Abstract
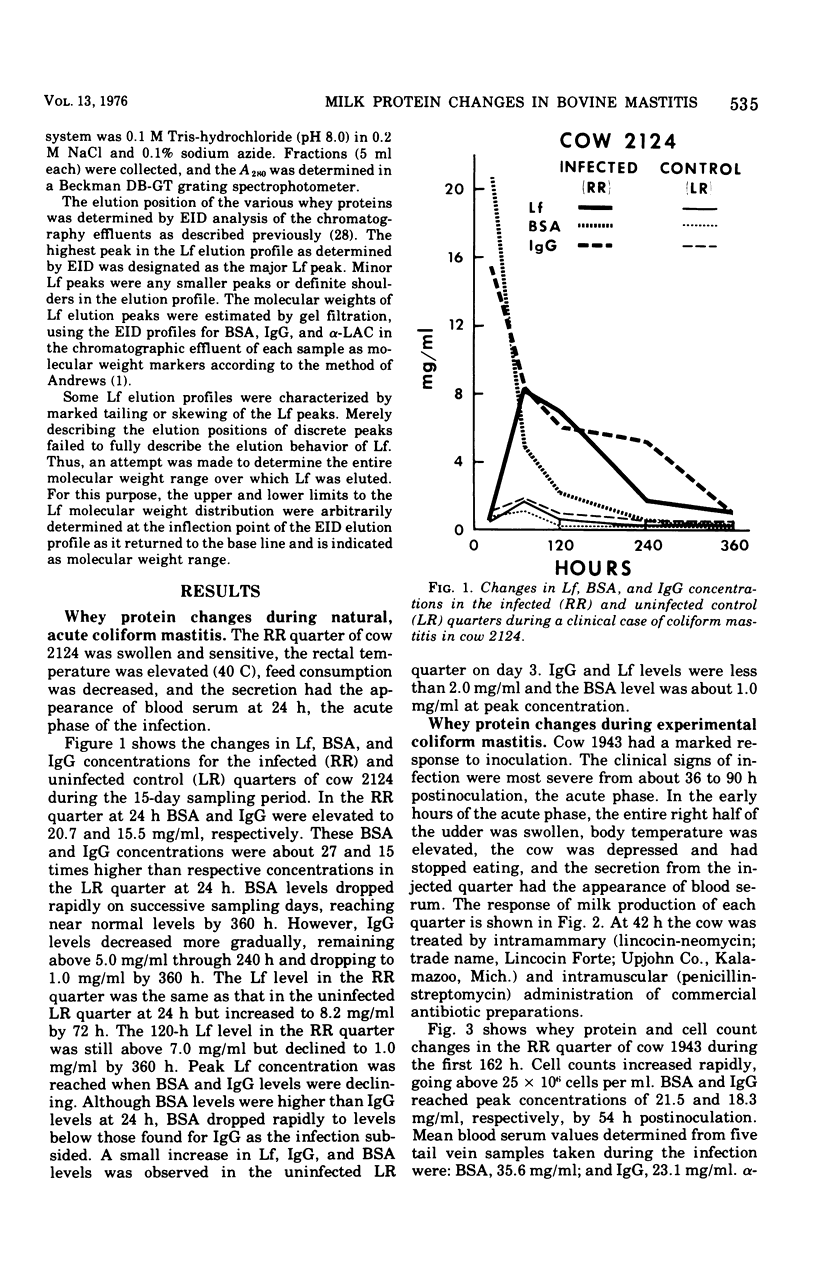
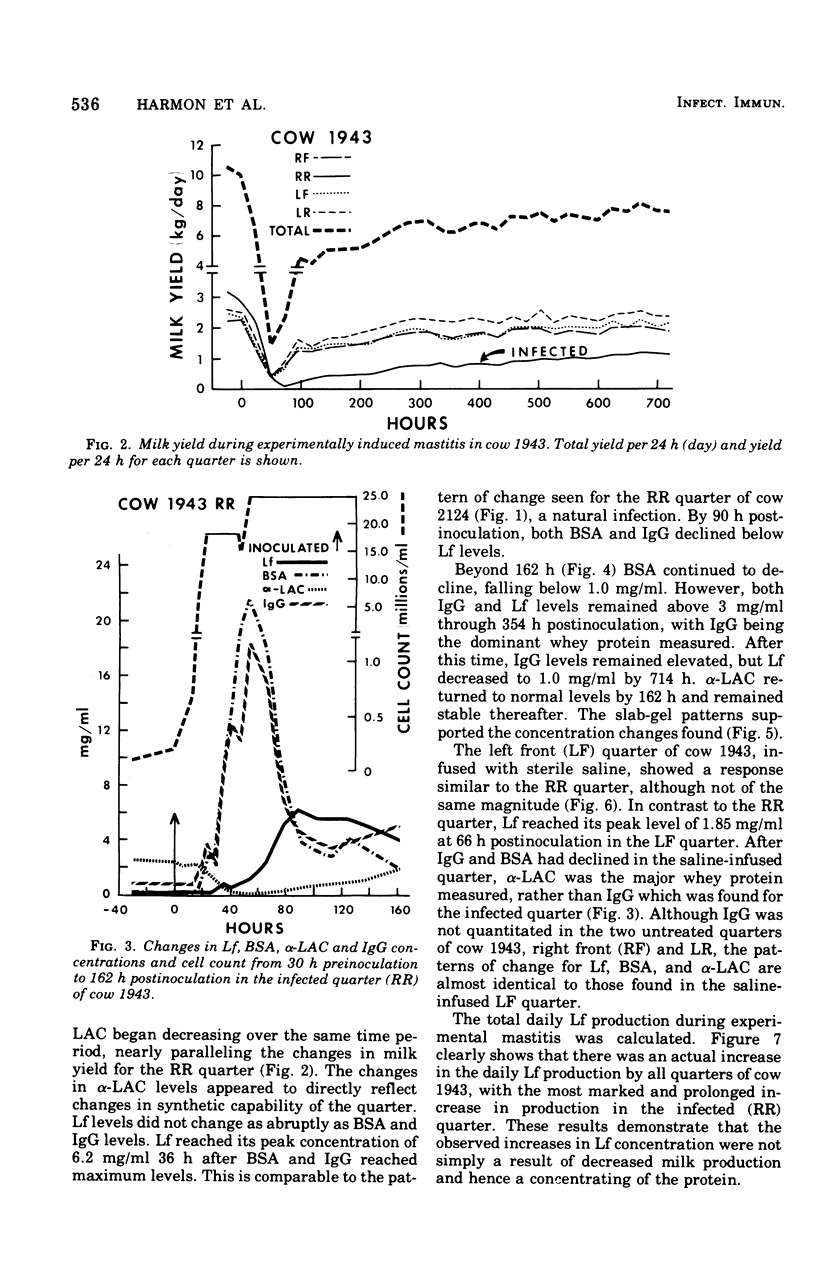
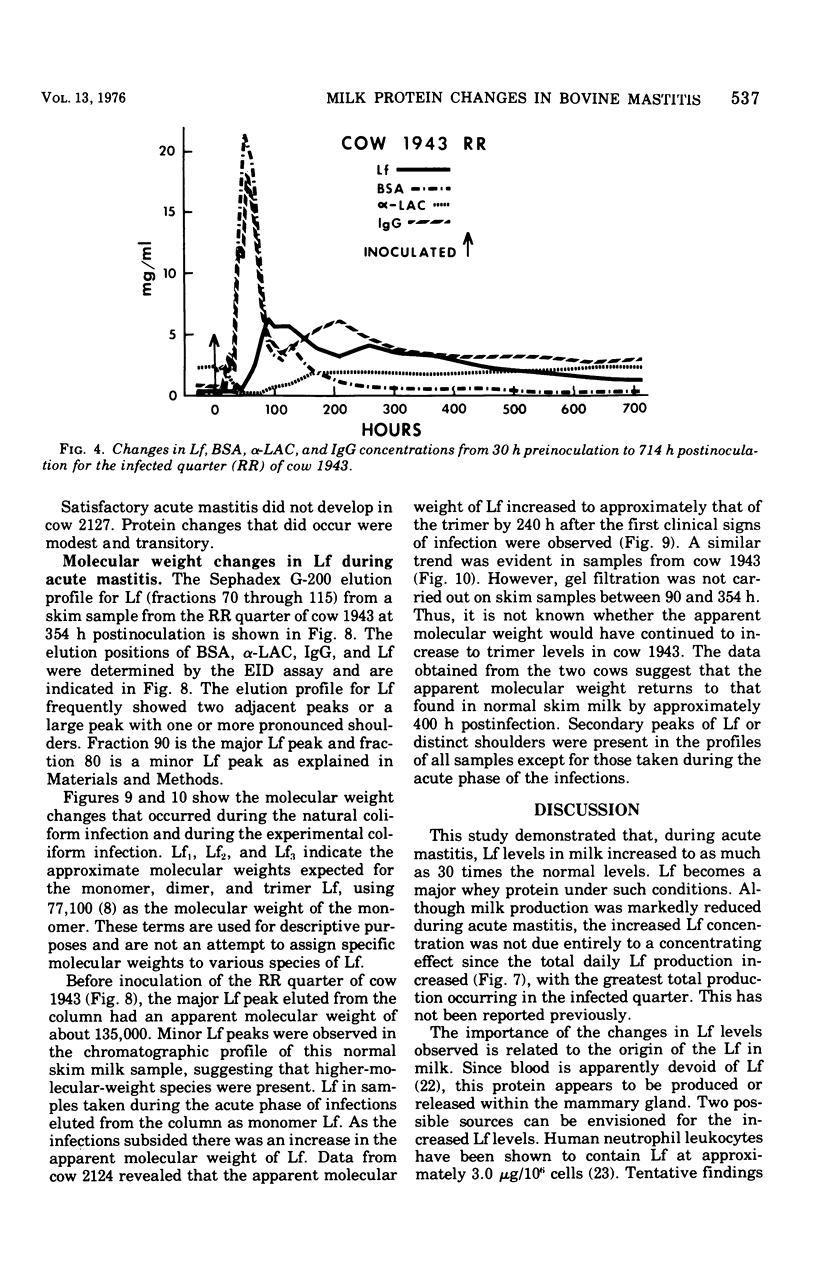
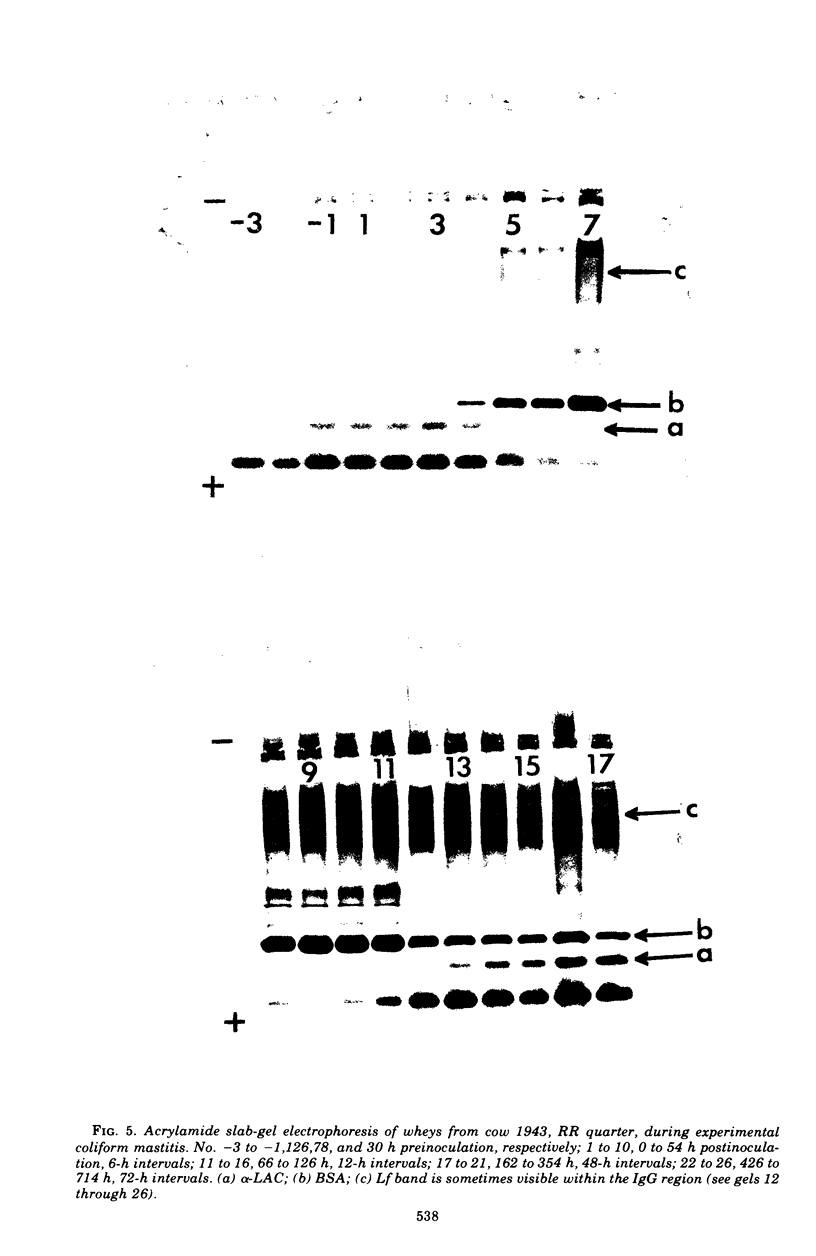
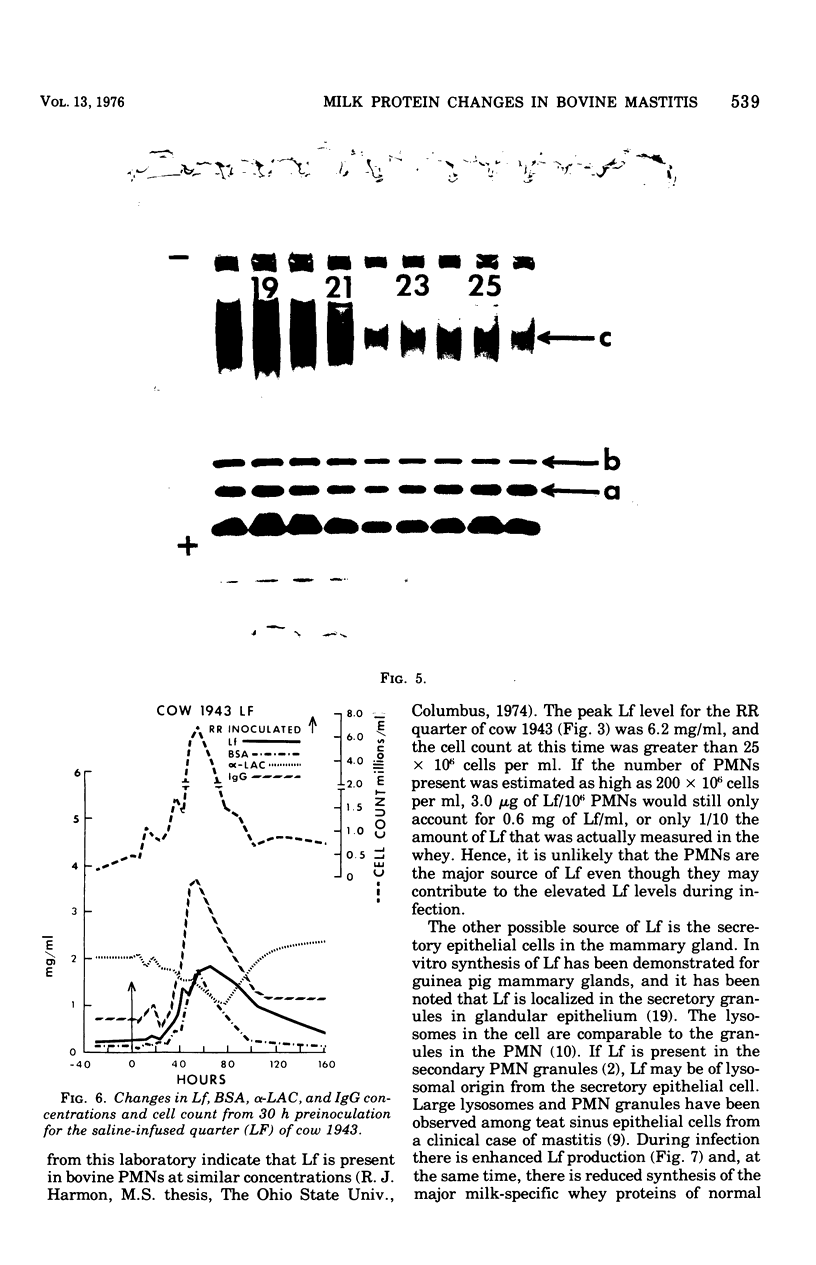
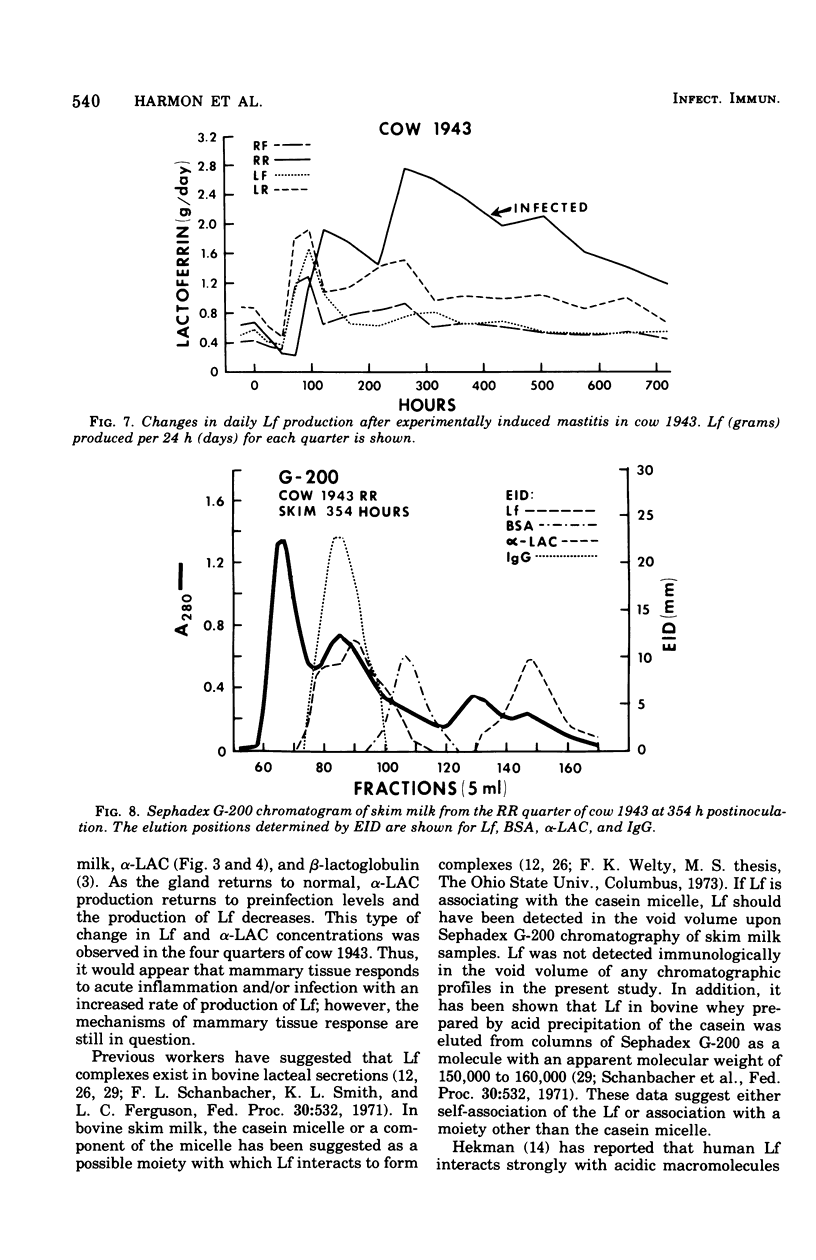
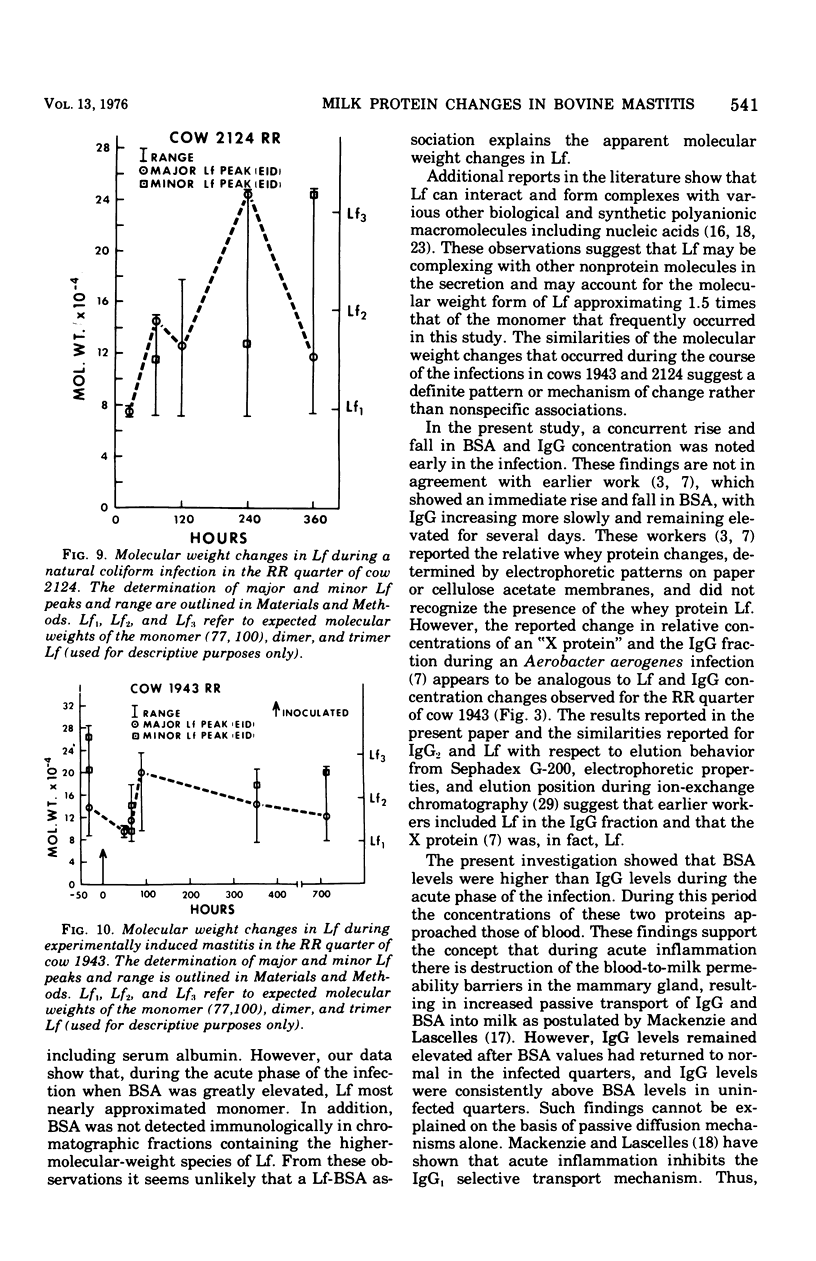
An experimentally induced Escherichia coli infection of a bovine mammary gland resulted in a 30-fold increase in lactoferrin (Lf) concentration in the mammary secretion by 90 h postinoculation and a 4-fold increase in total daily production of Lf by 264 h postinoculation in the infected quarter. A simultaneous rise and fall of bovine serum albumin (BSA) and immunoglobulin G (IgG) concentrations occurred during the acute phase of the infection. Peak BSA and IgG levels were reached 36 h before peak Lf levels. BSA concentrations declined rapidly after the acute phase, whereas IgG and Lf levels remained elevated and decreased slowly as the infection subsided. A decline in alpha-lactalbumin concentration by 48 h postinoculation indicated decreased synthetic capability. The increased Lf production may be a result of a specific response of secretory tissue to inflammatory agents and thus the infectious process. Analogous changes in Lf, IgG, and BSA were observed during a natural coliform infection. Sephadex G-200 chromatography of mastitis skim milk showed that Lf approximated the monomer (molecular weight 77,100) early in infections progressed and abated, the apparent molecular weight of Lf increased to approximately that of the trimer and subsequently decreased to about 1.5 times that of the monomer.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baggiolini M., De Duve C., Masson P. L., Heremans J. F. Association of lactoferrin with specific granules in rabbit heterophil leukocytes. J Exp Med. 1970 Mar 1;131(3):559–570. doi: 10.1084/jem.131.3.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J., Leigh L. Iron-binding proteins in milk and resistance to Escherichia coli infection in infants. Br Med J. 1972 Jan 8;1(5792):69–75. doi: 10.1136/bmj.1.5792.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler J. E., Maxwell C. F., Pierce C. S., Hylton M. B., Asofsky R., Kiddy C. A. Studies on the relative synthesis and distribution of IgA and IgG1 in various tissues and body fluids of the cow. J Immunol. 1972 Jul;109(1):38–46. [PubMed] [Google Scholar]
- Castellino F. J., Fish W. W., Mann K. G. Structural studies on bovine lactoferrin. J Biol Chem. 1970 Sep 10;245(17):4269–4275. [PubMed] [Google Scholar]
- Chandler R. L., Reid I. M. Ultrastructural and associated observations on clinical cases of mastitis in cattle. J Comp Pathol. 1973 Apr;83(2):233–241. doi: 10.1016/0021-9975(73)90047-9. [DOI] [PubMed] [Google Scholar]
- DE DUVE C. The lysosome. Sci Am. 1963 May;208:64–72. doi: 10.1038/scientificamerican0563-64. [DOI] [PubMed] [Google Scholar]
- GROVES M. L. PREPARATION OF SOME IRON-BINDING PROTEINS AND ALPHA-LACTALBUMIN FROM BOVINE MILK. Biochim Biophys Acta. 1965 Apr 12;100:154–162. doi: 10.1016/0304-4165(65)90438-1. [DOI] [PubMed] [Google Scholar]
- Harmon R. J., Schanbacher F. L., Ferguson L. C., Smith K. L. Concentration of lactoferrin in milk of normal lactating cows and changes occurring during mastitis. Am J Vet Res. 1975 Jul;36(7):1001–1007. [PubMed] [Google Scholar]
- Hekman A. Association of lactoferrin with other proteins, as demonstrated by changes in electrophoretic mobility. Biochim Biophys Acta. 1971 Dec 28;251(3):380–387. doi: 10.1016/0005-2795(71)90126-7. [DOI] [PubMed] [Google Scholar]
- Kirkpatrick C. H., Green I., Rich R. R., Schade A. L. Inhibition of growth of Candida albicans by iron-unsaturated lactoferrin: relation to host-defense mechanisms in chronic mucocutaneous candidiasis. J Infect Dis. 1971 Dec;124(6):539–544. doi: 10.1093/infdis/124.6.539. [DOI] [PubMed] [Google Scholar]
- Loisillier F., Burtin P., Grabar P. Isolement et caractérisation de l'auto-antigène responsable de la formation d'auto-anticorps chez les malades atteints de lésions mammaires (cancéruses ou non) Ann Inst Pasteur (Paris) 1968 Nov;115(5):829–840. [PubMed] [Google Scholar]
- Mackenzie D. D., Lascelles A. K. The transfer of [131-I]-labelled immunoglobulins and serum albumin from blood into milk of lactating ewes. Aust J Exp Biol Med Sci. 1968 Jun;46(3):285–294. doi: 10.1038/icb.1968.23. [DOI] [PubMed] [Google Scholar]
- Malmquist J., Johansson B. G. Interaction of lactoferrin with agar gels and with Trypan Blue. Biochim Biophys Acta. 1971 Apr 27;236(1):38–46. doi: 10.1016/0005-2795(71)90146-2. [DOI] [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F. Lactoferrin in milk from different species. Comp Biochem Physiol B. 1971 May 15;39(1):119–129. doi: 10.1016/0305-0491(71)90258-6. [DOI] [PubMed] [Google Scholar]
- Masson P. L., Heremans J. F., Schonne E. Lactoferrin, an iron-binding protein in neutrophilic leukocytes. J Exp Med. 1969 Sep 1;130(3):643–658. doi: 10.1084/jem.130.3.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norcross N. L. Immune response in the mammary gland. J Dairy Sci. 1971 Dec;54(12):1880–1885. doi: 10.3168/jds.S0022-0302(71)86129-5. [DOI] [PubMed] [Google Scholar]
- Oram J. D., Reiter B. Inhibition of bacteria by lactoferrin and other iron-chelating agents. Biochim Biophys Acta. 1968 Dec 23;170(2):351–365. doi: 10.1016/0304-4165(68)90015-9. [DOI] [PubMed] [Google Scholar]
- Schanbacher F. L., Smith K. L. Electroimmunodiffusion on cellulose acetate: a rapid method for analysis of bovine lactoferrin in chromatography effluents. Anal Biochem. 1974 May;59(1):235–247. doi: 10.1016/0003-2697(74)90029-3. [DOI] [PubMed] [Google Scholar]
- Smith K. L., Conrad H. R., Porter R. M. Lactoferrin and IgG immunoglobulins from involuted bovine mammary glands. J Dairy Sci. 1971 Oct;54(10):1427–1435. doi: 10.3168/jds.S0022-0302(71)86043-5. [DOI] [PubMed] [Google Scholar]