Abstract
Background
There is intense interest in the role of programmed death 1 (PD-1) in causing persistent T-cell dysfunction in HIV infection. However, the impact of HIV infection and antiretroviral treatment (ART) on the expression of PD-1 on T cells is still poorly defined.
Methods
PD-1 was measured longitudinally in a cohort of recently HIV-infected individuals (n = 121) who started ART early (<6 months after infection) vs. later (≥2 years after infection). PD-1 was also measured cross-sectionally in a diverse cohort of chronically HIV-infected adults (n = 206).
Results
PD-1 expression levels were high on CD8+ T cells during early HIV infection. PD-1 levels increased on both CD4+ and CD8+ T cells populations in those who delayed therapy (11 and 10%/year, respectively). PD-1 levels declined and were similar in those treated early vs. late after 1 year of ART. In both cohorts, PD-1 expression on CD4+ T cells was associated with CD4+ T-cell activation (CD38+HLA-DR+) and inversely with CD4+ cell count. In contrast, PD-1 expression on CD8+ T cells was most strongly associated with CD8+ T-cell activation and with plasma viral load in viremic individuals.
Conclusion
Across two large cohorts of untreated and treated individuals, we found consistent associations between HIV RNA levels, CD8+ T-cell activation and PD-1 expression on CD8+ T cells. In contrast, CD4+ T-cell counts and CD4+ T-cell activation were more consistent correlates of PD-1 expression on CD4+ T cells. PD-1 expression appears to be driven by both direct antigen and homeostatic pathways.
Keywords: CD4+ lymphocyte count, early antiretroviral therapy, HIV antiretroviral therapy, HIV-1/immunology/*physiology, humans, programmed death-1, T-cell activation, T lymphocytes/immunology/*physiology, virus replication/physiology
Introduction
Combination antiretroviral therapy (ART) can suppress HIV viremia indefinitely in most individuals. As a consequence of viral suppression, the immune system is reconstituted, with many patients achieving sustained increases in CD4+ T-cell counts and a reduced risk of morbidity and mortality. The degree of immune reconstitution, however, is highly variable. A substantial proportion of treated adults fail to normalize their CD4+ T-cell counts [1,2]. Even those who do achieve normal CD4+ T-cell counts have residual immune dysfunction [3] and perhaps a higher than expected risk of HIV and non-HIV related morbidity [4,5]. The mechanism of this association between suboptimal immune reconstitution and risk of disease is not known.
There is intense interest in the role of inhibitory molecules, especially programmed death-1 (PD-1), in causing persistent T-cell dysfunction in HIV infection. PD-1 is upregulated on the surface of activated CD4+ and CD8+ T cells in untreated HIV and simian immunodeficiency virus infection, resulting in decreased proliferation and production of cytokines [6–11]. Many studies have shown that blocking the interaction between PD-1 and its ligand can reverse these effects [6,7,10, 12–15]. In addition, CD4+ T cells expressing high levels of PD-1 are enriched for HIV DNA [16] and the size of the HIV reservoir during ART correlates with the frequency of PD-1 expressing cells [17].
The effect of ART on PD-1 expression on CD4+ and CD8+ T cells is not fully understood, as previous studies have had a very limited sample size and have been mainly cross-sectional [17–22]. We therefore conducted two analyses to characterize PD-1 expression in untreated and treated HIV-infected individuals. First, to assess the impact of ART and its timing, we conducted a longitudinal study in a cohort of individuals identified in early HIV infection who initiated ART early vs. later. Second, to more fully explore the correlates of PD-1 expression across the entire spectrum of HIV infection, we performed a comprehensive cross-sectional analysis of PD-1 expression in untreated and treated individuals, including untreated HIV controllers and ART-suppressed individuals with varying degrees of CD4+ T-cell recovery.
Materials and methods
Study participants
Participants were identified from the University of California-San Francisco (UCSF) Options cohort to assess longitudinal patterns of PD-1 expression. This is a prospective cohort of recently infected HIV-infected individuals (defined as <12 months after estimated infection; since 2003 this was changed to ≤6 months) and at-risk individuals who sought HIV testing for possible acute infection, but were found to be uninfected. Early HIV was diagnosed as previously described and estimated infection dates were calculated using standard methods [23]. Participants who initiated therapy early vs. later were selected as previously described [23]. Briefly, the early ART group consisted of those participants who initiated therapy less than 6 months after the estimated date of HIV infection, while the later ART group initiated therapy at least 2 years after estimated HIV infection. Both groups had sustained virologic suppression (plasma viral load <75 copies/ml) for at least 2 years. A group of individuals who remained ART-naive and a group of HIV-uninfected individuals were also included in the analysis.
The majority of individuals in the later ART group in Options started therapy before the onset of advanced immunosuppression. Thus, this group has limited representation of persons who initiated treatment in advanced HIV infection. Therefore, we also examined PD-1 expression in a cross-sectional analysis of participants from the UCSF SCOPE cohort. From SCOPE, we selected a group of chronically treated individuals who achieved virologic suppression. A subset of this group had CD4+ cell count nadirs less than 350 cells/ml and had either robust CD4+ T cells gains with counts more than 500 cells/µl (’immunologic responders’) or suboptimal gains with CD4+ T cells counts less than 350 cells/µl (’immunologic nonresponders’). We also studied long-term untreated controllers (defined as an HIV RNA <500 copies/ml) and chronically untreated HIV-infected individuals with high viremia. All individuals provided written informed consent. This study was approved by the UCSF Committee on Human Research.
T-cell immunophenotyping
Peripheral blood mononuclear cells (PBMCs) were isolated from whole blood, cryopreserved and stored at the UCSF AIDS Specimen Bank. Assays were performed at the UCSF Core Immunology Laboratory using previously described optimized methods [24]. Briefly, cells were thawed, washed and stained with LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (Invitrogen Life Technologies, Grand Island, New York, USA) to exclude nonviable cells and then stained with the following fluorescently conjugated mAbs: CD8-QDOT605 and CD4-PE-Texas Red (Invitrogen); CD3-Pacific Blue, CCR5-PE-Cy5, CD38-PE, HLA-DR-FITC, PD-1 Alexa Fluor 647, CD45RA-PECY7 (BD Biosciences, San Jose, California, USA) and CCR7-APCeFluor780 (eBioscience, San Diego, California, USA).
Data were compensated and analysed using FlowJo V9 (TreeStar, Ashland, Oregon, USA). Naive and memory T-cell subsets were defined by quadrant gating with FMO controls on a CD45RA vs. CCR7 plot. The following populations were identified: naive (CCR7+, CD45RA+), central memory (CCR7+, CD45RA–), effector memory (CCR7+, CD45RA–) and terminally differentiated T cells (CCR7+, CD45RA+). The mean fluorescent intensity (MFI) of PD-1 expression was measured by obtaining the geometric mean for this parameter on total CD4+ and CD8+ T cells and on each of the subsets described above. The frequency of PD-1+ cells was also determined and correlated very strongly with the MFI data (data not shown). Analysis using the frequencies rather than the MFI yielded similar results (Supplemental Figure 1, http://links.lww.com/QAD/A529). Activation was defined as the frequency of CD38+HLA-DR+ cells for both CD4+ and CD8+ T-cell compartments.
Programmed death-1 measurement time points
In the early vs. later treatment study, PD-1 expression was measured during acute/recent infection for all HIV-infected individuals. In those in the early and later ART groups, PD-1 expression was also measured at 1 year following ART initiation and at the last observed time point in the cohort. In the untreated group, PD-1 expression was measured after 1 year of untreated HIV infection and at participants’ last observed untreated time point. For HIV-negative individuals, PD-1 expression was measured at baseline and at a follow-up time point, 12–48 weeks after baseline. For SCOPE participants, PD-1 was measured during chronic infection.
Statistical methods
The Wilcoxon rank-sum test was used to compare PD-1 expression levels between groups. The Wilcoxon signed-rank test was used to compare PD-1 expression at different time points within groups. Spearman rank correlation coefficients were calculated to assess associations between virologic and immunologic measurements. Mixed effects modelling was performed to assess the impact of ART timing, clinical factors and immunologic factors on PD-1 expression. All statistical analyses were performed using STATA/SE 12 (StataCorp, College Station, Texas, USA).
Results
Individual characteristics
A total of 327 individuals was included in this study: 121 from the Options cohort and 206 from the SCOPE cohort (Tables 1 and 2). In most of the subgroups, participants were predominantly men, although women comprised 34% of the controller subset of the SCOPE cohort. SCOPE cohort participants were older than those in Options, with interquartile ages ranging from 42 to 51 years compared with 33 to 42 years, respectively.
Table 1.
Baseline characteristics of participants in the Options cohort.
| Early ART (n = 34) | Later ART (n = ) | Untreated (n = 36) | HIV negative (n = 19) | P | |
|---|---|---|---|---|---|
| Age | 37 (33–42) | 36 (34–42.5) | 33 (26.5–36.5) | 37 (30–42) | 0.87 |
| Male | 33 (97%) | 32 (100%) | 35 (97%) | 17 (89%) | 0.33 |
| HIV duration at diagnosis, months | 2.4 (1.0–2.4) | 2.4 (2.4–3.7) | 2.4 (2.4–3.5) | – | <0.01 |
| Baseline CD4+ cell count (cells/µl) | 533 (434–742) | 562 (499–704) | 581 (512–680) | – | 0.61 |
| Baseline log VL (copies/ml) | 5.2 (4.5–5.7) | 4.2 (3.5–4.7) | 4.2 (3.3–5.0) | – | <0.01 |
| Baseline CD4+ PD-1, MFI | 134 (116–179) | 118 (104–137) | 130 (96–150) | 125 (98–153) | 0.02 |
| Baseline CD8+ PD-1, MFI | 206 (160–278) | 162 (129–245) | 182 (135–223) | 115 (94–135) | 0.13 |
| Baseline CD4+38+DR+ | 6.5% (5.2–10.8) | 6.3% (5.2–8.6) | 6.3 (5.3–10.5) | 3.8% (2.8–7.4) | 0.92 |
| Baseline CD8+38+DR+ | 52.1% (35.5–70.5) | 45.8 (33.0–57.3) | 46.1% (36.5–57.7) | 15.7% (9.1–24.2) | 0.29 |
| Immediate pre-ART CD4+ cell count (cells/µl) | 526 (375–748) | 298 (244–379) | – | – | <0.01 |
| Immediate pre-ART CD4+ PD-1, MFI | 134 (116–179) | 146 (119–183) | – | – | 0.44 |
| Immediate pre-ART CD8+ PD-1, MFI | 206 (160–278) | 229 (192 – 293) | – | – | 0.32 |
Table 2.
Baseline characteristics of participants in the SCOPE cohort.
| HIV negative (n = 24) | Controllers (n = 47) | Untreated (n = 18) | ART suppressed (n = 117) | |
|---|---|---|---|---|
| Age | 44 (34–46) | 50 (46–54) | 43 (39–47) | 50 (46–54) |
| Male | 23 (96) | 31 (66) | 17 (94) | 100 (85) |
| CD4+ cell count (cells/µl) | 863 (745–1198) | 719 (572–1013) | 388 (303–489) | 532 (346–690) |
| VL (copies/ml) | – | 40 (40–73) | 41243 (30901–125279) | 59 (40–75) |
Data are medians (IQR) or numbers (%). Baseline refers to the first time point upon entry into the cohort during acute/early infection. Immediate pre-ART refers to the time point just prior to ART initiation. For those who started ART during early/acute infection, the baseline and pre-ART time point are the same. P values are for pairwise comparisons between Early ART group and Later ART group using a Wilcoxon Rank Sum Test. ART, antiretroviral therapy; IQR, interquartile range; MFI, mean fluorescent intensity; PD-1, programmed death-1; VL, plasma HIV viral load.
In the Options Cohort, members of both the early and later ART groups were estimated to have acquired HIVa median 2.4 months prior to cohort enrolment. The median time to ART initiation after estimated infection in the later ART group was 38.2 months (interquartile range, IQR 30.4–54.6) compared with 2.6 months (IQR 1.5–3.2) in the early ART group. Median CD4+ T-cell counts and ages were similar between the two groups at baseline. However, the baseline median plasma HIV RNA level and PD-1 expression in both CD4+ and CD8+ T cells was higher in the early ART group than in the later ART group. It may be that factors such as viral load affected the decision of the patient and their provider to treat earlier.
Impact of acute infection on programmed death-1 expression
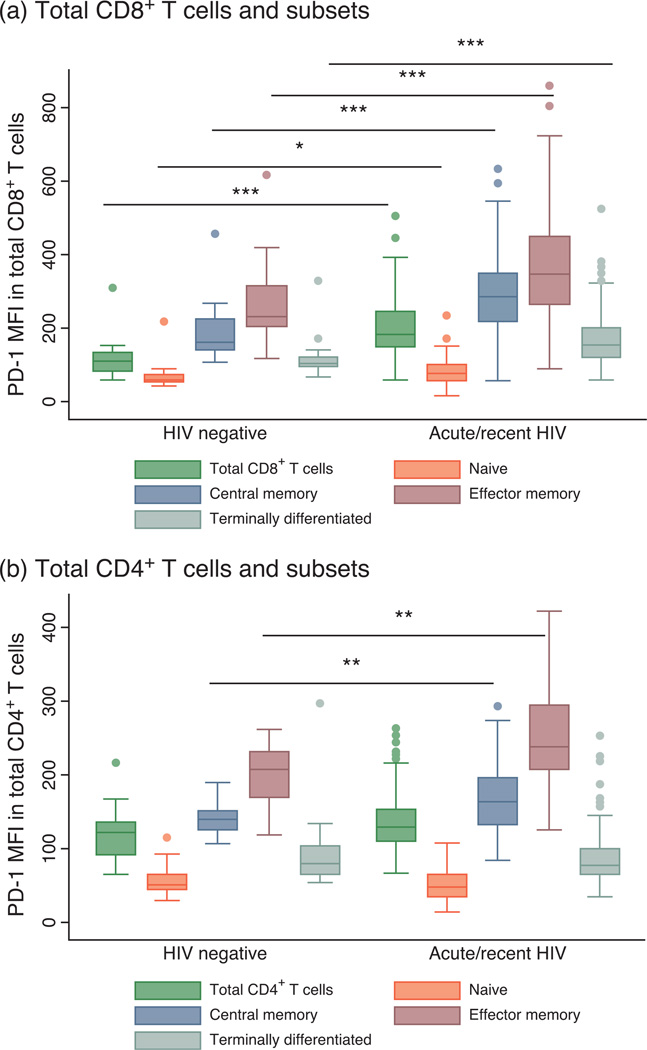
A total of 102 individuals was studied during acute/recent infection. The median MFI of PD-1 expression on total CD8+ T cells and all CD8+ subsets in patients during early HIV infection was substantially higher than HIV-negative individuals (total CD8+ T cells: 184 vs. 115, P < 0.001 for all subsets except naive T cells P = 0.01) (Fig. 1a). In contrast, the MFI of PD-1 on total CD4+ T cells in recently infected individuals was not significantly different than the HIV-uninfected group. PD-1 levels were, however, significantly higher in the central and effector memory CD4+ T-cell subsets of those with acute HIV infection than of those who were not infected (central memory: 164 vs. 140, P = 0.007, effector memory: 238 vs. 206, P = 0.003) (Fig. 1b).
Fig. 1. Programmed death-1 expression levels in total CD8+ and CD4+ T cells and naive and memory subsets in individuals with acute/recent HIV infection compared with HIV-negative individuals.
Box plots show median proportions (line), IQR (box), adjacent values (1.5× IQR; whiskers) and outside values (dots). (a) The median MFI of PD-1 expression on total CD8+ T cells and all CD8+ subsets in patients during early HIV infection was significantly higher than HIV-negative individuals. (b) In CD4+ T cells, PD-1 levels were only significantly higher in the central and effector memory CD4+ T-cell subsets of those with acute/recent HIV infection than in HIV-negative individuals. *P<0.05, **P<0.01, ***P< 0.001 for pairwise comparisons.
During early untreated HIV infection, viral load was only modestly associated with PD-1 levels on total CD8+ T cells (ρ = 0.27, P = 0.006) and was not significantly associated with PD-1 expression on CD4+ T cells (ρ = 0.14, P = 0.15). There was also no association between CD4+ cell count and PD-1 expression on total CD4+ or CD8+ T cells. However, T-cell activation was strongly associated with PD-1 expression on both CD4+ and CD8+ T cells (CD8+: ρ = 0.59, P<0.001, CD4+: ρ = 0.43, P < 0.001). Furthermore, after controlling for age, CD4+ T-cell count, plasma viral load and T-cell activation, the percentage of CD38+HLA-DR+ T cells within each population (CD4+ or CD8+) was the only factor significantly associated with PD-1 expression on the total CD4+ and CD8+ T-cell populations during early infection (P < 0.001).
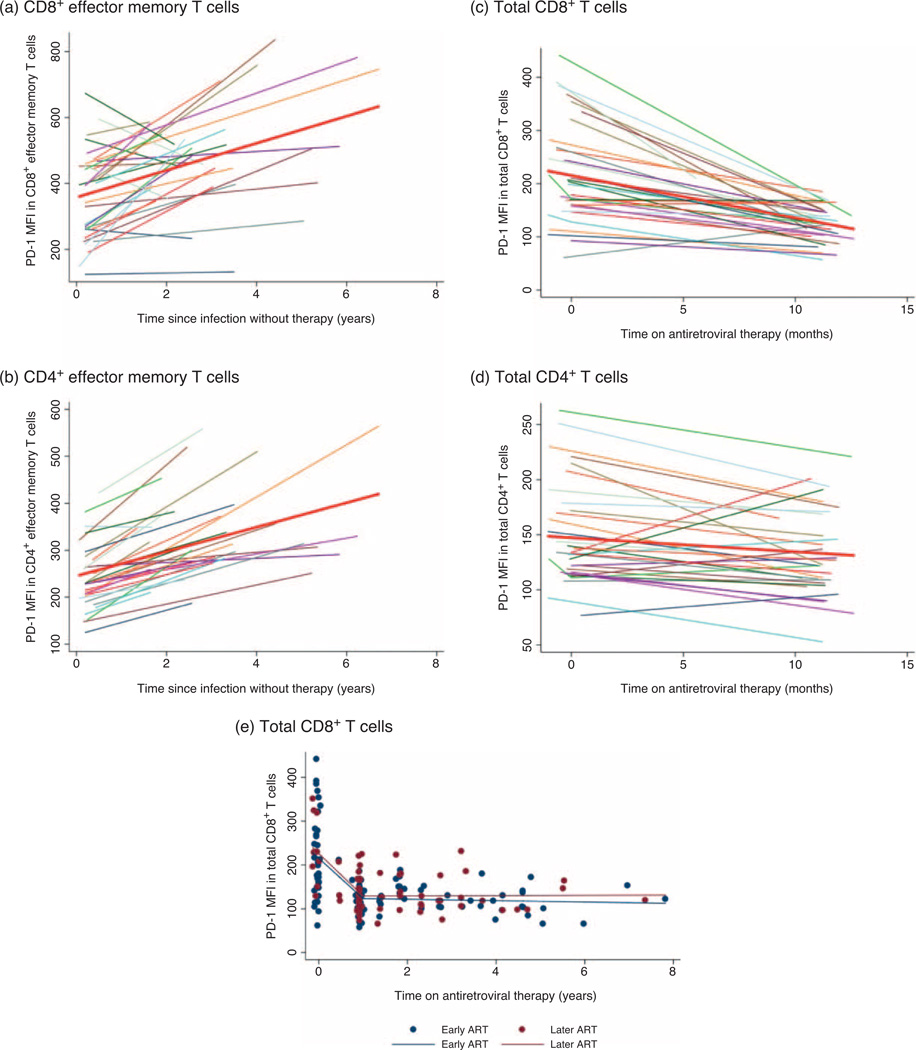
We next examined the natural history of PD-1 expression prior to initiation of therapy among those presenting with recent infection. In those who delayed therapy, PD-1 expression on both total CD4+ and CD8+ T cells increased over time. PD-1 expression also increased over time in the T-cell subsets; however, it was most pronounced in the effector memory subset in both populations (Fig. 2a and 2b). In CD8+ and CD4+ effector memory T-cell subsets, the PD-1 MFI increased by an average of 10 and 11%, respectively, every year without therapy.
Fig. 2. Programmed death-1 expression levels prior to and after antiretroviral therapy initiation.
PD-1 expression in (a) CD8+ and (b) CD4+ effector memory T cells in the later ART group prior to initiating ART. Values from baseline (acute/recent infection) until the last pretherapy time point are shown. A linear regression line is shown in red. (c) PD-1 expression levels on total CD8+ T cells and (d) total CD4+ T cells after ART initiation. Values from baseline (acute/recent infection) until 1 year following ART initiation are shown and a linear regression line is shown in red. (e) Scatter plot of PD-1 expression on total CD8+ T cells in the early (blue dots) and later (red dots) ART groups. Values from baseline (acute/recent infection), 1 year following ART initiation and last observed time point in the cohort, are shown for both groups. The change in PD-1 expression is modelled with a linear spline for the early (blue solid line) and later ART groups (red solid line). PD-1 levels decrease rapidly over the first 12 months on ART. After 1 year of ART, levels are relatively stable and similar between the early and later ART groups.
Impact of early vs. later antiretroviral therapy on programmed death-1 expression
Participants were observed for a median 2.8 years on therapy in the early ART group and a median of 2.3 years on therapy in the later ART group. Baseline PD-1 levels were higher in the early ART group than in the later ART group, but were similar by the time of ART initiation (Table 1).
In the early ART group, PD-1 expression on total CD8+ T cells decreased by 41% after 1 year of ART [95% confidence interval (CI) –49 to –34, P < 0.001, Fig. 2c]. A similar decrease was also seen in the later ART group (data not shown). In contrast, although statistically significant, the decline in PD-1 expression on total CD4+ T cells in both groups was smaller (Fig. 2d). The decline of PD-1 expression on both CD4+ and CD8+ T cells appeared to be nonlinear with a rapid decline in PD-1 expression over the first 12 months on ART and relatively stable levels after 1 year of ART (Fig. 2e).
The median PD-1 expression on total CD8+ T cells in the early and later ART groups was similar after 1 year of therapy (125 vs. 128, P = 0.55) and at final observed time point (Fig. 2e). Similarly, there was no significant difference in PD-1 expression on total CD4+ T cells between the two groups at either time point. Among both early and later ART groups, PD-1 expression on total CD4+ and CD8+ T cells after several years of therapy was comparable to levels seen in the HIV-uninfected controls. However, median PD-1 MFI remained elevated on the CD4+ effector memory T cells, even in the early ART group (234 vs. 206, P = 0.007).
Mixed effects modelling was used to assess determinants of PD-1 expression in the later ART group as individuals transitioned from untreated to ART-suppressed. The CD4+ T-cell count continued to predict PD-1 expression on total CD4+ T cells even after accounting for viral load and duration of HIV infection. When CD4+ T-cell activation was added to the model, the effect of CD4+ cell count was attenuated (CD4+ cell count, model without activation: P< 0.001, model with activation: P = 0.07) and T cell activation was significant (P < 0.001). In CD8+ T cells, both viral load and CD8+ T-cell activation were statistically significant predictors of PD-1 expression (both P<0.001).
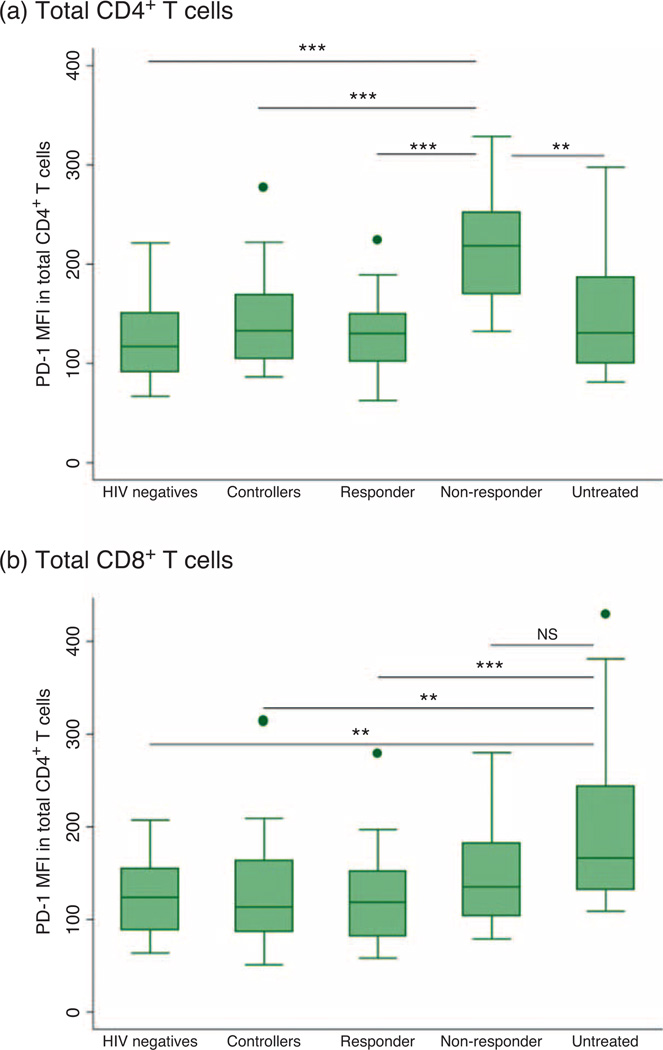
Programmed death-1 expression in immunologic nonresponders
In the SCOPE cohort, we compared PD-1 expression in the immunologic responders with immunologic non-responders. Nonresponders had significantly higher levels of PD-1 than responders on total CD4+ T cells (218 vs. 130, P < 0.001) (Fig. 3a). This difference was statistically significant in all CD4+ memory subsets. Nonresponders had elevated PD-1 expression on total CD8+ T cells (199 vs. 135, P = 0.15); however, this was not statistically significant and was not seen in the CD8+ T-cell subsets. There was a strong association between PD-1 expression on CD4+ T cells and T-cell activation in the responders (ρ = 0.45, P = 0.002), but this relationship was absent in nonresponders (ρ = 0.07, P = 0.80).
Fig. 3. Programmed death-1 expression in total CD4+ and CD8+ T cells within the five individual groups in the SCOPE cohort: HIV-negative individuals, controllers (including both elite and viremic controllers), immunologic responders, immunologic nonresponders and chronically untreated individuals.
Box plots show median proportions (line), IQR (box), adjacent values (1.5x IQR; whiskers) and outside values (dots). (a) PD-1 expression on total CD8+ T cells was highest among chronically untreated individuals. (b) PD-1 expression on total CD4+ T cells was highest among the immunologic nonresponders. *P<0.05, **P<0.01, ***P<0.001, NS – nonsignificant (P = 0.053).
Programmed death-1 expression in HIV controllers
PD-1 expression was assessed in 47 long-term untreated controllers, including 37 ‘elite controllers’ (plasma viral load <75 copies/ml) and 10 ‘viremic controllers’ (median viral load 146 copies/ml, IQR 119–364). Overall, controllers had levels of PD-1 expression on both total CD4+ and CD8+ T cells that were similar to those found in HIV-uninfected individuals. PD-1 expression on total CD4+ T cells in elite controllers was also comparable to that of viremic controllers. However, there was a trend towards increased PD-1 expression on total CD8+ T cells in viremic controllers compared with elite controllers, which was statistically significant in the effector memory subset (291 vs. 186, P = 0.01). There was a strong association between PD-1 expression in all controllers and T-cell activation on total CD4+ T cells (ρ = 0.41, P = 0.005). No correlation was observed between CD8+ T-cell activation and PD-1 expression on CD8+ T cells in the controllers.
Comparison of SCOPE cohort participants
On total CD4+ T cells, the MFI of PD-1 expression was similar amongst HIV-negative individuals, controllers, ART-suppressed immunologic responders and untreated individuals. However, PD-1 expression was highest amongst those participants who were ART-suppressed but were immunologic nonresponders (Fig. 3a). In contrast, on CD8+ T cells, untreated individuals had markedly elevated PD-1 expression compared with all other groups (Fig. 3b).
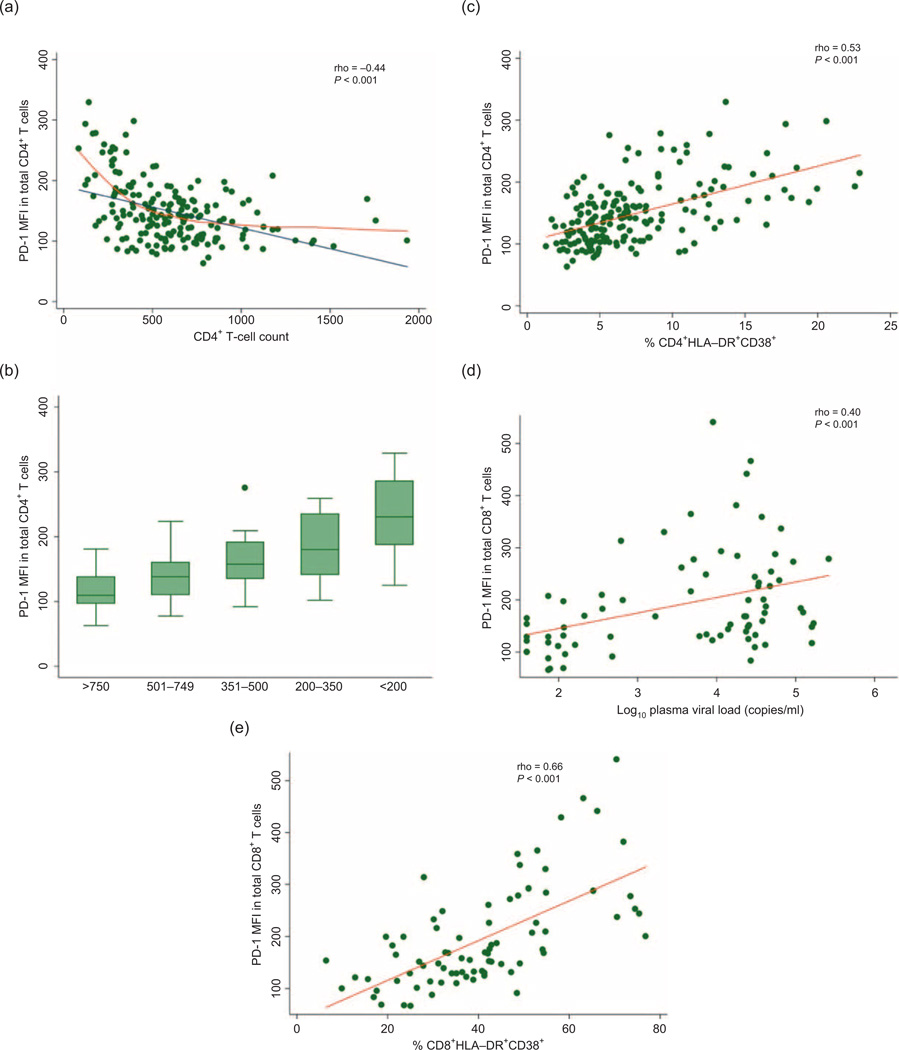
Across all HIV-infected participants in SCOPE, PD-1 expression on CD4+ T cells was significantly inversely associated with CD4+ cell count (ρ = –0.44, P < 0.001); however, the relationship was not linear (Fig. 4a). This inverse relationship persisted even at ‘normal’ CD4+ cell counts (Fig. 4b). CD4+ T-cell activation was also strongly associated with PD-1 expression on CD4+ T cells (ρ = 0.53, P<0.001, Fig. 4c). The CD4+ cell count nadir was not associated with PD-1 expression. There was no association between CD4+ cell count and PD-1 expression on CD8+ T cells (ρ = –0.04, P = 0.58). Furthermore, there was no association between PD-1 expression on CD4+ or CD8+ T cells and CD4+ cell count in HIV-uninfected individuals. In contrast, PD-1 expression on CD8+ T cells was strongly associated with viral load amongst individuals with detectable viremia (ρ = 0.40, P < 0.001, Fig. 4d) and CD8+ T-cell activation (ρ = 0.66, P < 0.001, Fig. 4e).
Fig. 4. The relationship between programmed death-1 expression on total CD4+ T cells and CD4+ T-cell count amongst all HIV-infected participants in the SCOPE cohort.
(a) PD-1 expression on total CD4+ T cells is significantly inversely associated with CD4+ cell count (ρ = –0.44, P< 0.001). The relationship may not be linear (blue line) as shown by the lowess regression line (orange line). (b) PD-1 expression in ART-suppressed individuals in SCOPE by CD4+ cell count strata. (c) CD4+ T-cell activation is strongly associated with PD-1 expression on CD4+ T cells (ρ = 0.53, P<0.001). (d) PD-1 expression on CD8+ T-cells is associated with plasma viral load amongst individuals with detectable viremia (ρ = 0.40, P<0.001). (e) PD-1 expression on CD8+ T cells is strongly associated with CD8+ T-cell activation (ρ = 0.66, P<0.001).
Discussion
There is currently a strong focus on how PD-1 expression may contribute to an active and potentially reversible T-cell dysfunction during ART. We therefore conducted a longitudinal study of PD-1 expression in a cohort of individuals identified in early HIV infection and a comprehensive cross-sectional analysis of PD-1 expression in a chronically infected cohort of individuals to understand the effect of ART on PD-1 expression and better elucidate the determinants of PD-1 expression. We found that PD-1 expression levels were high during early HIV infection (particularly on CD8+ T cells), increased on all T cells over time in the absence of therapy and rapidly improved after ART initiation. Individuals who delayed therapy to advanced stages and failed to normalize their CD4+ T-cell counts had persistently elevated PD-1 levels on all CD4+ T-cell subsets. Across all individuals, the host and viral factors driving PD-1 expression appeared to differ between the CD8+ and CD4+ T-cell compartments and varied by the stage of HIV infection.
Recent HIV infection resulted in markedly elevated PD-1 expression on total CD8+ T cells and CD8+ memory subsets, as found in previous studies [6,25] and was strongly associated with T-cell activation [26]. Among those with early infection who delayed initiating therapy, both ongoing viremia and T-cell activation were associated with an increased PD-1 expression on CD8+ T cells, a finding that is consistent with prior observations linking viremia with PD-1 on these cells [7,26,27]. Similarly, controllers who had detectable viremia had elevated PD-1 on CD8+ T cells, while ‘elite’ controllers had apparently normal PD-1 expression levels. Treatment-mediated reductions in viremia generally normalized PD-1 levels on CD8+ T cells; this effect was even observed among immunologic nonresponders. The lack of any observed difference in PD-1 expression in those who started ART early compared with those who started later in infection suggests that it is not the cumulative antigen or activation exposure that dictates PD-1 levels, but rather the current level of circulating virus and/or of CD8+ T-cell activation.
Although HIV viremia and CD8+ T-cell activation were the most consistent predictors of PD-1 expression on CD8+ T cells, we found that CD4+ T-cell count and CD4+ T-cell activation were the strongest predictors of PD-1 on CD4+ T cells in most subgroups. During early HIV infection, when immune function is likely less compromised, PD-1 expression on CD4+ T cells was not dramatically different from that observed in uninfected adults. During untreated HIV infection, PD-1 expression on CD4+ T cells increased over time and mirrored the concurrent decline in CD4+ T-cell count. Among those individuals who initiated ART before the onset of advanced immunodeficiency, both CD4+ T-cell counts and PD-1 expression generally returned to normal levels. In contrast, PD-1 expression was highest among those individuals who had successful suppression of viremia with ART but nonetheless failed to regain a normal CD4+ cell count (’immunologic nonresponders’). These individuals had higher PD-1 expression on CD4+ T cells than immune responders, as we, and others previously reported [28,29]. Notably, PD-1 expression on CD4+ T cells of the nonresponders was even higher than that observed in chronically untreated individuals. In an analysis of those participants followed longitudinally after ART initiation, CD4+ T-cell activation was the most significant predictor of PD-1 expression on total CD4+ T cells, suggesting that the same factors that influence these activation markers might influence PD-1. Surprisingly, however, an association between CD4+ T-cell activation and PD-1 expression on CD4+ T cells was not seen in the immune nonresponders, suggesting that there may be unique mechanisms contributing to immune dysfunction in this population.
Although it is difficult in observational studies to determine whether increased PD-1 expression is the cause or the consequence of poor CD4+ cell count recovery, the striking association between CD4+ T-cell activation and PD-1 on these cells suggests that the extensive knowledge gained regarding the mechanism of CD4+ T-cell activation might be informative. Indeed, our findings are generally consistent with a series of studies by Catalfamo et al. who found that CD4+ T-cell depletion caused an increase in homeostatic proliferation, which in turn led to increased CD4+ T-cell proliferation and elevated expression of CD38 and HLA-DR [30,31]. One mechanism for this is via the common γ-chain cytokines such as interleukin (IL)-7 [32,33]. It is also possible that immunodeficiency contributes to high pathogen burden via microbial translocation and/or cytomegalovirus viremia, which may increase CD4+ T -cell activation and PD-1 expression in an antigen-dependent mechanism through engagement of the T-cell receptor [34,35], although the lack of a strong effect of CD4+ T-cell counts on CD8+ T-cell activation and PD-1 makes this pathway less likely. Further studies are needed to define whether PD-1 expression on CD4+ T cells is a cause or a consequence of CD4+ T-cell lymphopenia. These studies may be especially informative in the immunologic nonresponders.
Our study has certain limitations. Our population is predominantly composed of middle-aged men. Further studies should be performed in women and older adults, as there may be effects of sex and age on PD-1 expression. It is also challenging to compare persons with HIV vs. those without HIV, as these groups almost certainly differ in ways that are difficult to control for and analyse. Our use of an at-risk population partially addresses this concern. Also, as noted above, the observational nature of our study makes it challenging to conclude cause and effect. Interventional studies with agents that directly affect the biology of PD-1 are needed to better understand how HIV and the host response interact to influence this important pathway.
In summary, we used a large and diverse population of HIV-infected adults to describe the impact of viremia, immune activation, immunodeficiency and treatment on PD-1 expression in total T cells and individual T-cell subsets. We found that viremia predicted PD-1 expression on CD8+ T cells, although CD4+ T-cell counts predicted PD-1 expression on CD4+ T cells. T-cell activation had the strongest associations with PD-1 expression, suggesting that the pathophysiology of ‘activation’ might be informative for studies on expression of PD-1.
Supplementary Material
Acknowledgements
This work was supported by grants from the National Institute of Allergy and Infectious Disease (K24 AI069994), the American Foundation for AIDS Research (106710-40-RGRL), the Delaney AIDS Research Enterprise (DARE; U19 AI0961090), the UCSF/Gladstone Institute of Virology & Immunology CFAR (P30 AI027763), the National Center for Advancing Transla-tional Sciences, National Institutes of Health, through UCSF’s Clinical and Translational Science Institute (KL2 TR000143, UL1 RR024131), the Center for AIDS Prevention Studies (P30 MH62246) and the CFAR Network of Integrated Systems (R24 AI067039).
The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Footnotes
L.R.C., V.J., S.G.D., F.M.H., J.M.M. conceived and designed the study. E.S. performed the experiments. L.R.C., V.J., W.H., D.G. analysed the data. J.N.M., C.D.P., F.M.H., S.G.D., H.H., P.W.H., R.S. contributed materials/analysis tools. L.R.C., S.G.D. wrote the article.
This study was presented in part at the 21st Conference on Retroviruses and Opportunistic Infections, Boston, Massachusetts, USA, 3–6 March 2014 (Abstract 283).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Battegay M, Nuesch R, Hirschel B, Kaufmann GR. Immunological recovery and antiretroviral therapy in HIV-1 infection. Lancet Infect Dis. 2006;6:280–287. doi: 10.1016/S1473-3099(06)70463-7. [DOI] [PubMed] [Google Scholar]
- 2.Kaufmann GR, Furrer H, Ledergerber B, Perrin L, Opravil M, Vernazza P, et al. Characteristics, determinants, and clinical relevance of CD4 T cell recovery to <500 cells/microL in HIV type 1-infected individuals receiving potent antiretroviral therapy. Clin Infect Dis. 2005;41:361–372. doi: 10.1086/431484. [DOI] [PubMed] [Google Scholar]
- 3.Hunt PW, Martin JN, Sinclair E, Bredt B, Hagos E, Lampiris H, et al. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–1543. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- 4.Cockerham L, Scherzer R, Zolopa A, Rimland D, Lewis CE, Bacchetti P, et al. Association of HIV infection, demographic and cardiovascular risk factors with all-cause mortality in the recent HAART era. J Acquir Immune Defic Syndr. 2010;53:102–106. doi: 10.1097/QAI.0b013e3181b79d22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lodwick RK, Sabin CA, Porter K, Ledergerber B, van Sighem A, Cozzi-Lepri A, et al. Death rates in HIV-positive antiretroviral-naive patients with CD4 count greater than 350 cells per microL in Europe and North America: a pooled cohort observational study. Lancet. 2010;376:340–345. doi: 10.1016/S0140-6736(10)60932-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 7.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 8.D’Souza M, Fontenot AP, Mack DG, Lozupone C, Dillon S, Meditz A, et al. Programmed death 1 expression on HIV-specific CD4+ T cells is driven by viral replication and associated with T cell dysfunction. J Immunol. 2007;179:1979–1987. doi: 10.4049/jimmunol.179.3.1979. [DOI] [PubMed] [Google Scholar]
- 9.Petrovas C, Price DA, Mattapallil J, Ambrozak DR, Geldmacher C, Cecchinato V, et al. SIV-specific CD8+ T cells express high levels of PD1 and cytokines but have impaired proliferative capacity in acute and chronic SIVmac251 infection. Blood. 2007;110:928–936. doi: 10.1182/blood-2007-01-069112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dyavar Shetty R, Velu V, Titanji K, Bosinger SE, Freeman GJ, Silvestri G, et al. PD-1 blockade during chronic SIV infection reduces hyperimmune activation and microbial translocation in rhesus macaques. J Clin Invest. 2012;122:1712–1716. doi: 10.1172/JCI60612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Breton G, Chomont N, Takata H, Fromentin R, Ahlers J, Filali-Mouhim A, et al. Programmed death-1 is a marker for abnormal distribution of naive/memory T cell subsets in HIV-1 infection. J Immunol. 2013;191:2194–2204. doi: 10.4049/jimmunol.1200646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porichis F, Kwon DS, Zupkosky J, Tighe DP, McMullen A, Brockman MA, et al. Responsiveness of HIV-specific CD4 T cells to PD-1 blockade. Blood. 2011;118:965–974. doi: 10.1182/blood-2010-12-328070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amancha PK, Hong JJ, Rogers K, Ansari AA, Villinger F. In vivo blockade of the programmed cell death-1 pathway using soluble recombinant PD-1-Fc enhances CD4+ and CD8+ T cell responses but has limited clinical benefit. J Immunol. 2013;191:6060–6070. doi: 10.4049/jimmunol.1302044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seung E, Dudek TE, Allen TM, Freeman GJ, Luster AD, Tager AM. PD-1 blockade in chronically HIV-1-infected humanized mice suppresses viral loads. PLoS One. 2013;8:e77780. doi: 10.1371/journal.pone.0077780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458:206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chomont N, El-Far M, Ancuta P, Trautmann L, Procopio FA, Yassine-Diab B, et al. HIV reservoir size and persistence are driven by T cell survival and homeostatic proliferation. Nat Med. 2009;15:893–900. doi: 10.1038/nm.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatano H, Jain V, Hunt PW, Lee TH, Sinclair E, Do TD, et al. Cell-based measures of viral persistence are associated with immune activation and programmed cell death protein 1 (PD-1)-expressing CD4+ T cells. Infect Dis. 2013;208:50–56. doi: 10.1093/infdis/jis630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spitsin S, Tustin NB, Riedel E, Tustin R3rd, Murray JB, Peck LM, et al. Programmed death 1 receptor changes ex vivo in HIV-infected adults following initiation of highly active antiretro-viral therapy. Clin Vacc Immunol. 2012;19:752–756. doi: 10.1128/CVI.00093-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grabmeier-Pfistershammer K, Steinberger P, Rieger A, Leitner J, Kohrgruber N. Identification of PD-1 as a unique marker for failing immune reconstitution in HIV-1-infected patients on treatment. J Acquir Immune Defic Syndr. 2011;56:118–124. doi: 10.1097/QAI.0b013e3181fbab9f. [DOI] [PubMed] [Google Scholar]
- 20.Piconi S, Trabattoni D, Gori A, Parisotto S, Magni C, Meraviglia P, et al. Immune activation, apoptosis, and Treg activity are associated with persistently reduced CD4+ T-cell counts during antiretroviral therapy. AIDS. 2010;24:1991–2000. doi: 10.1097/QAD.0b013e32833c93ce. [DOI] [PubMed] [Google Scholar]
- 21.Conrad JA, Ramalingam RK, Duncan CB, Smith RM, Wei J, Barnett L, et al. Antiretroviral therapy reduces the magnitude and T cell receptor repertoire diversity of HIV-specific T cell responses without changing T cell clonotype dominance. J Virol. 2012;86:4213–4221. doi: 10.1128/JVI.06000-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakanjako D, Ssewanyana I, Mayanja-Kizza H, Kiragga A, Colebunders R, Manabe YC, et al. High T-cell immune activation and immune exhaustion among individuals with suboptimal CD4 recovery after 4 years of antiretroviral therapy in an African cohort. BMC Infect Dis. 2011;11:43. doi: 10.1186/1471-2334-11-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jain V, Hartogensis W, Bacchetti P, Hunt PW, Hatano H, Sinclair E, et al. Antiretroviral therapy initiated within 6 months of HIV infection is associated with lower T-cell activation and smaller HIV reservoir size. J Infect Dis. 2013;208:1202–1211. doi: 10.1093/infdis/jit311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, et al. T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J Infect Dis. 2011;203:452–463. doi: 10.1093/infdis/jiq071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breton G, Chomont N, Takata H, Fromentin R, Ahlers J, Filali-Mouhim A, et al. Programmed death-1 is a marker for abnormal distribution of naive/memory T cell subsets in HIV-1 infection. J Immunol. 2013;191:2194–2204. doi: 10.4049/jimmunol.1200646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sauce D, Almeida JR, Larsen M, Haro L, Autran B, Freeman GJ, et al. PD-1 expression on human CD8 T cells depends on both state of differentiation and activation status. AIDS. 2007;21:2005–2013. doi: 10.1097/QAD.0b013e3282eee548. [DOI] [PubMed] [Google Scholar]
- 27.Zhang JY, Zhang Z, Wang X, Fu JL, Yao J, Jiao Y, et al. PD-1 up-regulation is correlated with HIV-specific memory CD8+ T-cell exhaustion in typical progressors but not in long-term nonprogressors. Blood. 2007;109:4671–4678. doi: 10.1182/blood-2006-09-044826. [DOI] [PubMed] [Google Scholar]
- 28.Grabmeier-Pfistershammer K, Steinberger P, Rieger A, Leitner J, Kohrgruber N. Identification of PD-1 as a unique marker for failing immune reconstitution in HIV-1-infected patients on treatment. J Acquir Immune Defic Syndr. 2011;56:118–124. doi: 10.1097/QAI.0b013e3181fbab9f. [DOI] [PubMed] [Google Scholar]
- 29.Hatano H, Jain V, Hunt PW, Lee TH, Sinclair E, Do TD, et al. Cell-based measures of viral persistence are associated with immune activation and programmed cell death protein 1 (PD-1)-expressing CD4+ T cells. J Infect Dis. 2013;208:50–56. doi: 10.1093/infdis/jis630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Catalfamo M, Di Mascio M, Hu Z, Srinivasula S, Thaker V, Adelsberger J, et al. HIV infection-associated immune activation occurs by two distinct pathways that differentially affect CD4 and CD8 T cells. Proc Natl Acad Sci U S A. 2008;105:19851–19856. doi: 10.1073/pnas.0810032105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Catalfamo M, Wilhelm C, Tcheung L, Proschan M, Friesen T, Park JH, et al. CD4 and CD8 T cell immune activation during chronic HIV infection: roles of homeostasis, HIV, type I IFN, and IL-7. J Immunol. 2011;186:2106–2116. doi: 10.4049/jimmunol.1002000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Napolitano LA, Grant RM, Deeks SG, Schmidt D, De Rosa SC, Herzenberg LA, et al. Increased production of IL-7 accompanies HIV-1-mediated T-cell depletion: implications for T-cell homeostasis. Nat Med. 2001;7:73–79. doi: 10.1038/83381. [DOI] [PubMed] [Google Scholar]
- 33.Kinter AL, Godbout EJ, McNally JP, Sereti I, Roby GA, O’Shea MA, et al. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J Immunol. 2008;181:6738–6746. doi: 10.4049/jimmunol.181.10.6738. [DOI] [PubMed] [Google Scholar]
- 34.Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med. 2006;12:1365–1371. doi: 10.1038/nm1511. [DOI] [PubMed] [Google Scholar]
- 35.Naeger DM, Martin JN, Sinclair E, Hunt PW, Bangsberg DR, Hecht F, et al. Cytomegalovirus-specific T cells persist at very high levels during long-term antiretroviral treatment of HIV disease. PLoS One. 2010;5:e8886. doi: 10.1371/journal.pone.0008886. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.