients. This test measures the time required for a patient to stand up from a 46-cm-high chair, at the cue of ‘start’ and walk 3 m in front of them, and return to the chair. Patients wore their usual shoes, and used gait-assistance tools. The intra-rater credibility was.99, and inter-rater credibility was.98. A timer was used for 3 repeated measurements.
Gait ability
A gait analyzer (OptoGait, Microgate S.r.l, Italy, 2010) was used to test the gait pattern of patients and quantity of gait analysis. Temporal and spatial gait were measured. The gait analyzer was 3 m in length and had 2 transmitting bars and a webcam (Logitech Webcam Pro 9000). The distance between the 2 bars was 1 m, bars were 1 cm from each other, and were continuously receiving signals from a light-emitting diode in the transmitter. Participants gait was sensed and transmitted through the infrared ray sensor, temporal and spatial variables were collected, and participant walking order was stored in the webcam and later synced with the perception error for accurate gait measurement. Collected data were processed using OptoGait, Version 1.5.0. 0 software (Microgate S.r.l, Italy, 2010). To ensure data collection accuracy, the device was calibrated before the test. In order to minimize muscle fatigue, a 1-minute break was provided between measurements; measurement was repeated 3 times, and the average was used.
Data analysis
For data analysis, SPSS ver. 16.0 was used for averages and standard deviations. Data normality was tested using Shapiro-Wilk test, and all variables showed normal distribution. Independent t-tests and chi-squared tests were used for homogeneity testing. A paired t-test was used for comparison of within group exercise. An independent t-test was used for comparison of exercise differences between groups. Significance level was set at 0.05 for all analyses.
Results
Thirty-four participants with stroke participated in the study. Five participants dropped out: 2 participants from the experimental group and 3 participants from the control group (Figure 1).
Participants in the TENS group and the placebo TENS group were the same with regard to general characteristics. No significant difference in general characteristics was noted between groups (Table 1).
Table 1.
General characteristics of the subjects.
| TENS (n=15) | Placebo TENS (n=14) | X2/t | |
|---|---|---|---|
| Post-stroke months | 18.66 (2.46) | 18.57 (1.74) | .119 |
| Sex (male/female) | 12/3 | 8/6 | 1.768 |
| Age (years) | 71.20 (3.46) | 71.14 (3.82) | .829 |
| Paretic side (left/right) | 10/5 | 8/7 | .042 |
| Height (cm) | 167.70 (4.81) | 166.67 (7.32) | .441 |
| Weight (kg) | 61.95 (5.07) | 62.25 (8.74) | −.111 |
Values are presented as mean(standard deviations).
The TENS group showed more reductions of MAS than the placebo TENS group (p<.05) (Table 2).
Table 2.
Changes in the MAS, TUG of outcome measures.
| MAS | TUG | |||
|---|---|---|---|---|
| TENS (n=15) | Placebo TENS (n=14) | TENS (n=15) | Placebo TENS (n=14) | |
| Pre | 2.60 (.63) | 2.50 (.76) | 26.16 (11.71) | 25.70 (12.41) |
| Post | 1.80 (.41)* | 2.36 (.74) | 21.84 (9.28)* | 24.61 (11.61)* |
| Difference | −.80 (.56) | −.14 (.36)** | −4.32 (3.50) | −1.09 (1.83)** |
Values are presented as mean(standard deviations). MAS – Modified Ashworth scale; TUG – timed up and go.
Within-group p<0.05 by paired t-test value;
between-group p<0.05 by independent t-test value.
On the static balance test, a significant difference in eyes closed and opened, anterior posterior, medial lateral postural sway velocity, and velocity moment was observed in the TENS group before and after the test (p<.05), and in mean difference from pre- and posttest between the 2 groups (p<.05) (Table 3).
Table 3.
Changes in the static balance of outcome measures.
| Eye open | Eye close | |||
|---|---|---|---|---|
| TENS (n=15) | Placebo TENS (n=14) | TENS (n=15) | Placebo TENS (n=14) | |
| AP (mm/s) | ||||
| Pre | 11.33 (5.67) | 8.19 (4.61) | 13.47 (6.69) | 12.03 (7.93) |
| Post | 5.52 (2.40)* | 7.79 (3.92) | 7.61 (3.56)* | 11.33 (7.96) |
| Difference | −5.81 (4.42) | −0.40 (0.97)** | −5.85 (4.92) | −0.70 (1.64)** |
| ML (mm/s) | ||||
| Pre | 24.99 (7.17) | 22.36 (10.01) | 29.57 (10.53) | 30.02 (18.44) |
| Post | 17.01 (3.95)* | 21.28 (9.51) | 21.49 (6.75)* | 28.11 (16.44) |
| Difference | −7.97 (4.44) | −1.07 (2.29)** | −8.08 (7.26) | −1.09 (3.47)** |
| VM (mm2/s) | ||||
| Pre | 39.6 (21.19) | 39.10 (39.02) | 63.16 (42.01) | 84.78 (132.78) |
| Post | 25.84 (16.55)* | 33.78 (27.55) | 46.82 (37.68)* | 51.44 (46.82) |
| Difference | −13.79 (15.61) | −5.31 (13.92)** | −16.36 (9.12) | −33.35 (93.51)** |
Values are presented as mean(standard deviations). AP – anterior posterior velocity; ML – medial lateral velocity; VM – velocity moment.
Within-group p<0.05 by paired t-test value;
between-group p<0.05 by independent t-test value.
In TUG of the dynamic balance test, a significant difference in before and after the test was observed in the TENS group (p<.05) and the TENS group was more improved than the placebo TENS group (p<.05) (Table 2).
On the gait analysis test, significant differences in velocity, cadence, and step length and stride length of the paretic side were observed in the TENS group before and after the test (p<.05), but in the Placebo TENS group, only velocity showed a significant difference before and after the test (p<.05) and the TENS group showed more improvements of cadence, step length of the paretic side, and stride length of the paretic side than the placebo TENS group (p<.05) (Table 4).
Table 4.
Changes in the gait analysis of outcome measures.
| TENS (n=15) | Placebo TENS (n=14) | |
|---|---|---|
| Velocity (cm/s) | ||
| Pre | 45.81 (15.22) | 46.85 (20.07) |
| Post | 52.89 (17.43)* | 49.40 (20.50)* |
| Difference | 7.07 (4.58) | 2.55 (2.76) |
| Cadence (steps/min) | ||
| Pre | 73.71 (14.48) | 72.92 (21.75) |
| Post | 83.79 (17.05)* | 72.44 (22.21) |
| Difference | 10.07 (7.65) | −.48 (2.84)** |
| Paretic step length (cm) | ||
| Pre | 17.75 (7.29) | 16.19 (6.63) |
| Post | 24.24 (6.73)* | 16.26 (6.89) |
| Difference | 6.49 (2.30) | .07 (1.54)** |
| Paretic stride length (cm) | ||
| Pre | 52.17 (14.57) | 50.95 (14.36) |
| Post | 61.95 (13.38)* | 51.61 (14.32) |
| Difference | 9.77 (3.96) | 66 (3.05)** |
Values are presented as mean(standard deviations).
Within-group p<0.05 by paired t-test value;
between-group p<0.05 by independent t-test value.
Discussion
Our results suggest that the combination of TENS and exercise improves spasticity, balance, and gait in chronic stroke patients. In previous studies using motor level stimulation threshold, application of TENS resulted in decreased spasticity in patients with spinal cord injury [17] and chronic stroke patients [18], and decreased H-reflex size and spasticity in patients with hemiplegia [10].
Robbins et al. [7] reported that motor level stimulation has to be far above threshold for attainment of muscle contraction related to proprioception feedback in skin, muscles, and joints; and that sensory-level stimulation has to affect the afferent fibers of skin with no muscle contraction. Gravelle et al. [19] reported that sensory-level stimulation of a lower threshold stimulates skin or proprioceptors to increase skin or proprioception to increase standing posture stability. Dickstein et al. [20] reported that use of TENS led to an increase in somatosensory flow from the lower extremity.
In this study, after TENS and exercise, a greater decrease in MAS was observed in the TENS group relative to the Placebo TENS group. In this study, sensory level TENS was applied on the gastrocnemius and quadriceps, and exercise was performed to decrease spasticity.
Decreased spasticity led to improved asymmetric alignment, decreased extremity function, and (possibly) effective energy consumption.
Proprioceptive sensory damage causes postural control difficulties because it alters one’s perception of changes in body orientation in the environment [13]; various types of sensory stimulation, such as proprioception, induce development of minimum muscle contraction and activation of the cortex and cerebellum, which affects balance [21]. This evidence demonstrates that exercise with TENS promotes activation of the network that mediates proprioception and balance.
Gravelle et al. [19] reported that standing on 1 foot decreased postural sway in elderly individuals who received electrical stimulation, and Pérennou et al. [22] reported a decrease in postural sway in stroke patients who received TENS on the cervical area.
In this study, TENS was applied to chronic stroke patients at 100 Hz below threshold on the gastrocnemius to decrease postural sway; the results were the same as those of previous studies [4].
The TENS group showed greater improvement in balance relative to the Placebo TENS group. This result is the same as that of a study reporting that TENS improved somatosensory function in the lower extremity [23]. This study conducted an exercise program with TENS to improve standing postural control, and maintenance on quadriceps and gastrocnemius increased somatosensory function in the lower extremity.
The improvement in balance might have been due to changes in the distance of cadence or step length on the affected side. Gait is a complex movement consisting of balance, coordination, proprioception, and integrated harmony between joints and muscles [24]. Ng and Hui-Chan [18] applied TENS on acupuncture points and reported improved gait speed and endurance. Chen [25] applied sensory electrical stimulation on the Achilles tendon and gastrocnemius 6 times per week for 1 month, and reported significantly increased gait speed in stroke patients.
The TENS group showed improved gait speed, step length, and cadence in the affected leg during gait, compared with the Placebo TENS group. Previous studies used TENS for measurement of balance, but not gait, or did not report significant improvements in gait; however, correlations were observed between balance and gait function. Most chronic stroke patients have an asymmetric gait pattern, resulting in decreased speed, increased double stance, and short step length [26]. In this study, the combination of exercise and TENS effectively improved proprioception to each muscle, resulting in increased body orientation. Participant gait pattern became more symmetrical, as evidenced by the measured gait parameters.
Our study has several limitations. First, the mechanism by which TENS is effective cannot be adequately explained. Second, the sample size was small, which limits the generalizability of the data. Third, there was no long-term follow-up. Further research is needed to develop a more objective design.
Conclusions
Given the findings of this study, we can logically assume that exercise therapy with TENS improves spasticity, balance, and gait in chronic stroke patients and could be actively used in clinic settings as an adjunct to conventional physical therapy.
Footnotes
Conflict of interests
The authors declare that there are no conflicts of interest.
Source of support: This research was supported by a Sahmyook University Research Grant
References
- 1.Mirbagheri MM, Ladouceur M, Barbeau H, et al. The effects of long-term FES-assisted walking on intrinsic and reflex dynamic stiffness in spastic spinal-cord-injured subjects. IEEE Trans Neural Syst Rehabil Eng. 2002;10(4):280–89. doi: 10.1109/TNSRE.2002.806838. [DOI] [PubMed] [Google Scholar]
- 2.van der Salm A, Veltink PH, Ijzerman MJ, et al. Comparison of electric stimulation methods for reduction of triceps surae spasticity in spinal cord injury. Arch Phys Med Rehabil. 2006;87(2):222–28. doi: 10.1016/j.apmr.2005.09.024. [DOI] [PubMed] [Google Scholar]
- 3.Rothwell J, Burke D, Hicks R, et al. Transcranial electrical stimulation of the motor cortex in man: further evidence for the site of activation. J Physiol. 1994;481(Pt 1):243–50. doi: 10.1113/jphysiol.1994.sp020435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dickstein R, Laufer Y, Katz M. TENS to the posterior aspect of the legs decreases postural sway during stance. Neurosci Lett. 2006;393(1):51–55. doi: 10.1016/j.neulet.2005.09.039. [DOI] [PubMed] [Google Scholar]
- 5.Pizzi A, Carlucci G, Falsini C, et al. Evaluation of upper-limb spasticity after stroke: A clinical and neurophysiologic study. Arch Phys Med Rehabil. 2005;86(3):410–15. doi: 10.1016/j.apmr.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 6.Hultborn H, Illert M, Nielsen J, et al. On the mechanism of the post-activation depression of the H-reflex in human subjects. Exp Brain Res. 1996;108(3):450–62. doi: 10.1007/BF00227268. [DOI] [PubMed] [Google Scholar]
- 7.Robbins SM, Houghton PE, Woodbury MG, et al. The therapeutic effect of functional and transcutaneous electric stimulation on improving gait speed in stroke patients: a meta-analysis. Arch Phys Med Rehabil. 2006;87(6):853–59. doi: 10.1016/j.apmr.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 8.Sinkjaer T, Magnussen I. Passive, intrinsic and reflex-mediated stiffness in the ankle extensors of hemiparetic patients. Brain. 1994;117(Pt 2):355–63. doi: 10.1093/brain/117.2.355. [DOI] [PubMed] [Google Scholar]
- 9.Hamdy S, Rothwell JC, Aziz Q, et al. Long-term reorganization of human motor cortex driven by short-term sensory stimulation. Nat Neurosci. 1998;1(1):64–68. doi: 10.1038/264. [DOI] [PubMed] [Google Scholar]
- 10.Bakhtiary AH, Fatemy E. Does electrical stimulation reduce spasticity after stroke? A randomized controlled study. Clin Rehabil. 2008;22(5):418–25. doi: 10.1177/0269215507084008. [DOI] [PubMed] [Google Scholar]
- 11.Dong H-W, Wang L-H, Zhang M, et al. Decreased dynorphin A (1–17) in the spinal cord of spastic rats after the compressive injury. Brain Res Bull. 2005;67(3):189–95. doi: 10.1016/j.brainresbull.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 12.Bakhtiary AH, Fatemy E. Does electrical stimulation reduce spasticity after stroke? A randomized controlled study. Clin Rehabil. 2008;22(5):418–25. doi: 10.1177/0269215507084008. [DOI] [PubMed] [Google Scholar]
- 13.Leibowitz N, Levy N, Weingarten S, et al. Automated measurement of proprioception following stroke. Disabil Rehabil. 2008;30(24):1829–36. doi: 10.1080/09638280701640145. [DOI] [PubMed] [Google Scholar]
- 14.Saghaei M. Random allocation software for parallel group randomized trials. BMC Med Res Methodol. 2004;4:26. doi: 10.1186/1471-2288-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 16.Machin D, Campbell MJ, Tan S-B, et al. Sample size tables for clinical studies. John Wiley & Sons; 2011. [Google Scholar]
- 17.Ping Ho Chung B, Kam Kwan Cheng B. Immediate effect of transcutaneous electrical nerve stimulation on spasticity in patients with spinal cord injury. Clin Rehabil. 2010;24(3):202–10. doi: 10.1177/0269215509343235. [DOI] [PubMed] [Google Scholar]
- 18.Ng SS, Hui-Chan CW. Transcutaneous electrical nerve stimulation combined with task-related training improves lower limb functions in subjects with chronic stroke. Stroke. 2007;38(11):2953–59. doi: 10.1161/STROKEAHA.107.490318. [DOI] [PubMed] [Google Scholar]
- 19.Gravelle DC, Laughton CA, Dhruv NT, et al. Noise-enhanced balance control in older adults. Neuroreport. 2002;13(15):1853–56. doi: 10.1097/00001756-200210280-00004. [DOI] [PubMed] [Google Scholar]
- 20.Dickstein R, Laufer Y, Katz M. TENS to the posterior aspect of the legs decreases postural sway during stance. Neurosci Lett. 2006;393(1):51–55. doi: 10.1016/j.neulet.2005.09.039. [DOI] [PubMed] [Google Scholar]
- 21.Decety J, Perani D, Jeannerod M, et al. Mapping motor representations with positron emission tomography. Nature. 1994;371(6498):600–2. doi: 10.1038/371600a0. [DOI] [PubMed] [Google Scholar]
- 22.Pérennou DA, Leblond C, Amblard B, et al. Transcutaneous electric nerve stimulation reduces neglect-related postural instability after stroke. Arch Phys Med Rehabil. 2001;82(4):440–48. doi: 10.1053/apmr.2001.21986. [DOI] [PubMed] [Google Scholar]
- 23.Laessoe U, Hoeck HC, Simonsen O, et al. Fall risk in an active elderly population – can it be assessed? J Negat Results Biomed. 2007;6:2. doi: 10.1186/1477-5751-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teasell RW, Bhogal SK, Foley NC, et al. Gait retraining post stroke. Top Stroke Rehabil. 2003;10(2):34–65. doi: 10.1310/UDXE-MJFF-53V2-EAP0. [DOI] [PubMed] [Google Scholar]
- 25.Chen SC, Chen YL, Chen CJ, et al. Effects of surface electrical stimulation on the muscle-tendon junction of spastic gastrocnemius in stroke patients. Disabil Rehabil. 2005;27(3):105–10. doi: 10.1080/09638280400009022. [DOI] [PubMed] [Google Scholar]
- 26.Hesse S, Werner C, Bardeleben A, et al. Body weight-supported treadmill training after stroke. Curr Atheroscler Rep. 2001;3(4):287–94. doi: 10.1007/s11883-001-0021-z. [DOI] [PubMed] [Google Scholar]