Abstract
Auditory cortical maps have been a long-standing focus of studies that assess the expression, mechanisms, and consequences of sensory plasticity. Here we discuss recent progress in understanding how auditory experience transforms spatially organized sound representations at higher levels of the central auditory pathways. New insights into the mechanisms underlying map changes have been achieved and more refined interpretations of various map plasticity effects and their consequences in terms of behavioral corollaries and learning as well as other cognitive aspects have been offered. The systematic organizational principles of cortical sound processing remains a key-aspect in studying and interpreting the role of plasticity in hearing.
Introduction
The auditory cortex sits at the nexus of several distinct processing networks. It performs an exquisitely detailed decoding of spectral, temporal, and spatial information embedded in the ascending stream of auditory signal representation [1–3]. It is also the source of a vast, but poorly understood, network of descending corticofugal projections that are thought to adjust the dynamic range and selectivity within midbrain and brainstem nuclei [4, 5]. The auditory cortex is also deeply interconnected with limbic networks that imbue sound with learned emotional significance [6**]. Finally, the auditory cortex participates in an extended executive control network, where attention can powerfully modify cortical response properties, thus, biasing auditory-driven behavioral decisions [7**, 8–10]. The cortical map, sometimes viewed as a contrived construct derived from coarse spatial sampling of near-threshold tuning for rudimentary sounds in anesthetized animals [11, 12], continues to provide a valuable basis for understanding each of these processes.
Auditory maps retain a fundamental plasticity throughout the lifespan that enables highly specific adjustments in the spatial domain and tuning properties for distinct signal types. Maps represent a repository of an individual’s long-term history with sound as well as an ingenious biological solution to meet the competing demands of stability and lability. On the one hand, topographically mapped auditory feature representations provide a robust and stable scheme for decoding the acoustic content of afferent signals. On the other hand, inputs from higher cortical areas or neuromodulatory nuclei can override the biological controls that maintain feature stability and enable rapid, specific, and lasting spatial modifications in support of adaptive behavior. A key issue for understanding the limits of such systems-level plasticity is to develop a theory of neural substrates that plausibly encode experience while maintaining a viable network state.
One view of cortical maps is that they might represent an armature upon which functional subdomains are arrayed. This scaffold enables concurrent processing of different auditory tasks. It permits sequential operations, and it minimizes connectional path length in a system where spatial constraints are severe and connectivity is most valuable [13]. Interleaved with the tonotopic map of core auditory cortex are non-homogeneous representations of binaurality [14], and intensity information [12, 15, 16], and gradients for sharpness of tuning [17] or response timing [18–20]). In contrast, non-primary fields have, at best, only a coarse gradient of characteristic frequency [18, 21, 22], though their thalamic, corticocortical, and commissural connections exhibit the same degree of topographic precision as those in primary auditory cortex (AI) [23]. Other strong expressions of systematic parameter representations, beyond those found in highly specialized animals such as bats [3, 4], have not been encountered, explaining why plasticity studies have largely focused on frequency maps in primary auditory areas.
The easily observable extent of frequency map changes make this an ideal substrate for study. However, in addition to encoding frequency characteristics, auditory cortex neurons are also sensitive to level, temporal envelope shape, and binaural relationship. Thus, the multi-dimensional nature of any auditory stimulus makes it difficult to disambiguate the essential effects on plasticity given the multi-dimensional representational space of any receptive field. In addition, learning may induce only subtle changes in single unit receptive fields that may fall short of the retuning necessary for macroscopic map plasticity to be evident. As a consequence, essential but difficult to assess plastic changes may go unnoticed and hide the actual nature of the reorganization. The following discussion focuses on recent observations of the mechanisms, expression, manipulation and interpretation of plasticity complementing several other recent reviews of related topics [24–26].
Modes of Map Plasticity
Adult cortical plasticity based on behavioral training or co-release of neuromodulatory transmitters is often not just linked to the main task-dependent stimulus property but also affects other aspects that may correspond to task-covariations or independent features within the multi-dimensional acoustic parameter space and within the information-bearing receptive field properties [9, 27–34].
In the following we point to a few parameters that have been observed to be affected by cortical plasticity in parallel or as a consequence of frequency map changes.
Tonotopicity
Plasticity of the frequency map can be induced by a variety of manipulations of the environment, the behavioral situation, as well as changes to the auditory system itself, including associative learning [9, 25], release of neuromodulatory transmitters [115–117], aging [105], extended exposure to sounds [98], or lesioning of the peripheral receptor surface [58, 64]. In this section, we focus on the organization and experience-dependent reorganization of acoustic features other than preferred frequency. We return to tonotopy in the following sections in the context of mechanisms and interpretation of map plasticity.
Spectral integration
A critical aspect of any functional map is the parameter resolution that the map can provide. This is captured by the range of parameter values represented by each neuron, e.g., the range of frequencies in the case of tonotopic map, and the overlap in that range among neighboring neurons. In the functional interpretation of tonotopic maps this creates some problems since the frequency bandwidth – or frequency integration expressed by each site – for most cortical neurons changes as a function of stimulus intensity and, for a fixed intensity, that range can vary from very sharp tuning of less than a third octave to very broad tuning of several octaves width. Strict, highly resolved tonotopicity usually is only discernable at response threshold values and not at sound intensities of natural vocalizations [18].
Extended frequency discrimination training can result in an increased cortical representation of the trained frequency range in the tonotopic map [9, 28, 35, 36], which often is accompanied by an increase in the sharpness of tuning in the range of the frequency trained or paired with nucleus basalis stimulation [28, 37]. Spectral integration bandwidth can be increased or decreased depending on the spatial variability and modulation rate of sensory inputs associated with a behavioral task or sound-paired nucleus basalis stimulation [38]. Modulated stimuli repeatedly delivered to one site on the receptor surface increase spectral bandwidth, while unmodulated stimuli delivered to different locations decrease RF size [28, 37, 39, 40]. Long-term exposure of adult animals to broad-band noise also can increase the spectral bandwidth of neurons across the entire frequency range [41]. By contrast, raising animals in an enriched acoustical environment induces a significant increase in spectral selectivity [42, 43].
Animals trained to discriminate between broad-band stimuli with different spectrally structured acoustic gratings also revealed distinct changes in the spectral integration capacity of cortical neurons [44]. The spectral bandwidth of tonal tuning curves became narrower and the preferred spectral modulations frequency (the spacing between amplitude peaks and troughs in a noise with a sinusoidal spectral envelope) shifted toward that present in the trained grating stimulus. However, spectral integration properties in AI were also influenced by tasks that were not explicitly based but only accompanied by spectral envelope properties. Animals that performed an auditory lateralization task that did not depend on the details of the spectral stimulus envelope also sharpened their spectral integration. Thus, the bandwidth of spectral integration filters is strongly influenced by the spectral envelope of the input stimuli when the stimulus is relevant for the animal, regardless of whether the spectral properties are informative or relevant to the task demands.
Response Magnitude
Significant firing rate changes in marmoset AI have been observed after altering the animal’s ability to produce normal vocalizations [45]. While the ascending auditory system remained unaltered, and responses to pure tones in AI showed normal response magnitude, the cortical responses to normal and altered marmoset vocalizations showed a significant reduction in firing rate. This was interpreted that cortical plasticity can be expressed in response magnitude changes alone without overt map changes. These chronic plasticity effects following altered vocalizations suggest a top-down initiation of plasticity to adjust specific aspects of the sensory-motor loop [45].
Sound Intensity
The assessment of plasticity effects on response magnitude within the frequency map is complicated by the fact that response magnitude is strongly related to sound intensity. However, associative plasticity can create or refine an intensity-specific maximal firing rate [31]. In animals trained on a sound intensity discrimination task, population-response strengths in AI following paired stimulus reinforcement and instrumental conditioning paradigms, became more strongly nonlinear. Individual AI responses, as expressed in firing rate, became selective to more restricted ranges of sound intensities and, as a population, represented a broader range of preferred sound levels with higher specificity. This demonstrates that the representation of stimulus magnitude can be powerfully reshaped by associative learning processes and suggest that the code for sound intensity within AI can be derived from intensity-tuned neurons that change, rather than simply increase, their firing rates in proportion to increases in sound intensity.
The primary sensory cortex is positioned at a confluence of bottom-up dedicated sensory inputs and top-down inputs related to higher-order sensory features, attentional state, and behavioral reinforcement. Polley and colleagues [9] tested whether topographic map plasticity is controlled by the statistics of bottom-up sensory inputs or by top-down task-dependent influences. Rats were trained to attend to independent parameters, either frequency or intensity, within an identical set of auditory stimuli. Rats trained to attend to frequency cues exhibited an expanded representation of the target frequency range within the tonotopic map but no change in sound intensity encoding compared with controls. Rats trained to attend to intensity cues expressed an increased proportion of nonmonotonic intensity response profiles preferentially tuned to the target intensity range but no change in tonotopic map organization relative to controls. The degree of topographic map plasticity within the task-relevant stimulus dimension was correlated with the degree of perceptual learning for rats in both tasks. These data suggest that enduring receptive field plasticity in the adult auditory cortex may be shaped by task-specific top-down inputs that interact with bottom-up sensory inputs and reinforcement-based neuromodulator release. Top-down inputs might confer the selectivity necessary to modify a single feature representation without affecting other spatially organized features embedded within the same neural circuitry.
Response Timing
The relative and absolute timing of cortical responses is a highly relevant aspect of cortical processing. Information content carried by stimulus-locked or other temporal patterns is often higher than that provided by rate-information alone [46–48]. Plasticity effects on the timing of cortical responses have been widely reported. Behavioral training and nucleus basalis stimulation can enhance the ability of cortical neurons to phase-lock to faster amplitude modulation signals [30, 49–52]. Enriching the auditory environment of animals can result in faster and briefer responses to sound onsets and with enhanced phase-locking to modulated sounds [43]. Increased behavioral significance of pup vocalizations in mouse mothers also results in faster and more precise temporal responses [53]. Long-term exposure to broad-band noise resulted, by contrast, in longer peak latencies and longer response durations [41]. Given the limited integration window for converging inputs and the wide range of delays, timing plasticity may have particularly strong consequences when considering the propagation of information from one station to the next, a thoroughly understudied aspect of plasticity.
Sound Location
Although spatially organized modules of neurons with similar binaural response properties have been described in the auditory cortex of several mammalian species [14, 54], a systematic mapping of sound location has only been reported in the bat [3]. However, sound location is encoded by cortical neurons albeit largely in a non-topographical fashion [2, 55]. Training adult animals to discriminate subtle variations in sound source location can significantly enhance the representation of sound azimuths in rat primary auditory cortex [34]. This is reflected in sharper azimuth-selective tuning curves and more evenly distributed best angles of cortical neurons in anesthetized rats. Previously it had been shown that cortical azimuth representation was enhanced while animals were performing a spatial task [56]. This sharpening was interpreted as a generalized effect of arousal or of active listening. Extensive training or experience with a localization task may transfer these short-term gains into long-term benefits, as is, for example, also reflected in early-blind cats that show sharpened auditory spatial tuning of neurons [57].
Binaural processing of sounds is not only relevant for sound localization ability but also is involved in other sound processing aspects, such as detecting signals in background noise. Frequency alignment of binaural cortical inputs can undergo significant plasticity [58–60]. Mild asymmetric sensory-neural hearing loss (SNHL) in squirrel monkeys resulted initially in misalignment of the ear-specific inputs for frequency and response thresholds in primary auditory cortex in AI contralateral to the peripheral lesion: CF and thresholds of the ipsilateral (normal) input and contra-lateral (impaired) input to primary auditory cortex were misaligned in the SNHL-affected frequency range, resulting in a distorted frequency map for the contralateral input whereas the ipsilateral input map appeared normal. Slow reorganization of the cochleotopic map in AI following the SNHL over several months resulted in a gradual realignment of ipsi- and contra-lateral inputs in the hemisphere contralateral to the hearing loss [58]. This ‘reactive’ plasticity cannot be predicted by simple injury/deafferentation propagation models of the auditory periphery [61–64]. Realignment of inputs for restoring binaural integration may be enhanced by homeostatic as well as some associative influences on cortical plasticity. Re-establishing the alignment of corresponding parameters from the two ears in cortical binaural integration enabled by central plastic changes can overwrite a faithful replication of peripheral response properties such as response sensitivity or peripheral frequency mapping [58–60].
Categorical Representation
An important function expected to occur in cortical processing is the emergence of object-based processing in contrast to purely acoustics-based processing. Thus, auditory cortex might be involved in processing stimuli beyond simple feature detection, combining sound components across frequency and over time to generate representations of complex, potentially even categorical patterns in the auditory environment [65, 66*]. Plasticity in early auditory cortical stations may contribute to such a transition. Exposure to naturalistic complex sounds during maturation improves cortical selectivity for spectro-temporally complex features of specific sounds, and improves the stimulus processing in the cortex for their specific, selective representations [67*]. At the neuronal population level, more neurons were involved in representing the whole set of experienced complex sounds, although fewer neurons actually responded to each individual sound, but with greater response magnitudes. Cortical neurons also became more tolerant to natural acoustic variations associated with stimulus context and sound renderings, thus signifying an emergent “categorical” representation of complex experienced sounds [67*].
A similar plastic change in the encoding of species-specific vocalization, such as pup calls, was observed in the auditory cortex of mice with maternal experience [53]. In contrast to naïve female mice, the cortical representation of vocalizations in mothers was enhanced and provided a better detection and discrimination ability of these, suddenly behaviorally highly relevant, sounds. Cortical plasticity, even in primary auditory areas, does not appear solely reliant on sound statistics but also can contribute to the emergence of a more complex, category-sensitive form of sound representation that likely requires top-down regulation.
Synchrony
Correlated activity between neurons in auditory cortex has been widely observed and is likely related to important aspects of stimulus encoding and the propagation of information in auditory cortex [68–70]. Changes in the correlated activity or synchrony between pairs of neurons has been observed in learning, suggesting that a temporally tight interaction in local and distributed cell ensembles is a relevant feature of adaptive circuit plasticity [71, 72*]. Experience-dependent increases in synchrony appear to be most prominent at the beginning of the learning process but return to pre-training values weeks after the experience [73–77]. This initial increase in synaptic connections likely enables the network to restructure [77].
Broadening or shrinking a receptive field is often but not always associated with increasing or decreasing synchrony. Pairing tone trains of different carrier frequencies with nucleus basalis stimulation increases receptive field size without increasing synchronization, and environmental enrichment increases synchronization without increasing receptive field size [78]. The observation that receptive fields and synchronization can be manipulated independently suggests that common inputs are only one of many factors shaping the strength and temporal precision of cortical synchronization and supports the hypothesis that precise neural synchronization contributes to sensory information processing [78].
Mechanisms of Map Plasticity
Although representational maps of auditory features remain plastic throughout the lifespan, the “rules” for transforming particular patterns of auditory experience into map reorganization appears to change between infancy, adulthood, and old age. During a period beginning at the onset of hearing and ending at some time before sexual maturity, passive experience with particular patterns of sound in the ambient environment is sufficient to induce specific and enduring effects on spatially organized sound features. For example, rearing rodents in an acoustic environment containing repeating bursts of pure tones at a single frequency is associated with a specific and enduring over-representation of the exposure frequency in AI [79, 80]. The developmental plasticity mediating the effect of short-term passive tone exposure occurs during a brief window beginning on postnatal day 11 and ending on day 14 [81, 82*]. Although pure tone rearing has also been associated with tonotopic remapping in subcortical auditory nuclei [83, 84], these changes can require longer exposure periods and are more transient than tonotopic map plasticity in AI, suggesting that the cortex may be the primary site of plasticity [82*, 85]. In contrast to the early and brief sensitive period for tonotopic map reorganization, the developmental windows governing plasticity for higher-order auditory features, such as frequency modulated directional tuning or binaural sound localization cues, are comparatively delayed and protracted [59, 60, 86–90]. This organization suggests an unsupervised bootstrapping process where auditory cortical maps are fine-tuned by experience through a cascade of overlapping sensitive periods, with each successive window modifying representational features of greater complexity, as has also been demonstrated both for primary visual cortex development [91, 92] and infant language acquisition [93].
Whereas statistical patterns in passively experienced sound can radically change map organization in the developing cortex, the repeated presentation of artificial and intrinsically meaningless sounds in adult animals generally have no long-term effect on map organization unless they reliably predict aversive or appetitive reinforcement [28, 30, 31, 94]. These observations prompted a reconceptualization of developmental and adult plasticity as more accurately representing successive epochs of exposure-based and reinforcement-based plasticity, respectively [95, 96]. However, as the exception that ultimately proves the rule, Eggermont and colleagues have shown a surprising and profound tonotopic reorganization in adult cats passively exposed to moderate intensity acoustic stimuli that aggregate spectral energy into a restricted frequency band [97–102]. Though ostensibly at odds with findings that underscore the stability of the adult map, both sets of findings can be reconciled by a unified framework for topographic map plasticity founded on neurobiological mechanisms rather than teleological categories.
Recent evidence suggests that GABAergic tone, rather than age per se, regulates the effects of passive sound exposure on cortical map organization. Shortly after the onset of hearing, GABA-mediated two-tone suppression is weak [103], GABAA receptor subunit composition is immature [104], and inhibitory synaptic currents are sluggish [105] with frequency tuning that is not yet precisely co-registered with excitation [106]. As sound-evoked inhibition becomes sharper and more robust, repeated exposure to pure tones is no longer able to induce a long-term remodeling of frequency tuning [106]. Rearing young rats in unmodulated narrow band noise prevents the normal maturation of GABAergic parvalbumin-positive interneurons in map regions corresponding to the frequency range of the noise band and extends the sensitive period window for tonotopic map plasticity [107]. Importantly, chronic moderate intensity noise exposure in adult animals, like that used in the Eggermont studies, also decreases cortical parvalbumin expression levels back to that observed in young animals and reinstates the tonotopic plasticity induced by pure tone rearing that is otherwise only observed during the first days after hearing onset [41, 104]. Thus, the diminishing effect of passive sound statistics on map organization in older animals is better conceptualized as a readout for the maturational state of GABA circuits rather than a switch between unsupervised passive exposure and reinforced attended learning. Unlike trains of tone bursts, which feature sharply defined onsets and offsets, chronic exposure to unmodulated noise can specifically reduce GABAergic tone in the cortex, thereby permitting map reorganization according to the statistics of passively experienced sound. GABAergic tone ebbs once again in the auditory cortex of rats near the end of their lifespan [108]. This suggests that the basic functional architecture of the auditory cortex may be particularly susceptible to the statistical properties of ambient sound in the very young, very old, in young adults chronically exposed to moderate levels of featureless noise (e.g., factory floors), and potentially in neurological conditions typified by an imbalance in cortical inhibition such as aging [105], autism [109], schizophrenia [110], brain trauma [111], and hearing loss [112*, 113].
Intriguingly, several recent findings suggest that a brief and specific relaxation of GABAergic tone may also enable sustained shifts in sound frequency tuning that accompany reinforced learning paradigms in normal adult animals. It is now well established that pairing passive tone presentation with electrical stimulation of nucleus basalis, a heterogenous collection of cells in the basal forebrain believed to encode novel or behaviorally meaningful stimuli [114], can induce striking changes in AI tuning curves and tonotopic maps [115, 116]. By measuring synaptic receptive fields of AI neurons before and after repeated pairing of a single tone frequency with basalis stimulation, Froemke and colleagues demonstrated that acetylcholine release from basalis neurons rapidly reduced the strength of inhibition at the paired tone frequency, followed by a progressive enhancement of excitatory currents at the paired frequency [117]. Thus, a surge of acetylcholine in the auditory cortex was shown to plastically remodel the frequency tuning of an AI neuron, yet the first stage in this process was a highly specific and rapid reduction in synaptic inhibition at what would become the newly acquired preferred frequency. The neural circuitry integrating environmental reinforcement signals, acetylcholine release, intracortical inhibition, and AI tuning dynamics was recently illuminated in a landmark study by Letzkus and colleagues [6**]. By isolating a local network of layer 1 interneurons, layer 2/3 parvalbumin-positive interneurons, and layer 2/3 pyramidal neurons, Letzkus and colleagues revealed a disinhibitory microcircuit that translates the co-occurrence of acoustic stimulation and footshock into a specific remodeling of cortical frequency tuning associated with the behavioral manifestation of learned fear. Thus, while cholinergic input from the basal forebrain may play a critical role in remodeling adult auditory cortex receptive fields and maps under conditions of heightened attention and reinforced learning, its influence may ultimately be mediated through the regulation of GABA circuits.
Although inhibitory synapses may be a critical gatekeeper of plasticity in the developing and adult cortex, it is not the only mechanism involved in remodeling cortical maps. Shortly after hearing onset, non-neuronal extracellular matrix elements may also play an important role in stabilizing the physical geometry of developing synapses and closing sensitive period windows for cortical map plasticity. For example, perineuronal nets of extracellular matrix proteins such as chondroitin sulfate proteoglycans progressively envelop cortical neurons during an early period of hearing, thereby restricting neurite motility [118] and imposing a molecular brake on experience-dependent plasticity [119]. The early sensitive period tonotopic map plasticity corresponds to a period of marked dendritic spine maturation on thalamorecipient AI neurons. Gene-targeted deletion of Icam5, a negative regulator of spine maturation in the forebrain, accelerates the time course of AI spine development and reduces the normal 3 day window for tonotopic map plasticity to a single day [82*]. The brief developmental window for AI tonotopic map plasticity may also be regulated by long-term potentiation and depression of thalamocortical synapses. Recent evidence shows that thalamocortical synaptic plasticity is lost but not gone after an early period of infant development [120]. Thalamocortical synaptic long-term potentiation can be rescued in acute slices from the adult auditory cortex when cortical disinhibition is paired with concomitant activation of cholingeric nucleus basalis axons, suggesting a separate type of cholinergic gating mechanism for map reorganization in developing and adult brains [121*].
Interpreting Map Plasticity
Plastic changes in cortical neurons have been associated with learning, expression of memory and modified sensory perception. In developing animals, passive exposure to a repeated sound frequency is associated with a loss of perceptual acuity for frequencies within the expanded map region and enhanced discrimination ability for under-represented sound frequencies positioned near the edges of the topographic distortion [80]. By contrast, adult plasticity associated with behavioral conditioning, reflected in the extent of map expansion, also appears to correlate with improvements in behavioral performance and resistance to behavioral extinction, suggesting that the degree of plasticity may determine the strength of learning [9, 28, 35, 36, 122, 123*]. While frequency map expansion, as we discussed, captures only a fraction of the changes that plasticity impresses on the functional properties of neurons and the whole network, the interpretation of plasticity through map expansion alone does serve as a simple metaphor.
Learning and Map Plasticity
Plasticity mechanisms encompass aspects of both associative re-tuning of receptive fields and homeostasis of cells and networks. Changes to specific inputs must be coordinated within neural networks to ensure that excitability and feature selectivity are appropriately configured for perception of the sensory environment. Pairing acoustic stimuli with microstimulation of nucleus basalis can induce long-lasting enhancements and decrements to rat primary auditory cortical excitatory synaptic strength. Positive and negative synaptic modifications were shown to maintain a zero-sum across the entire receptive field, thereby changing tuning functions while balancing mean excitation and relative functional stability of the neuron’s activity and its role in the network [124*]. In addition, associative plasticity was shown to reduce overall response variability in later stages of the plastic process while increasing variability in the initial stages. The decreased response variability is associated with increased detection and recognition of near-threshold or previously imperceptible stimuli. This was demonstrated in behavioral tests of a tone detection task [124*]. Thus, brief modification of cortical inputs can lead to wide-scale synaptic changes, which enable improved sensory perception and enhanced behavioral performance, while maintaining a homeostatically viable network.
Cortical map plasticity has been considered a key substrate of perceptual and skill learning. A recent study measured perceptual ability after pairing tones with stimulation of the cholinergic nucleus basalis to induce auditory cortex map plasticity outside of a behavioral context [36]. Pairing nucleus basalis stimulation with a low-frequency tone before discrimination training began was sufficient to accelerate learning of the task. However, nucleus basalis stimulation in already well-trained animals did not improve discrimination performance. Furthermore, several weeks after the training, the initial map expansion faded and the tonotopic map reverted to normal size but the discrimination performance did not decline. The result was interpreted as map expansion improving learning, perhaps enabling the acquisition of the improved discrimination behavior, but that the expansion was not necessary for maintaining good performance in the perceptual discrimination task in the long run [36]. The authors propose a map expansion-renormalization model of plasticity and learning that necessitates changes in the network beyond expansion alone, such as synaptic and dendritic changes [75, 76], that maintain the altered functional improvements of the neurons even after the expansion has vanished. Therefore, it is proposed that map plasticity is involved in learning but not memory [25]. Temporary map expansion may increase the diversity of auditory filter types as well as response variance [125, 126] thus enabling associative selections to form a sparse code with low response variability following renormalization and network stabilization. The observed increase in synchrony that can accompany map expansion and reduction of synchrony when the expansion has faded [78] underscores the potential value of diversity in feature tuning properties in the initial stages of associative learning.
Memory and Map plasticity
Arguments that map plasticity is not so much related to perceptual learning but is rather linked with the formation of memory have also received further experimental evidence. For example, learned associations between a particular tone frequency and reward results in a over-representation of the conditioned frequency in the tonotopic map [28], where the degree of expansion is correlated with the degree of proficiency in the auditory task [9, 28], but also on how motivated the animal was to receive reward [122]. This relationship supports the proposal that sound representations at the cortex are continuously modulated by past experience, and indicates that the amount of gain in representational area is a likely candidate for the salience of associative memory [26].
Determination of tonotopic maps after an extinction procedure (withholding the expected reward and, thus, gradually eliminating the conditioned behavior) revealed that the strength of the memory (expressed in the speed of the extinction progress) was positively correlated with the area representing the frequency of the training stimulus [123*]. A further link between memory expression and map size expansion was demonstrated by expanding the tonotopic map in AI of rats by pairing a tone with activation of NB, mimicking the effects of natural associative learning [127*]. Remodeling of AI produced de novo specific behavioral memory, but neither memory nor plasticity was consistently at the frequency of the paired tone. Rather, the authors found a specific match between individual subjects’ area of expansion and the tone that was strongest in each animal’s memory, as determined by post-training frequency generalization gradients. This was interpreted as a demonstration that directly remodeling sensory cortical maps is sufficient for the specificity of memory formation [26, 127*].
Central Auditory Pathologies and Map Plasticity
Given the links between cortical organization and sound perception, pathophysiological reorganization of the auditory cortex has been directly linked to several hearing disorders. Otitis media is the most commonly diagnosed pathology in children [128]. In approximately 12% of children with otitis media, the accumulation of mucin in the middle ear cavity is severe enough to cause bouts of moderate conductive hearing loss that can last for weeks to months at a time [129]. These children are at significantly greater risk to develop a constellation of brain-based binaural hearing impairments in later life collectively known as amblyaudia (named after its visual analog, amblyopia, for review see [130]). Importantly, binaural impairments often persist long after the middle ear effusion has cleared and children are judged to be audiometrically normal. By introducing a reversible monaural occlusion at various stages of postnatal development in rats, researchers were able to observe a large-scale remapping in AI – but not the inferior colliculus - that greatly enhanced tonotopically organized inputs from the non-deprived ipsilateral ear, suppressed and distorted the tonotopic organization from the developmentally deprived contralateral ear, and strongly disrupted normal sensitivity for interaural level differences [59]. A recent follow-up study induced a brief middle ear disruption at several experimentally determined milestones during the first week of hearing in postnatal life. By measuring binaural selectivity in AI 1 week after normal hearing was restored, it was revealed that binaural integration was shaped by imbalanced binaural experience during two early sensitive periods, such that monaural deprivation before P16 disrupted the co-registration of interaural frequency tuning, whereas monaural deprivation on P16, but not before or after, disrupted ipsilateral interaural level difference sensitivity contained in long-latency spikes [60]. Thus, much like the effects of monaural lid suture on the development of coordinated binocular tuning in the primary visual cortex, early binaural experience may calibrate the central auditory circuits that support spatial hearing during sensitive periods for binaural integration in the auditory cortex.
AI map dysregulation has also been linked to tinnitus, the phantom ringing of the ears that can accompany the degeneration of cochlear hair cells and/or spiral ganglion neurons. Lesioning hair cells at the high-frequency base of the cochlea is associated with an expansion of spared frequencies at the edge of the lesioned zone and disorganized, high-threshold receptive fields in deafferented map regions. Whole cell recordings from neurons within the deafferented zone exhibit hyperexcitability and an abnormal balance of synaptic excitation and inhibition [131, 132]. Transferring acoustically traumatized cats to an acoustic environment containing high intensity tone complex centered on the lesion frequencies prevents the tonotopic distortion in AI [133]. Alternatively, pairing tonal stimuli near the lesion frequency with electrical stimulation of the vagus nerve, an upstream activator of the cholinergic basal forebrain and other neuromodulatory centers, also normalizes AI tonotopy and mitigates behavior consistent with tinnitus [134*].
Network Stability and Map Plasticity
Associative learning and Hebbian plasticity alter and potentially destabilize the properties of neuronal networks. Destabilizing influences can be counteracted by a number of homeostatic plasticity mechanisms with the goal to stabilize neuronal activity. Homeostatic regulation of neuronal excitability refers to the collective phenomena by which neurons alter their intrinsic or synaptic properties to maintain a target level of electrical activity [135]. Hebbian and homeostatic plasticity often target the same molecular substrates, such as synaptic strengths, changes in neuronal excitability, and the regulation of synapse number, but may have opposing effects on synaptic or neuronal properties [136].
A recent study of the role of homeostatic synaptic plasticity in the development and refinement of frequency representations in mouse primary auditory cortex used the tumor necrosis factor-α (TNF-α) knockout (KO), a mutant mouse with impaired homeostatic but normal Hebbian plasticity [137]. These mice develop weaker tonal responses and incomplete frequency representations. TNF-α KOs lacked homeostatic adjustments of cortical responses following exposure to multiple frequencies. This sensory over-stimulation resulted in competitive refinement of frequency tuning in wild-type controls, but broadened frequency tuning in TNF-α KOs. Thus, homeostatic plasticity plays an important role in gain control and competitive interaction in sensory cortical development.
Another study used Dlx1–/f;I12b-Cre mutant mice (cKO) to study the homeostatic plasticity effects of a partial loss of dendrite-targeting interneurons on auditory cortical processing [138]. These animals have normal peripheral hearing but an ~30% reduction in mostly dendrite-targeting interneurons (DTIs), including SOM+, NPY+, CR+, and VIP+ interneurons, that develops near the end of the sensitive period in auditory cortex. After the interneuronal loss, receptive field sizes were slightly reduced in single units from core areas of auditory cortex in cKO mutants due to higher thresholds and narrower bandwidths. The reduction in cortical receptive field size may be a general response to the loss of dendrite-targeting inhibition and may reflect a decreased ability of cortico-cortical connections to drive responses. Overactivity to normal stimuli developed following the loss of DTIs and responses became less sparse combined with an increase in baseline firing rates and seizure-like activity. To prevent extraneous hyperactivity that would render the network unstable, this compensation would replicate a state of tonic DTI inhibition. When faced with a change in the homeostatic state, such as an occurrence of hyperactivity, cortical rebalancing plasticity of the network activity may sacrifice connectivity and computational power for stability [138]. These processes may interfere with potential goals of other bottom-up or top-down plasticity purposes, such as memory formation or enhancing perceptual performances.
Conclusions and Future Directions
All neocortical areas feature a patchwork of gradients and modules that provide a spatial framework for organizing neurons with similar feature tuning. The auditory cortex features a smooth one-dimensional gradient of preferred frequency studded with circumscribed islands of neurons with similar spectral bandwidths, intensity preferences, or binaural selectivity. As animals accumulate experience with sound, the boundaries of these feature representations dilate and contract and the tuning properties for individual neurons contained therein can shift rather dramatically in their preference and tolerance.
The cause and control of these plastic changes is diverse (see Figure 1), including bottom-up maturational plasticity, top-down associative/learning/reward-based plasticity, bottom-up ‘reactive’ plasticity (e.g., following distal deafferentiation, or long-term sound exposure), and local homeostatic plasticity. Although there is overlap between these not very strict categories, the control loops/recurrent networks that govern these processes are likely different with specific sensing, control, and actuating elements and networks. It remains to be seen whether their control and expression are independent and whether pathology and aging affect them differently.
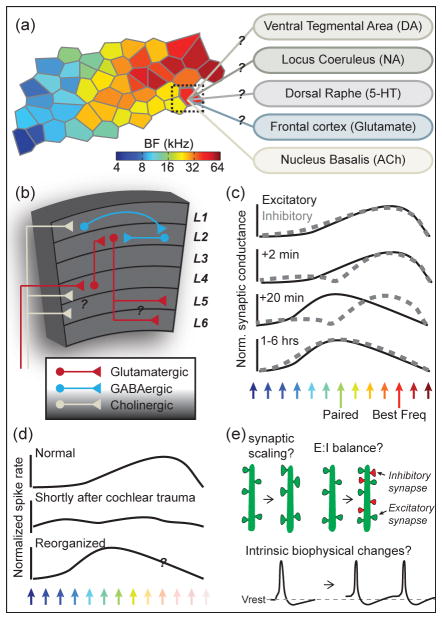
Figure 1.
Mechanisms and unsolved mysteries underlying auditory cortical map reorganization. (a) Tonotopic best frequency (BF) map reconstructed from ~50 extracellular multiunit recording sites from the middle layers of mouse AI, each spaced ~100 μm apart (data from [18]) In addition to receiving heavy feedforward sensory input from the medial geniculate body, AI tonotopic organization is influenced by long-range neuromodulatory inputs such as dopaminergic (DA) inputs from the ventral tegmental area [141], noradrenergic (NA) inputs from locus coeruleus [142], serotinergic inputs from the dorsal raphe (5-HT) [143], glutamatergic inputs from the frontal cortex [144], and cholinergic (ACh) input from nucleus basalis [49]. Of these systems, retuning of auditory response properties by cholingeric modulation is by far the best understood. (b) Recent research has described a cortical microcircuit that translates associative learning cues from nucleus basalis into lasting reorganization of auditory response properties. During auditory fear learning, nociceptive inputs activate basalis afferents innervating layer I of auditory cortex, which excite layer I interneurons via nicotinic ACh receptors. These interneurons, in turn, inhibit parvalbumin+ interneurons in layer 2/3, thereby disinhibiting layer 2/3 pyramidal neurons and enabling plastic reorganization of sound-related excitatory inputs conveyed from layer IV neurons. However, basalis afferents also convey associative learning signals to deeper layers of the auditory cortex, where their effects are thought to be mediated by muscarinic ACh receptors. More work will be needed to reconstruct the organization of parallel microcircuits that translate basalis signals into plasticity of the deeper input/output layers of AI. (c) The synaptic basis for associative retuning of frequency selectivity has been characterized in experiments that isolate excitatory and inhibitory synaptic conductances onto AI neurons before and after a single tone frequency is repeatedly paired with electrical stimulation of nucleus basalis [117]. Before pairing, tone-evoked synaptic excitation and inhibition are precisely co-tuned for frequency. Within minutes of pairing, sound-evoked inhibition is selectively weakened at the paired frequency, followed by an intermediate unbalanced period when excitation has shifted to the paired frequency but inhibition is disorganized. Within an hour after pairing, synaptic excitation and inhibition have co-registered and remain tuned to the paired frequency for at least several hours before returning to their pre-pairing baseline tuning absent further bouts of associative learning cues from basalis. (d) Auditory maps can also be reorganized through non-associative plasticity mechanisms. For instance, within minutes following exposure to intense noise, spectral and temporal organization of sound-evoked inhibitory synaptic inputs are dysregulated, producing poorly selective ‘noisy’ receptive field organization [145]. Over the course of several weeks, AI neurons become re-tuned to sound frequencies bordering the cochlear lesion [64, 131] in a manner that may depend on homeostatic plasticity mechanisms [137] rather than associative plasticity mechanisms such as modulation from nucleus basalis [146]. (e) Additional work will be needed to unveil the specific homeostatic mechanisms that enable receptive field renormalization following auditory deafferentation. For instance, compensatory plasticity could be supported by scaling up postsynaptic responses to a reduced afferent signal, by changing the balance of synaptic excitation (E) and inhibition (I), or by altering the intrinsic electrical excitability of neurons through changing the levels or type of voltage-gated ion channels, as has been demonstrated in the auditory brainstem following changes in afferent activity levels [147, 148], but not in the cortex.
The functional interpretations of the various map plasticity effects and their consequences in terms of behavioral corollaries and learning as well as other cognitive aspects also remain unsettled and require further attention in studies that combine mechanistic, functional and cognitive components.
Organizational constructs such as topographic maps and modules are not the only types of spatially organized neuronal networks, they just happen to be the ones most amenable to microelectrode recordings from the middle layers of anesthetized animals. Newer approaches to optical physiology are revealing dynamic neuronal assemblies for auditory feature representations in multiple cortical layers that are organized at finer spatial scales and reorganize on more rapid temporal scales than can be resolved through traditional microelectrode mapping [66*, 77, 139]. Studies of this ilk are bound to reveal new circuit architectures built from dozens of neurons that form and reform on more rapid time scales to support long-term modifications of modules and maps.
Recent findings drive home the point that the auditory cortex does not sit at the top of a sensory processing hierarchy, but instead is linked into a distributed heterarchical network of brain areas that complement and in many ways complete and enhance the function of the classically defined central auditory pathways. For instance, tonotopically organized projections from AI innervate discrete modules of striatal neurons with well defined auditory tuning that directly enable auditory-based decision making [7**]. Similarly, neurons in the frontal cortex rapidly acquire auditory sensitivity over the course of behavioral conditioning [8] that may enable rapid amplification or attenuation of auditory features in AI according to their hedonic value [140]. More information is needed about the various brain areas that provide modulatory input to the auditory cortex during active listening and how these perceptual representations are reformatted into motor commands. By studying local microcircuits within the auditory cortex and distributed networks across multiple brain areas, we will come to appreciate how the functional organization of the auditory cortex enables a variety of adaptive behaviors. Spatially organized maps of auditory feature space provide an essential framework to investigate these diverse causes and consequences.
Highlights.
Cortical maps of different stimulus and response aspects undergo plastic changes.
Expression and mechanisms of different plastic processes are discussed.
Functional interpretations of map plasticity in terms of behavioral, learning, and cognitive aspects have progressed but remain unsettled.
Acknowledgments
The work of the authors has been supported by the National Institute of Health (CES: DC02260 and MH077970; DBP: DC00983 and DC012894).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
* of special interest
** of outstanding interest
- 1.Bendor D, Osmanski MS, Wang X. Dual-pitch processing mechanisms in primate auditory cortex. Journal of Neuroscience. 2012;32:16149–16161. doi: 10.1523/JNEUROSCI.2563-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Middlebrooks JC, Bremen P. Spatial stream segregation by auditory cortical neurons. Journal of Neuroscience. 2013;33:10986–11001. doi: 10.1523/JNEUROSCI.1065-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Razak KA. Systematic representation of sound locations in the primary auditory cortex. Journal of Neuroscience. 2011;31:13848–13859. doi: 10.1523/JNEUROSCI.1937-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suga N, Xiao Z, Ma X, Ji W. Plasticity and corticofugal modulation for hearing in adult animals. Neuron. 2002;36:9–18. doi: 10.1016/s0896-6273(02)00933-9. [DOI] [PubMed] [Google Scholar]
- 5.Winer JA. Decoding the auditory corticofugal systems. Hearing Research. 2006;212:1–8. doi: 10.1016/j.heares.2005.06.014. [DOI] [PubMed] [Google Scholar]
- 6**.Letzkus JJ, Wolff SBE, Meyer EMM, Tovote P, Courtin J, Herry C, Lüthi A. A disinhibitory microcircuit for associative fear learning in the auditory cortex. Nature. 2011;480:331–335. doi: 10.1038/nature10674. The authors provide an unprecedented view into the step-by-step transformation of foot shock-induced acetylcholine release, local interneuron disinhibition, and shifts in AI pyramidal cell acoustic selectivity that enables auditory fear conditioning. [DOI] [PubMed] [Google Scholar]
- 7**.Znamenskiy P, Zador AM. Corticostriatal neurons in auditory cortex drive decisions during auditory discrimination. Nature. 2013;497:482–485. doi: 10.1038/nature12077. This study shows that tonotopically organized projections from the auditory cortex to the striatum convey signals that bias behavioral choices during performance of an auditory discrimination task and, thus, indicates a general mechanism for control of motor decisions by sensory context. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fritz JB, David SV, Radtke-Schuller S, Yin P, Shamma SA. Adaptive, behaviorally gated, persistent encoding of task-relevant auditory information in ferret frontal cortex. Nature Neuroscience. 2010;13:1011–1019. doi: 10.1038/nn.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polley DB, Steinberg EE, Merzenich MM. Perceptual learning directs auditory cortical map reorganization through top-down influences. Journal of Neuroscience. 2006;26:4970–4982. doi: 10.1523/JNEUROSCI.3771-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee AKC, Larson E, Maddox RK, Shinn-Cunningham BG. Using neuroimaging to understand the cortical mechanisms of auditory selective attention. Hearing Research. 2013 doi: 10.1016/j.heares.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Castro JB, Kandler K. Changing tune in auditory cortex. Nature Neuroscience. 2010;13:271–273. doi: 10.1038/nn0310-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips DP, Semple MN, Calford MB, Kitzes LM. Level-dependent representation of stimulus frequency in cat primary auditory cortex. Exp Brain Res. 1994;102:210–226. doi: 10.1007/BF00227510. [DOI] [PubMed] [Google Scholar]
- 13.Klyachko VA, Stevens CF. Connectivity optimization and the positioning of cortical areas. Proc Natl Acad Sci USA. 2003;100:7937–7941. doi: 10.1073/pnas.0932745100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Middlebrooks JC, Dykes RW, Merzenich MM. Binaural response-specific bands in primary auditory cortex (AI) of the cat: topographical organization orthogonal to isofrequency contours. Brain Res. 1980;181:31–48. doi: 10.1016/0006-8993(80)91257-3. [DOI] [PubMed] [Google Scholar]
- 15.Polley DB, Read HL, Storace DA, Merzenich MM. Multiparametric auditory receptive field organization across five cortical fields in the albino rat. Journal of Neurophysiology. 2007;97:3621–3638. doi: 10.1152/jn.01298.2006. [DOI] [PubMed] [Google Scholar]
- 16.Schreiner CE, Mendelson JR, Sutter ML. Functional topography of cat primary auditory cortex: representation of tone intensity. Exp Brain Res. 1992;92:105–122. doi: 10.1007/BF00230388. [DOI] [PubMed] [Google Scholar]
- 17.Read HL, Winer JA, Schreiner CE. Modular organization of intrinsic connections associated with spectral tuning in cat auditory cortex. Proc Natl Acad Sci USA. 2001;98:8042–8047. doi: 10.1073/pnas.131591898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo W, Chambers AR, Darrow KN, Hancock KE, Shinn-Cunningham BG, Polley DB. Robustness of cortical topography across fields, laminae, anesthetic states, and neurophysiological signal types. Journal of Neuroscience. 2012;32:9159–9172. doi: 10.1523/JNEUROSCI.0065-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carrasco A, Lomber SG. Neuronal activation times to simple, complex, and natural sounds in cat primary and nonprimary auditory cortex. Journal of Neurophysiology. 2011;106:1166–1178. doi: 10.1152/jn.00940.2010. [DOI] [PubMed] [Google Scholar]
- 20.Cheung SW, Bedenbaugh PH, Nagarajan SS, Schreiner CE. Functional organization of squirrel monkey primary auditory cortex: responses to pure tones. Journal of Neurophysiology. 2001;85:1732–1749. doi: 10.1152/jn.2001.85.4.1732. [DOI] [PubMed] [Google Scholar]
- 21.Schreiner CE, Cynader MS. Basic functional organization of second auditory cortical field (AII) of the cat. Journal of Neurophysiology. 1984;51:1284–1305. doi: 10.1152/jn.1984.51.6.1284. [DOI] [PubMed] [Google Scholar]
- 22.Schreiner CE. Order and disorder in auditory cortical maps. Current Opinion in Neurobiology. 1995;5:489–496. doi: 10.1016/0959-4388(95)80010-7. [DOI] [PubMed] [Google Scholar]
- 23.Lee CC, Winer JA. Principles governing auditory cortex connections. Cereb Cortex. 2005;15:1804–1814. doi: 10.1093/cercor/bhi057. [DOI] [PubMed] [Google Scholar]
- 24.Pienkowski M, Eggermont JJ. Cortical tonotopic map plasticity and behavior. Neurosci Biobehav Rev. 2011;35:2117–2128. doi: 10.1016/j.neubiorev.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 25.Shepard KN, Kilgard MP, Liu RC. Experience-dependent plasticity and the auditory cortex. In: Cohen Y, Fay RR, editors. Neural Correlates of Auditory Cognition. Springer Handbook of Auditory Research; 2012. [Google Scholar]
- 26.Weinberger NM. Plasticity in the Primary Auditory Cortex, Not What You Think it is: Implications for Basic and Clinical Auditory Neuroscience. Otolaryngology. 2012;2:1–8. doi: 10.4172/2161-119X.S3-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bakin JS, Weinberger NM. Classical conditioning induces CS-specific receptive field plasticity in the auditory cortex of the guinea pig. Brain Res. 1990;536:271–286. doi: 10.1016/0006-8993(90)90035-a. [DOI] [PubMed] [Google Scholar]
- 28.Recanzone GH, Schreiner CE, Merzenich MM. Plasticity in the frequency representation of primary auditory cortex following discrimination training in adult owl monkeys. J Neurosci. 1993;13:87–103. doi: 10.1523/JNEUROSCI.13-01-00087.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fritz J, Shamma S, Elhilali M, Klein D. Rapid task-related plasticity of spectrotemporal receptive fields in primary auditory cortex. Nature Neuroscience. 2003;6:1216–1223. doi: 10.1038/nn1141. [DOI] [PubMed] [Google Scholar]
- 30.Bao S, Chang EF, Woods J, Merzenich MM. Temporal plasticity in the primary auditory cortex induced by operant perceptual learning. Nature Neuroscience. 2004;7:974–981. doi: 10.1038/nn1293. [DOI] [PubMed] [Google Scholar]
- 31.Polley DB, Heiser MA, Blake DT, Schreiner CE, Merzenich MM. Associative learning shapes the neural code for stimulus magnitude in primary auditory cortex. Proc Natl Acad Sci USA. 2004;101:16351–16356. doi: 10.1073/pnas.0407586101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou X, Merzenich MM. Intensive training in adults refines A1 representations degraded in an early postnatal critical period. Proc Natl Acad Sci USA. 2007;104:15935–15940. doi: 10.1073/pnas.0707348104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou X, Merzenich MM. Developmentally degraded cortical temporal processing restored by training. Nature Neuroscience. 2009;12:26–28. doi: 10.1038/nn.2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y, Zhao Y, Zhu X, Sun X, Zhou X. Refining cortical representation of sound azimuths by auditory discrimination training. Journal of Neuroscience. 2013;33:9693–9698. doi: 10.1523/JNEUROSCI.0158-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bieszczad KM, Weinberger NM. Representational gain in cortical area underlies increase of memory strength. Proceedings of the National Academy of Sciences. 2010;107:3793–3798. doi: 10.1073/pnas.1000159107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reed A, Riley J, Carraway R, Carrasco A, Perez C, Jakkamsetti V, Kilgard MP. Cortical map plasticity improves learning but is not necessary for improved performance. Neuron. 2011;70:121–131. doi: 10.1016/j.neuron.2011.02.038. [DOI] [PubMed] [Google Scholar]
- 37.Kilgard MP, Pandya PK, Vazquez J, Gehi A, Schreiner CE, Merzenich MM. Sensory input directs spatial and temporal plasticity in primary auditory cortex. Journal of Neurophysiology. 2001;86:326–338. doi: 10.1152/jn.2001.86.1.326. [DOI] [PubMed] [Google Scholar]
- 38.Moucha R, Pandya PK, Engineer ND, Rathbun DL, Kilgard MP. Background sounds contribute to spectrotemporal plasticity in primary auditory cortex. Exp Brain Res. 2005;162:417–427. doi: 10.1007/s00221-004-2098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Recanzone GH, Merzenich MM, Jenkins WM. Frequency discrimination training engaging a restricted skin surface results in an emergence of a cutaneous response zone in cortical area 3a. Journal of Neurophysiology. 1992;67:1057–1070. doi: 10.1152/jn.1992.67.5.1057. [DOI] [PubMed] [Google Scholar]
- 40.Bao S, Chang EF, Davis JD, Gobeske KT, Merzenich MM. Progressive degradation and subsequent refinement of acoustic representations in the adult auditory cortex. Journal of Neuroscience. 2003;23:10765–10775. doi: 10.1523/JNEUROSCI.23-34-10765.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhou X, Merzenich MM. Environmental noise exposure degrades normal listening processes. Nature Communications. 2012;3:843. doi: 10.1038/ncomms1849. [DOI] [PubMed] [Google Scholar]
- 42.Engineer ND, Percaccio CR, Pandya PK, Moucha R, Rathbun DL, Kilgard MP. Environmental enrichment improves response strength, threshold, selectivity, and latency of auditory cortex neurons. Journal of Neurophysiology. 2004;92:73–82. doi: 10.1152/jn.00059.2004. [DOI] [PubMed] [Google Scholar]
- 43.Jakkamsetti V, Chang KQ, Kilgard MP. Reorganization in processing of spectral and temporal input in the rat posterior auditory field induced by environmental enrichment. Journal of Neurophysiology. 2012;107:1457–1475. doi: 10.1152/jn.01057.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keeling MD, Calhoun BM, Krüger K, Polley DB, Schreiner CE. Spectral integration plasticity in cat auditory cortex induced by perceptual training. Exp Brain Res. 2008;184:493–509. doi: 10.1007/s00221-007-1115-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cheung SW, Nagarajan SS, Schreiner CE, Bedenbaugh PH, Wong A. Plasticity in primary auditory cortex of monkeys with altered vocal production. Journal of Neuroscience. 2005;25:2490–2503. doi: 10.1523/JNEUROSCI.5289-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Imaizumi K, Priebe NJ, Sharpee TO, Cheung SW, Schreiner CE. Encoding of temporal information by timing, rate, and place in cat auditory cortex. PLoS ONE. 2010;5:e11531. doi: 10.1371/journal.pone.0011531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou Y, Mesik L, Sun YJ, Liang F, Xiao Z, Tao HW, Zhang LI. Generation of spike latency tuning by thalamocortical circuits in auditory cortex. Journal of Neuroscience. 2012;32:9969–9980. doi: 10.1523/JNEUROSCI.1384-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malone BJ, Beitel RE, Vollmer M, Heiser MA, Schreiner CE. Spectral context affects temporal processing in awake auditory cortex. Journal of Neuroscience. 2013;33:9431–9450. doi: 10.1523/JNEUROSCI.3073-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kilgard MP, Merzenich MM. Plasticity of temporal information processing in the primary auditory cortex. Nature Neuroscience. 1998;1:727–731. doi: 10.1038/3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beitel RE, Schreiner CE, Cheung SW, Wang X, Merzenich MM. Reward-dependent plasticity in the primary auditory cortex of adult monkeys trained to discriminate temporally modulated signals. Proc Natl Acad Sci USA. 2003;100:11070–11075. doi: 10.1073/pnas.1334187100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vollmer M, Beitel RE. Behavioral training restores temporal processing in auditory cortex of long-deaf cats. Journal of Neurophysiology. 2011;106:2423–2436. doi: 10.1152/jn.00565.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beitel RE, Vollmer M, Raggio MW, Schreiner CE. Behavioral training enhances cortical temporal processing in neonatally deafened juvenile cats. Journal of Neurophysiology. 2011;106:944–959. doi: 10.1152/jn.00731.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu RC, Schreiner CE. Auditory cortical detection and discrimination correlates with communicative significance. PLoS Biol. 2007;5:e173. doi: 10.1371/journal.pbio.0050173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Higgins NC, Storace DA, Escabí MA, Read HL. Specialization of binaural responses in ventral auditory cortices. Journal of Neuroscience. 2010;30:14522–14532. doi: 10.1523/JNEUROSCI.2561-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Middlebrooks JC, Clock AE, Xu L, Green DM. A panoramic code for sound location by cortical neurons. Science. 1994;264:842–844. doi: 10.1126/science.8171339. [DOI] [PubMed] [Google Scholar]
- 56.Lee C-C, Middlebrooks JC. Auditory cortex spatial sensitivity sharpens during task performance. Nature Neuroscience. 2011;14:108–114. doi: 10.1038/nn.2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Korte M, Rauschecker JP. Auditory spatial tuning of cortical neurons is sharpened in cats with early blindness. Journal of Neurophysiology. 1993;70:1717–1721. doi: 10.1152/jn.1993.70.4.1717. [DOI] [PubMed] [Google Scholar]
- 58.Cheung SW, Bonham BH, Schreiner CE, Godey B, Copenhaver DA. Realignment of interaural cortical maps in asymmetric hearing loss. Journal of Neuroscience. 2009;29:7065–7078. doi: 10.1523/JNEUROSCI.6072-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Popescu MV, Polley DB. Monaural deprivation disrupts development of binaural selectivity in auditory midbrain and cortex. Neuron. 2010;65:718–731. doi: 10.1016/j.neuron.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Polley DB, Thompson JH, Guo W. Brief hearing loss disrupts binaural integration during two early critical periods of auditory cortex development. Nature Communications. 2013;4:2547. doi: 10.1038/ncomms3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Snyder RL, Sinex DG, McGee JD, Walsh EW. Acute spiral ganglion lesions change the tuning and tonotopic organization of cat inferior colliculus neurons. Hearing Research. 2000;147:200–220. doi: 10.1016/s0378-5955(00)00132-5. [DOI] [PubMed] [Google Scholar]
- 62.Syka J, Rybalko N. Threshold shifts and enhancement of cortical evoked responses after noise exposure in rats. Hearing Research. 2000;139:59–68. doi: 10.1016/s0378-5955(99)00175-6. [DOI] [PubMed] [Google Scholar]
- 63.Snyder RL, Sinex DG. Immediate changes in tuning of inferior colliculus neurons following acute lesions of cat spiral ganglion. Journal of Neurophysiology. 2002;87:434–452. doi: 10.1152/jn.00937.2000. [DOI] [PubMed] [Google Scholar]
- 64.Irvine DRF, Rajan R, Smith S. Effects of restricted cochlear lesions in adult cats on the frequency organization of the inferior colliculus. J Comp Neurol. 2003;467:354–374. doi: 10.1002/cne.10921. [DOI] [PubMed] [Google Scholar]
- 65.King AJ, Nelken I. Unraveling the principles of auditory cortical processing: can we learn from the visual system? Nature Neuroscience. 2009;12:698–701. doi: 10.1038/nn.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66*.Bathellier B, Ushakova L, Rumpel S. Discrete neocortical dynamics predict behavioral categorization of sounds. Neuron. 2012;76:435–449. doi: 10.1016/j.neuron.2012.07.008. Using in vivo two-photon calcium imaging in mouse auditory cortex this study demonstrates that local nonlinear dynamics in the auditory cortex generate spontaneous sound categories which can be selected for behavioral or perceptual decisions. [DOI] [PubMed] [Google Scholar]
- 67*.Bao S, Chang EF, Teng C-L, Heiser MA, Merzenich MM. Emergent categorical representation of natural, complex sounds resulting from the early post-natal sound environment. Neuroscience. 2013;248:30–42. doi: 10.1016/j.neuroscience.2013.05.056. Rearing rats in a naturalistic, complex acoustic environment shows that cortical neurons became more selective to spectrotemporal features in the experienced sounds with an increased tolerance for acoustic variations suggestive for the emergence of categorical sound representation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.deCharms RC, Merzenich MM. Primary cortical representation of sounds by the coordination of action-potential timing. Nature. 1996;381:610–613. doi: 10.1038/381610a0. [DOI] [PubMed] [Google Scholar]
- 69.Eggermont JJ. Correlated neural activity as the driving force for functional changes in auditory cortex. Hearing Research. 2007;229:69–80. doi: 10.1016/j.heares.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 70.Atencio CA, Schreiner CE. Columnar connectivity and laminar processing in cat primary auditory cortex. PLoS ONE. 2010;5:e9521. doi: 10.1371/journal.pone.0009521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Komiyama T, Sato TR, O’Connor DH, Zhang Y-X, Huber D, Hooks BM, Gabitto M, Svoboda K. Learning-related fine-scale specificity imaged in motor cortex circuits of behaving mice. Nature. 2010;464:1182–1186. doi: 10.1038/nature08897. [DOI] [PubMed] [Google Scholar]
- 72*.Jeanne JM, Sharpee TO, Gentner TQ. Associative learning enhances population coding by inverting interneuronal correlation patterns. Neuron. 2013;78:352–363. doi: 10.1016/j.neuron.2013.02.023. The authors show that learning produces stimulus-specific changes in the pattern of interneuronal correlations that enhance the ability of neural populations to recognize signals relevant for behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Knott GW, Quairiaux C, Genoud C, Welker E. Formation of dendritic spines with GABAergic synapses induced by whisker stimulation in adult mice. Neuron. 2002;34:265–273. doi: 10.1016/s0896-6273(02)00663-3. [DOI] [PubMed] [Google Scholar]
- 74.Holtmaat A, Svoboda K. Experience-dependent structural synaptic plasticity in the mammalian brain. Nat Rev Neurosci. 2009;10:647–658. doi: 10.1038/nrn2699. [DOI] [PubMed] [Google Scholar]
- 75.Xu T, Yu X, Perlik AJ, Tobin WF, Zweig JA, Tennant K, Jones T, Zuo Y. Rapid formation and selective stabilization of synapses for enduring motor memories. Nature. 2009;462:915–919. doi: 10.1038/nature08389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang G, Pan F, Gan W-B. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462:920–924. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rothschild G, Cohen L, Mizrahi A, Nelken I. Elevated correlations in neuronal ensembles of mouse auditory cortex following parturition. Journal of Neuroscience. 2013;33:12851–12861. doi: 10.1523/JNEUROSCI.4656-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kilgard MP, Vazquez JL, Engineer ND, Pandya PK. Experience dependent plasticity alters cortical synchronization. Hearing Research. 2007;229:171–179. doi: 10.1016/j.heares.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang LI, Bao S, Merzenich MM. Persistent and specific influences of early acoustic environments on primary auditory cortex. Nature Neuroscience. 2001;4:1123–1130. doi: 10.1038/nn745. [DOI] [PubMed] [Google Scholar]
- 80.Han YK, Köver H, Insanally MN, Semerdjian JH, Bao S. Early experience impairs perceptual discrimination. Nature Neuroscience. 2007;10:1191–1197. doi: 10.1038/nn1941. [DOI] [PubMed] [Google Scholar]
- 81.de Villers-Sidani E, Chang EF, Bao S, Merzenich MM. Critical period window for spectral tuning defined in the primary auditory cortex (A1) in the rat. Journal of Neuroscience. 2007;27:180–189. doi: 10.1523/JNEUROSCI.3227-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82*.Barkat TR, Polley DB, Hensch TK. A critical period for auditory thalamocortical connectivity. Nature Neuroscience. 2011;14:1189–1194. doi: 10.1038/nn.2882. The authors identify a 3-day window shortly after hearing onset during which thalamic inputs strength becomes enhanced, tone rearing distorts the tonotopic map, and dendritic spines exhibit maturation. Genetic deletion of Icam 5, a forebrain-specific regulator of spine maturation, was sufficient to compress the critical period window to a single day, suggesting that the evolving postnatal connectivity between thalamus and cortex following hearing onset may thus determine a critical period for auditory processing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yu X, Sanes DH, Aristizabal O, Wadghiri YZ, Turnbull DH. Large-scale reorganization of the tonotopic map in mouse auditory midbrain revealed by MRI. Proc Natl Acad Sci USA. 2007;104:12193–12198. doi: 10.1073/pnas.0700960104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Oliver DLD, Izquierdo MAM, Malmierca MSM. Persistent effects of early augmented acoustic environment on the auditory brainstem. Neuroscience. 2011;184:75–87. doi: 10.1016/j.neuroscience.2011.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Miyakawa AA, Gibboni RR, Bao SS. Repeated exposure to a tone transiently alters spectral tuning bandwidth of neurons in the central nucleus of inferior colliculus in juvenile rats. Neuroscience. 2013;230:114–120. doi: 10.1016/j.neuroscience.2012.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gold JI, Knudsen EI. A site of auditory experience-dependent plasticity in the neural representation of auditory space in the barn owl’s inferior colliculus. Journal of Neuroscience. 2000;20:3469–3486. doi: 10.1523/JNEUROSCI.20-09-03469.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miller GL, Knudsen EI. Early auditory experience induces frequency-specific, adaptive plasticity in the forebrain gaze fields of the barn owl. Journal of Neurophysiology. 2001;85:2184–2194. doi: 10.1152/jn.2001.85.5.2184. [DOI] [PubMed] [Google Scholar]
- 88.Razak KA, Richardson MD, Fuzessery ZM. Experience is required for the maintenance and refinement of FM sweep selectivity in the developing auditory cortex. Proceedings of the National Academy of Sciences. 2008;105:4465–4470. doi: 10.1073/pnas.0709504105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Insanally MN, Köver H, Kim H, Bao S. Feature-dependent sensitive periods in the development of complex sound representation. Journal of Neuroscience. 2009;29:5456–5462. doi: 10.1523/JNEUROSCI.5311-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Keating P, Dahmen JC, King AJ. Context-Specific Reweighting of Auditory Spatial Cues following Altered Experience during Development. Curr Biol. 2013;23:1291–1299. doi: 10.1016/j.cub.2013.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Espinosa JS, Stryker MP. Development and plasticity of the primary visual cortex. Neuron. 2012;75:230–249. doi: 10.1016/j.neuron.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hensch TK. Critical period plasticity in local cortical circuits. Nat Rev Neurosci. 2005;6:877–888. doi: 10.1038/nrn1787. [DOI] [PubMed] [Google Scholar]
- 93.Werker JF, Yeung HH. Infant speech perception bootstraps word learning. Trends in Cognitive Sciences. 2005;9:519–527. doi: 10.1016/j.tics.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 94.Blake DT, Heiser MA, Caywood M, Merzenich MM. Experience-dependent adult cortical plasticity requires cognitive association between sensation and reward. Neuron. 2006;52:371–381. doi: 10.1016/j.neuron.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Polley DB, Hillock AR, Spankovich C, Popescu MV, Royal DW, Wallace MT. Development and Plasticity of Intra- and Intersensory Information Processing. Journal of the American Academy of Audiology. 2008;19:780–798. doi: 10.3766/jaaa.19.10.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.de Villers-Sidani E, Merzenich MM. Lifelong plasticity in the rat auditory cortex: basic mechanisms and role of sensory experience. Prog Brain Res. 2011;191:119–131. doi: 10.1016/B978-0-444-53752-2.00009-6. [DOI] [PubMed] [Google Scholar]
- 97.Noreña AJ, Gourévitch B, Gourevich B, Aizawa N, Eggermont JJ. Spectrally enhanced acoustic environment disrupts frequency representation in cat auditory cortex. Nature Neuroscience. 2006;9:932–939. doi: 10.1038/nn1720. [DOI] [PubMed] [Google Scholar]
- 98.Pienkowski M, Eggermont JJ. Long-term, partially-reversible reorganization of frequency tuning in mature cat primary auditory cortex can be induced by passive exposure to moderate-level sounds. Hearing Research. 2009;257:24–40. doi: 10.1016/j.heares.2009.07.011. [DOI] [PubMed] [Google Scholar]
- 99.Pienkowski M, Eggermont JJ. Passive exposure of adult cats to moderate-level tone pip ensembles differentially decreases AI and AII responsiveness in the exposure frequency range. Hearing Research. 2010;268:151–162. doi: 10.1016/j.heares.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 100.Pienkowski M, Eggermont JJ. Intermittent exposure with moderate-level sound impairs central auditory function of mature animals without concomitant hearing loss. Hearing Research. 2010;261:30–35. doi: 10.1016/j.heares.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 101.Pienkowski M, Munguia R, Eggermont JJ. Passive exposure of adult cats to bandlimited tone pip ensembles or noise leads to long-term response suppression in auditory cortex. Hearing Research. 2011;277:117–126. doi: 10.1016/j.heares.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 102.Pienkowski M, Munguia R, Eggermont JJ. Effects of passive, moderate-level sound exposure on the mature auditory cortex: spectral edges, spectrotemporal density, and real-world noise. Hearing Research. 2013;296:121–130. doi: 10.1016/j.heares.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 103.Chang EF, Bao S, Imaizumi K, Schreiner CE, Merzenich MM. Development of spectral and temporal response selectivity in the auditory cortex. Proc Natl Acad Sci USA. 2005;102:16460–16465. doi: 10.1073/pnas.0508239102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhou X, Panizzutti R, de Villers-Sidani E, Madeira C, Merzenich MM. Natural restoration of critical period plasticity in the juvenile and adult primary auditory cortex. Journal of Neuroscience. 2011;31:5625–5634. doi: 10.1523/JNEUROSCI.6470-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Takesian AE, Kotak VC, Sanes DH. Age-dependent effect of hearing loss on cortical inhibitory synapse function. Journal of Neurophysiology. 2012;107:937–947. doi: 10.1152/jn.00515.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dorrn AL, Yuan K, Barker AJ, Schreiner CE, Froemke RC. Developmental sensory experience balances cortical excitation and inhibition. Nature. 2010;465:932–936. doi: 10.1038/nature09119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.de Villers-Sidani E, Simpson KL, Lu Y-F, Lin RCS, Merzenich MM. Manipulating critical period closure across different sectors of the primary auditory cortex. Nature Neuroscience. 2008;11:957–965. doi: 10.1038/nn.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.de Villers-Sidani E, Alzghoul L, Zhou X, Simpson KL, Lin RCS, Merzenich MM. Recovery of functional and structural age-related changes in the rat primary auditory cortex with operant training. Proceedings of the National Academy of Sciences. 2010;107:13900–13905. doi: 10.1073/pnas.1007885107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gogolla N, Leblanc JJ, Quast KB, Südhof TC, Fagiolini M, Hensch TK. Common circuit defect of excitatory-inhibitory balance in mouse models of autism. J Neurodev Disord. 2009;1:172–181. doi: 10.1007/s11689-009-9023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fung SJ, Webster MJ, Sivagnanasundaram S, Duncan C, Elashoff M, Weickert CS. Expression of interneuron markers in the dorsolateral prefrontal cortex of the developing human and in schizophrenia. Am J Psychiatry. 2010;167:1479–1488. doi: 10.1176/appi.ajp.2010.09060784. [DOI] [PubMed] [Google Scholar]
- 111.Lowenstein DH, Thomas MJ, Smith DH, McIntosh TK. Selective vulnerability of dentate hilar neurons following traumatic brain injury: a potential mechanistic link between head trauma and disorders of the hippocampus. J Neurosci. 1992;12:4846–4853. doi: 10.1523/JNEUROSCI.12-12-04846.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112*.Sanes DH, Kotak VC. Developmental plasticity of auditory cortical inhibitory synapses. Hearing Research. 2011;279:140–148. doi: 10.1016/j.heares.2011.03.015. This review of recent findings regarding the function of inhibitory synapses shows that inhibitory synapses are exceptionally dynamic during development, and deafness-induced perturbation of inhibitory properties may have a profound impact on auditory processing. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sarro EC, Kotak VC, Sanes DH, Aoki C. Hearing loss alters the subcellular distribution of presynaptic GAD and postsynaptic GABAA receptors in the auditory cortex. Cereb Cortex. 2008;18:2855–2867. doi: 10.1093/cercor/bhn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Richardson RT, DeLong MR. Nucleus basalis of Meynert neuronal activity during a delayed response task in monkey. Brain Res. 1986;399:364–368. doi: 10.1016/0006-8993(86)91529-5. [DOI] [PubMed] [Google Scholar]
- 115.Bakin JS, Weinberger NM. Induction of a physiological memory in the cerebral cortex by stimulation of the nucleus basalis. Proc Natl Acad Sci USA. 1996;93:11219–11224. doi: 10.1073/pnas.93.20.11219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kilgard MP, Merzenich MM. Cortical map reorganization enabled by nucleus basalis activity. Science. 1998;279:1714–1718. doi: 10.1126/science.279.5357.1714. [DOI] [PubMed] [Google Scholar]
- 117.Froemke RC, Merzenich MM, Schreiner CE. A synaptic memory trace for cortical receptive field plasticity. Nature. 2007;450:425–429. doi: 10.1038/nature06289. [DOI] [PubMed] [Google Scholar]
- 118.Friauf E. Development of chondroitin sulfate proteoglycans in the central auditory system of rats correlates with acquisition of mature properties. Audiol Neurootol. 2000;5:251–262. doi: 10.1159/000013889. [DOI] [PubMed] [Google Scholar]
- 119.Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- 120.Blundon JA, Bayazitov IT, Zakharenko SS. Presynaptic gating of postsynaptically expressed plasticity at mature thalamocortical synapses. Journal of Neuroscience. 2011;31:16012–16025. doi: 10.1523/JNEUROSCI.3281-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121*.Chun S, Bayazitov IT, Blundon JA, Zakharenko SS. Thalamocortical long-term potentiation becomes gated after the early critical period in the auditory cortex. Journal of Neuroscience. 2013;33:7345–7357. doi: 10.1523/JNEUROSCI.4500-12.2013. This study shows that, in the auditory cortex, long-term potentiation, a major form of synaptic plasticity, is expressed at thalamo-cortical synapses in both young and mature mice but becomes gated with age by presynaptic cholinergic mechanisms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rutkowski RG, Weinberger NM. Encoding of learned importance of sound by magnitude of representational area in primary auditory cortex. Proc Natl Acad Sci USA. 2005;102:13664–13669. doi: 10.1073/pnas.0506838102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123*.Bieszczad KM, Weinberger NM. Extinction reveals that primary sensory cortex predicts reinforcement outcome. Eur J Neurosci. 2012;35:598–613. doi: 10.1111/j.1460-9568.2011.07974.x. The authors used extinction to investigate whether behavioral importance reflects a sound’s ability to predict reinforcement or whether it predicts change in the meaning of a sound. The findings show that primary sensory cortical representation can encode behavioral importance as a signal’s value to predict reinforcement, and that the number of cells tuned to a stimulus can dictate its ability to command behavior. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124**.Froemke RC, Carcea I, Barker AJ, Yuan K, Seybold BA, Martins ARO, Zaika N, Bernstein H, Wachs M, Levis PA, et al. Long-term modification of cortical synapses improves sensory perception. Nature Neuroscience. 2013;16:79–88. doi: 10.1038/nn.3274. Here it is demonstrated that modification of cortical inputs through nucleus basalis stimulation leads to wide-scale synaptic changes in auditory cortex that conserve mean excitation while reducing overall response variability. These changes are reflected in improved sensory perception and enhanced behavioral performance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Takahashi H, Yokota R, Funamizu A, Kose H, Kanzaki R. Learning-stage-dependent, field-specific, map plasticity in the rat auditory cortex during appetitive operant conditioning. Neuroscience. 2011;199:243–258. doi: 10.1016/j.neuroscience.2011.09.046. [DOI] [PubMed] [Google Scholar]
- 126.Takahashi H, Yokota R, Kanzaki R. Response variance in functional maps: neural darwinism revisited. PLoS ONE. 2013;8:e68705. doi: 10.1371/journal.pone.0068705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127*.Bieszczad KM, Miasnikov AA, Weinberger NM. Remodeling sensory cortical maps implants specific behavioral memory. Neuroscience. 2013;246:40–51. doi: 10.1016/j.neuroscience.2013.04.038. The findings of this study demonstrate a match between the artificial induction of specific neural representational plasticity and artificial induction of behavioral memory. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lanphear BP, Byrd RS, Auinger P, Hall CB. Increasing prevalence of recurrent otitis media among children in the United States. Pediatrics. 1997;99:E1. doi: 10.1542/peds.99.3.e1. [DOI] [PubMed] [Google Scholar]
- 129.Gravel JS, Wallace IF. Effects of otitis media with effusion on hearing in the first 3 years of life. J Speech Lang Hear Res. 2000;43:631–644. doi: 10.1044/jslhr.4303.631. [DOI] [PubMed] [Google Scholar]
- 130.Whitton JP, Polley DB. Evaluating the perceptual and pathophysiological consequences of auditory deprivation in early postnatal life: a comparison of basic and clinical studies. J Assoc Res Otolaryngol. 2011 doi: 10.1007/s10162-011-0271-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang S, Weiner BD, Zhang LS, Cho S-J, Bao S. Homeostatic plasticity drives tinnitus perception in an animal model. Proceedings of the National Academy of Sciences. 2011;108:14974–14979. doi: 10.1073/pnas.1107998108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang S, Su W, Bao S. Long-term, but not transient, threshold shifts alter the morphology and increase the excitability of cortical pyramidal neurons. Journal of Neurophysiology. 2012 doi: 10.1152/jn.00371.2012. no volume. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Noreña AJ, Eggermont JJ. Enriched acoustic environment after noise trauma reduces hearing loss and prevents cortical map reorganization. Journal of Neuroscience. 2005;25:699–705. doi: 10.1523/JNEUROSCI.2226-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134*.Engineer ND, Riley JR, Seale JD, Vrana WA, Shetake JA, Sudanagunta SP, Borland MS, Kilgard MP. Reversing pathological neural activity using targeted plasticity. Nature. 2011;470:101–104. doi: 10.1038/nature09656. Vagal nerve stimulation paired with a specific paradigm of tonal stimulation reversed changes in the organization of rat auditory cortex induced by noise exposure. Behavioral signs of tinnitus, a common corollary of noise induced hearing loss, were eliminated as well suggesting that tinnitus is related to alterations of excitability of cortical neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Williams AH, O’Leary T, Marder E. Homeostatic Regulation of Neuronal Excitability. Scholarpedia. 2013;8:1656. [Google Scholar]
- 136.Turrigiano GG, Nelson SB. Hebb and homeostasis in neuronal plasticity. Current Opinion in Neurobiology. 2000;10:358–364. doi: 10.1016/s0959-4388(00)00091-x. [DOI] [PubMed] [Google Scholar]
- 137.Yang S, Zhang LS, Gibboni R, Weiner B, Bao S. Impaired Development and Competitive Refinement of the Cortical Frequency Map in Tumor Necrosis Factor-α-Deficient Mice. Cereb Cortex. 2013 doi: 10.1093/cercor/bht053. [DOI] [PMC free article] [PubMed]
- 138.Seybold BA, Stanco A, Cho KKA, Potter GB, Kim C, Sohal VS, Rubenstein JLR, Schreiner CE. Chronic reduction in inhibition reduces receptive field size in mouse auditory cortex. Proceedings of the National Academy of Sciences. 2012;109:13829–13834. doi: 10.1073/pnas.1205909109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Winkowski DE, Kanold PO. Laminar transformation of frequency organization in auditory cortex. Journal of Neuroscience. 2013;33:1498–1508. doi: 10.1523/JNEUROSCI.3101-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.David SV, Fritz JB, Shamma SA. Task reward structure shapes rapid receptive field plasticity in auditory cortex. Proceedings of the National Academy of Sciences. 2012;109:2144–2149. doi: 10.1073/pnas.1117717109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bao S, Chan VT, Merzenich MM. Cortical remodelling induced by activity of ventral tegmental dopamine neurons. Nature. 2001;412:79–83. doi: 10.1038/35083586. [DOI] [PubMed] [Google Scholar]
- 142.Edeline J-M, Manunta Y, Hennevin E. Induction of selective plasticity in the frequency tuning of auditory cortex and auditory thalamus neurons by locus coeruleus stimulation. Hear Res. 2011;274:75–84. doi: 10.1016/j.heares.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 143.Ji W, Suga N. Serotonergic modulation of plasticity of the auditory cortex elicited by fear conditioning. Journal of Neuroscience. 2007;27:4910–4918. doi: 10.1523/JNEUROSCI.5528-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Winkowski DE, Bandyopadhyay S, Shamma SA, Kanold PO. Frontal cortex activation causes rapid plasticity of auditory cortical processing. Journal of Neuroscience. 2013;33:18134–18148. doi: 10.1523/JNEUROSCI.0180-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Scholl B, Wehr M. Disruption of balanced cortical excitation and inhibition by acoustic trauma. J Neurophysiol. 2008;100:646–656. doi: 10.1152/jn.90406.2008. [DOI] [PubMed] [Google Scholar]
- 146.Kamke MR, Brown M, Irvine DRF. Basal forebrain cholinergic input is not essential for lesion-induced plasticity in mature auditory cortex. Neuron. 2005;48:675–686. doi: 10.1016/j.neuron.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 147.Strumbos JG, Polley DB, Kaczmarek LK. Specific and rapid effects of acoustic stimulation on the tonotopic distribution of Kv3. 1b potassium channels in the adult rat. Neuroscience. 2010;167:567–572. doi: 10.1016/j.neuroscience.2010.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lu Y, Monsivais P, Tempel BL, Rubel EW. Activity-dependent regulation of the potassium channel subunits Kv1.1 and Kv3.1. J Comp Neurol. 2004;470:93–106. doi: 10.1002/cne.11037. [DOI] [PubMed] [Google Scholar]