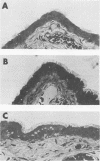
Abstract
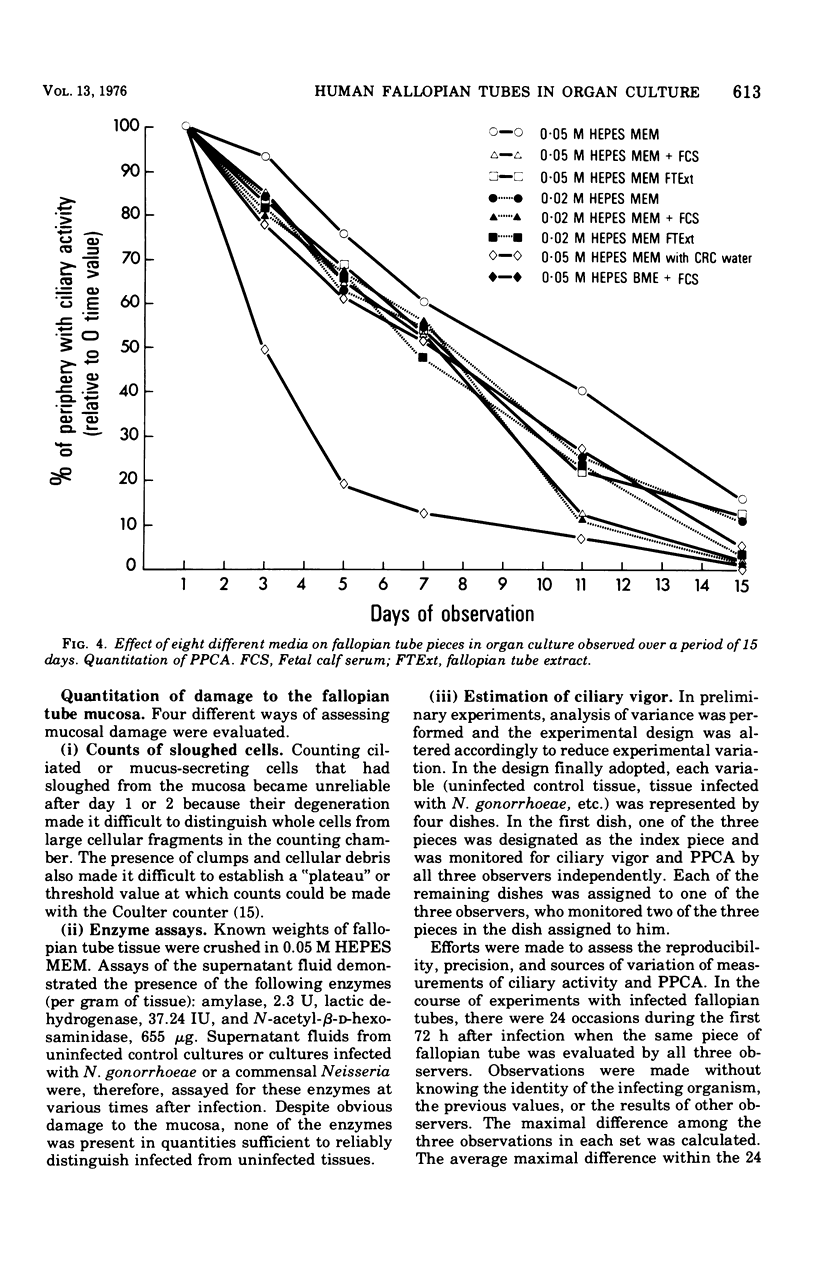
An experimental organ culture model has been developed in which rates of damage to fallopian tube mucosa by agents of sexually transmitted diseases can be quantitated. This required assessment of the effect on organ cultures of such variables as the anatomic area of the fallopian tube employed, endocrinological status of the donor, and composition of media. Organ cultures established from the ampulla of tubes of nonpregnant, premenopausal women and maintained with Eagle minimal essential medium containing 0.05 M N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid buffer were most suitable for quantitative studies. Various means of quantitating damage to fallopian tube mucosa were evaluated. Of these, sequential estimations of ciliary vigor and percentage of the periphery of fallopian tube pieces with active cilia proved the most successful. To assess the specificity of the model, fallopian tube organ cultures were infected with Neisseria gonorrhoeae or N. pharyngis, a commensal Neisseria. Damage by N. gonorrhoeae was significantly greater than that by N. pharyngis at each period of observation (P less than 0.001), suggesting that, by using the model, differences in virulence can be determined in vitro that reflect differences in virulence for humans. This model is a potentially valuable tool for studying the interaction of human genital mucosa with known or suspected agents of sexually transmitted diseases.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apicella M. A. Antigenically distinct populations of Neisseria gonorrhoeae: isolation and characterization of the responsible determinants. J Infect Dis. 1974 Dec;130(6):619–625. doi: 10.1093/infdis/130.6.619. [DOI] [PubMed] [Google Scholar]
- Arko R. J., Kraus S. J., Brown W. J., Buchanan T. M., Kuhn U. S. Neisseria gonorrhoeae: effects of systemic immunization on resistance of chimpanzees to urethral infection. J Infect Dis. 1974 Aug;130(2):160–164. doi: 10.1093/infdis/130.2.160. [DOI] [PubMed] [Google Scholar]
- Blaskovic P., Rohoman K., Rhodes A. J., Labzoffsky N. A. The effect of different media on maintenance of chick embryo tracheal organ cultures and replication of influenza virus. Brief report. Arch Gesamte Virusforsch. 1973;42(2):210–213. doi: 10.1007/BF01270842. [DOI] [PubMed] [Google Scholar]
- Brown W. J., Lucas C. T., Kuhn U. S. Gonorrhoea in the chimpanzee. Infection with laboratory-passed gonococci and by natural transmission. Br J Vener Dis. 1972 Jun;48(3):177–178. doi: 10.1136/sti.48.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan T. M. Antigenic heterogeneity of gonococcal pili. J Exp Med. 1975 Jun 1;141(6):1470–1475. doi: 10.1084/jem.141.6.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney F. E., Jr, Taylor-Robinson D. Growth and effect of Neisseria gonorrhoeae in organ cultures. Br J Vener Dis. 1973 Oct;49(5):435–440. doi: 10.1136/sti.49.5.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A., Kwapinski J. B., Ronald A. R. Isolation and immunological characterization of a unique toxic cytoplasmic polymer of type 1 Neisseria gonorrhoeae. Can J Microbiol. 1974 Aug;20(8):1163–1167. doi: 10.1139/m74-180. [DOI] [PubMed] [Google Scholar]
- Cherry J. D., Taylor-Robinson D. Growth and Pathogenesis of Mycoplasma mycoides var. capri in Chicken Embryo Tracheal Organ Cultures. Infect Immun. 1970 Oct;2(4):431–438. doi: 10.1128/iai.2.4.431-438.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier A. M., Clyde W. A. Relationships Between Mycoplasma pneumoniae and Human Respiratory Epithelium. Infect Immun. 1971 May;3(5):694–701. doi: 10.1128/iai.3.5.694-701.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny F. W. Effect of a toxin produced by Haemophilus influenzae on ciliated respiratory epithelium. J Infect Dis. 1974 Feb;129(2):93–100. doi: 10.1093/infdis/129.2.93. [DOI] [PubMed] [Google Scholar]
- HIRATA A. A. CYTOLYTIC ANTIBODY ASSAY BY TRYPTIC DIGESTION OF INJURED CELLS AND ELECTRONIC COUNTING. J Immunol. 1963 Nov;91:625–632. [PubMed] [Google Scholar]
- Holten E., Jyssum K. Activities of some enzymes concerning pyruvate metabolism in Neisseria. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Dec;82(6):843–848. doi: 10.1111/j.1699-0463.1974.tb02382.x. [DOI] [PubMed] [Google Scholar]
- Hu P. C., Collier A. M., Baseman J. B. Alterations in the metabolism of hamster tracheas in organ culture after infection by virulent Mycoplasma pneumoniae. Infect Immun. 1975 Apr;11(4):704–710. doi: 10.1128/iai.11.4.704-710.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jyssum S. Search for thymidine phosphorylase, nucleoside deoxyribosyltransferase and thymidine kinase in genus Neisseria. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Feb;82(1):53–56. doi: 10.1111/j.1699-0463.1974.tb02292.x. [DOI] [PubMed] [Google Scholar]
- Kellogg D. S., Jr, Cohen I. R., Norins L. C., Schroeter A. L., Reising G. Neisseria gonorrhoeae. II. Colonial variation and pathogenicity during 35 months in vitro. J Bacteriol. 1968 Sep;96(3):596–605. doi: 10.1128/jb.96.3.596-605.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lostroh A. J. Effect of testosterone and insulin in vitro on maintenance and repair of the secretory epithelium of the mouse prostate. Endocrinology. 1971 Feb;88(2):500–503. doi: 10.1210/endo-88-2-500. [DOI] [PubMed] [Google Scholar]
- Lucas C. T., Chandler F., Jr, Martin J. E., Jr, Schmale J. D. Transfer of gonococcal urethritis from man to chimpanzee. An animal model for gonorrhea. JAMA. 1971 Jun 7;216(10):1612–1614. [PubMed] [Google Scholar]
- McGEACHIN R. L., HARGAN L. A., POTTER B. A., DAUS A. T., Jr Amylase in fallopian tubes. Proc Soc Exp Biol Med. 1958 Oct;99(1):130–131. doi: 10.3181/00379727-99-24270. [DOI] [PubMed] [Google Scholar]
- Mercke U., Håkansson C. H., Toremalm N. G. A method for standardized studies of mucociliary activity. Acta Otolaryngol. 1974 Jul-Aug;78(1-2):118–123. doi: 10.3109/00016487409126335. [DOI] [PubMed] [Google Scholar]
- Peacock W. L., Jr, Schmale J. D. Toxic constituents of Neisseria gonorrhoeae. Nature. 1969 Feb 22;221(5182):760–761. doi: 10.1038/221760a0. [DOI] [PubMed] [Google Scholar]
- Pierce W. A., Jr, Leong J. K., Hough D. M. Strain-specific virulence-associated antigen of Neisseria gonorrhoeae. Infect Immun. 1975 May;11(5):898–903. doi: 10.1128/iai.11.5.898-903.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SOMOGYI M. Modifications of two methods for the assay of amylase. Clin Chem. 1960 Feb;6:23–35. [PubMed] [Google Scholar]
- Schmidt R. C., Maassab H. F. Local immunity to influenza virus in chicken tracheal organ cultures. J Infect Dis. 1974 Jun;129(6):637–643. doi: 10.1093/infdis/129.6.637. [DOI] [PubMed] [Google Scholar]
- Shipman C., Jr Evaluation of 4-(2-hydroxyethyl)-1-piperazineëthanesulfonic acid (HEPES) as a tissue culture buffer. Proc Soc Exp Biol Med. 1969 Jan;130(1):305–310. doi: 10.3181/00379727-130-33543. [DOI] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Taylor-Robinson D., Carney F. E., Jr Growth and effect of mycoplasmas in Fallopian tube organ cultures. Br J Vener Dis. 1974 Jun;50(3):212–216. doi: 10.1136/sti.50.3.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson D., Whytock S., Green C. J., Carney F. E., Jr Effect of Neisseria gonorrhoeae on human and rabbit oviducts. Br J Vener Dis. 1974 Aug;50(4):279–288. doi: 10.1136/sti.50.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward M. E., Watt P. J., Robertson J. N. The human fallopian tube: a laboratory model for gonococcal infection. J Infect Dis. 1974 Jun;129(6):650–659. doi: 10.1093/infdis/129.6.650. [DOI] [PubMed] [Google Scholar]
- Wiesner P. J., Handsfield H. H., Holmes K. K. Low antibiotic resistance of gonococci causing disseminated infection. N Engl J Med. 1973 Jun 7;288(23):1221–1222. doi: 10.1056/NEJM197306072882308. [DOI] [PubMed] [Google Scholar]